Abstract
The aim of the present study was to investigate the hepatoprotective and antioxidant effects of Lycium barbarum (LB) extract against paracetamol-induced acute oxidative stress and hepatotoxicity in rats. The subjects were divided into 6 groups of 8 rats each. The rats in the LB group were administered a dose of 100 mg/kg LB extract dissolved in saline via the intraperitoneal route for 7 days. Subsequently, after last dose of LB, PCT was given in a single dose of 1 g/kg diluted in saline via the oral route. Twenty-four hours later, blood samples were drawn from all of the subjects for serum Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Total antioxidant status (TAS) and Total oxidant status (TOS) tests, and liver tissue samples were obtained for histopathological evaluation. The mean TAS level of the group that was subjected to PCT intoxication was significantly lower than those of the other groups. Additionally, the mean TOS, Oxidative stress index (OSI), ALT and AST values were significantly higher in this group. Though the mean TAS level in the PCT + LB group was significantly higher than that of the PCT group, the TOS, OSI, ALT, and AST levels were significantly lower. When the PCT + LB group and the PCT only group were compared in terms of liver damage during the histopathological evaluation, a statistically significant difference was observed in Grade I and Grade III damage (P=0.013 and P=0.038, respectively). We conclude that Lycium barbarum extract leads to a significant improvement in PCT-induced acute hepatotoxicity in terms of the histopathological results, serum oxidative stress parameters, and serum liver function marker enzymes.
Keywords: Lycium barbarum, paracetamol intoxication, hepatic damage
Introduction
Acetaminophen (N-acetyl-p-amino-phenol, APAP), also known as paracetamol (PCT), is a drug with analgesic and antipyretic properties that can be safely used in therapeutic doses. However, overdoses of PCT may cause severe liver damage both in humans and experimental animal models [1]. The pharmacokinetics of acetaminophen show that approximately 90% of the oral dose is converted into its sulfate and glucuronide metabolites in the liver before it is excreted in the urine. Of the remaining part, 8% is metabolized into the more toxic N-acetyl-p-benzoquinone imine (NAPQI) via cytochrome p450 [2]. NAPQI covalently binds to the hepatocellular proteins and renders the cells more vulnerable to oxidative stress [3,4].
In the treatment of PCT toxicity, increasing the antioxidant concentration to prevent tissue damage associated with the increased oxidative stress plays an important role [5-7]. Used for this purpose, N-acetylcysteine (NAC) restores the glutathione (GSH) stores in the liver cells and leads to the detoxification of NAPQI and reduction of reactive oxygen species [8,9].
Historically consumed as a “super food” among the population, LB has become more popular in recent years as a beneficial nutrient and antioxidant (UK Food Standard Agency, http://www.food.gov.uk/news/news archive//jun/goji/). The bio-efficiency of LB arises from the polysaccharide complex with various fractions in its content. Recent studies have indicated a number of anti-aging, neuroprotective, anti-fatigue/endurance-increasing, and anti-oxidant biological effects of LB extract and the polysaccharides contained within it, which act as active ingredients, leading to increased metabolism, better glucose control in diabetes and a positive influence on glaucoma, immune-modulation, anti-tumor activity and cytoprotection [10,11].
The aim of the present study was to investigate the hepatoprotective and antioxidant effects of Lycium barbarum (LB) extract against paracetamol-induced acute oxidative stress and hepatotoxicity in rats.
Materials and methods
Preparation of the Lycium barbarum extract
To prepare the LB extract, 50 g of shade-dried, ground LB was soaked in 500 ml of ethanol for 7 days at ambient temperature. The mixture was filtered, and the obtained mass was desiccated to obtain 2 g of the extract [12]. The extraction of LB using ethanol yielded an extract with a high concentration of polysaccharides [13,14].
Animals and the study protocol
For the purposes of this experimental study, 48 males Wistar Albino rats weighing 250-300 grams, which were maintained at the Dicle University Health Sciences and Research Center (Diyarbakir, Turkey), were randomized into 6 groups. The rats were kept in wooden cages (14×9×8 cm) and were handled in a humane manner. Before beginning the experiment, all rats were supplied with standard rat chow and water ad libitum. The cages were located in an air-conditioned room maintained at 21°C, and light/dark cycles were alternated every 12 hours. The rats were fasted before the beginning of the experiment. The approval of the Dicle University Ethics Committee for Animal Studies (Diyarbakir, Turkey, 2013/35) was granted before the study.
The rats were divided into 6 groups with 8 rats in each group:
Control group: (distilled water; 10 ml/kg, perorally).
PCT group: The rats in this group received a single 1 g/kg oral dose of PCT diluted in saline through a gastric tube [15].
LB group: These rats were administered 100 mg/kg [16] of LB extract dissolved in saline through the intraperitoneal route for 7 days.
PCT + NAC group: Two hours after the administration of PCT, a single dose of 1.5 g/kg NAC [17] was administered intraperitoneally.
PCT + LB group: The rats in this group received 100 mg/kg of LB extract dissolved in saline through the intraperitoneal route for 7 days. Subsequently, they were administered PCT diluted in saline through a gastric tube in a single dose of 1 g/kg perorally.
PCT + NAC + LB group: These rats received 100 mg/kg LB extract dissolved in saline through the intraperitoneal route for 7 days. They were administered a single 1 g/kg oral dose of PCT diluted in saline through a gastric tube. After two hours, they were administered a single dose of 1.5 g/kg NAC intraperitoneally.
Drug administration and collection of the samples
Twenty-four hours after the administration of PCT, all rats were anesthetized with ketamine hydrochloride (50 mg/kg via the intramuscular route). The rats were placed in a supine position for the surgical procedure. A laparotomy was performed through a midline incision, and liver tissue biopsies and blood samples were obtained. At the end of the procedure, all rats were euthanized by exsanguination.
Biochemical analysis and oxidant-antioxidant parameters
Biochemical parameters
The maximum volume of blood was obtained through the intracardiac route and centrifuged, and the sera were stored at -70°C until the biochemical analysis. The serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) values were determined in U/L using the Abbott Architect c16000 Autoanalyzer.
The TAS level of the supernatant phase was analyzed using the novel automated measurement method developed by Erel [18]. Using this method, the most potent biological radical - the hydroxyl radical-is produced. In this assay, the ferrous ion solution in Reagent 1 is mixed with the hydrogen peroxide in Reagent 2. The sequentially produced radicals, including the brown-colored dianisidinyl radical cation produced by the hydroxyl radical, are also potent radicals. This method helps to measure the antioxidative effect of the sample against the potent free radical reactions initiated by the hydroxyl radical that is produced. The precision of the assay is within 3%. The obtained results are expressed as μmol H2O2 equivalent/L.
In the supernatant fractions, the TOS levels were measured by the novel automated measurement method developed by Erel [19]. In this method, the oxidants in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ions. The glycerol molecules, which are present in high amounts in the reaction medium, contribute to the oxidation reaction. In an acidic medium, the ferric ion leads to the formation of a colored complex with xylenol orange. The color intensity is measured by a spectrophotometer and depends on the total amount of oxidant molecules in the sample. Hydrogen peroxide is used to calibrate the assay, and the results are expressed in mmol Trolox equivalent/L.
OSI was defined as the percentage ratio of the TOS level to the TAS level. The calculation of the OSI value was based on the following formula: OSI (Arbitrary Units) = TOS (mmol Trolox equivalent/L)/TAC (μmol H2O2 equivalent/L) [20]. The results are expressed as Arbitrary Units.
Histopathological evaluation of liver damage
The tissue specimens were fixed in 10% formalin for 48 hours. Subsequently, they were embedded in paraffin and cut into 5 μm cross-sections. The slides were stained with hematoxylin-eosin. A liver pathologist blinded to the animal groups examined the cross-sections for hepatic injury under a light microscope with 100× or 200× magnification. Hepatic injury was classified as grade 0: minimal or no evidence of injury; grade 1: mild injury characterized by cytoplasmic vacuolization and focal nuclear pyknosis; grade 2: moderate injury exhibiting cytoplasmic vacuolization, confluent areas of hepatocyte ballooning but no frank necrosis, sinusoidal dilatation and congestion, and blurring of intercellular borders; grade 3: moderate to severe injury with areas of coagulative necrosis, cytoplasmic hypereosinophilia, extensive sinusoidal dilatation and congestion; grade 4: severe injury consisting of severe confluent coagulative necrosis and disintegration of and hemorrhage into hepatic chords leading to a loss of tissue architecture [21]. The results of the histopathological staging of the liver tissues are shown in Figure 1.
Figure 1.
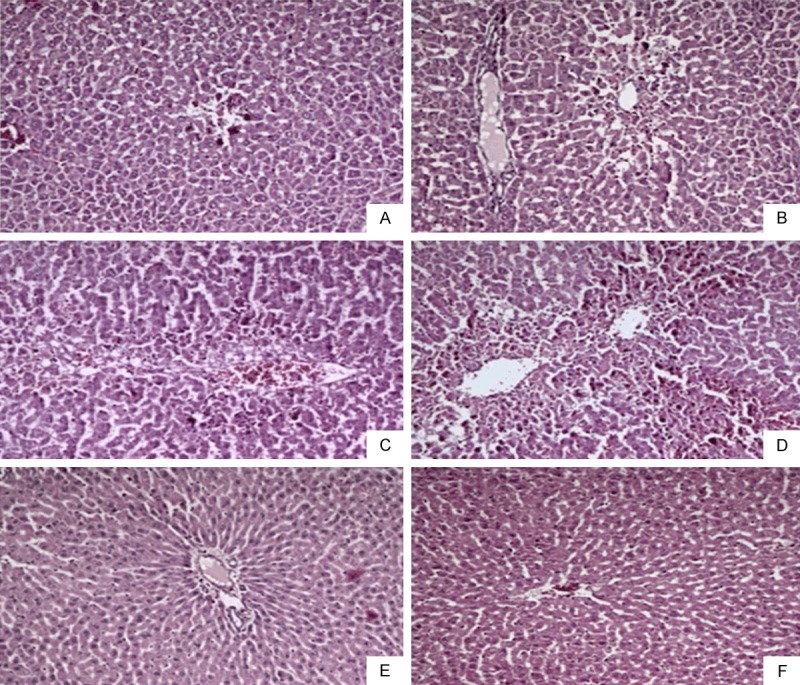
The histopathological evaluation of liver damage in each group. A: Control group, hepatic lobule (H&E, 100×); B: PCT group, massive hepatic lobule injury (H&E, 100×); C: LB group, protected hepatic lobule (H&E, 100×); D: PCT + NAC group, moderate hepatic lobule injury (H&E, 100×); E: PCT + LB group, moderate hepatic lobule injury (H&E, 100×); F: PCT + NAC + LB group, moderate hepatic lobule injury (H&E, 100×).
Statistical analysis
The statistical analyses were performed using the SPSS for Windows 11.0 (SPSS Inc., Chicago, IL, USA) software package. The categorical data was shown as percentages and the numerical data were presented as the median (minimum-maximum) values. A chi-square test was used to compare the categorical variables of the groups. The median values of numerical parameters of the study groups were compared using the nonparametric Kruskal-Wallis test. A Mann-Whitney U-test was used for pairwise comparisons. Spearman’s correlation test was employed to evaluate the correlations between the numerical variables. A p value below 0.05 was considered statistically significant.
Results
Biochemical parameters
When all of the groups were compared in terms of the serum AST, ALT, TOS, TAS and OSI (%) index, a statistically significant difference was observed between the groups. The mean serum AST, ALT, TOS, TAS and OSI index values are presented in Table 1.
Table 1.
The mean serum AST, ALT, TOS, TAS and OSI index values of the groups
| Parameters | CONTROL | PCT | LB | PCT + NAC | PCT + LB | PCT + NAC + LB | p |
|---|---|---|---|---|---|---|---|
| AST (IU/L) | 244.1±221.2 | 3452.9±991.4 | 131.1±16.6 | 734.3±433.4 | 678.8±624.7 | 702.8±646.0 | <0.001 |
| ALT (IU/L) | 80.1±45.4 | 3529.2±918.9 | 51.2±9.4 | 686.0±417.9 | 316.0±338.4 | 431.3±556.2 | <0.001 |
| TOS (mmol/L) | 80.0±93.1 | 218.6±166.4 | 35.2±13.2 | 102.9±60.3 | 51.1±48.8 | 71.6±53.5 | 0.02 |
| TAS (µmol/L) | 3.5±0.3 | 1.6±0.3 | 3.3±0.2 | 3.1±0.8 | 3.7±0.4 | 3.2±0.6 | <0.001 |
| OSI (%) | 2.3±1.9 | 14.5±9.7 | 3.3±1.7 | 3.2±1.4 | 1.3±1.1 | 2.2±1.7 | <0.001 |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TOS: Total oxidant status; TAS: Total antioxidant status; OSI: Oxidative stress index; PCT: Paracetamol; LB: Lycium barbarum; NAC: N-acetylcysteine.
When the groups in our study were assessed regarding the histopathological changes:
No statistically significant difference was observed between the PCT + LB group and the PCT + LB + NAC group. There was a statistically significant difference between the PCT group and the PCT + NAC group regarding Grade I and Grade III liver damage (P=0.013 and P=0.038, respectively).
No statistically significant difference was found between the PCT + NAC group and the PCT + NAC + LB group. A statistically significant difference was observed between the PCT group and the PCT + NAC + LB group regarding Grade I and Grade III liver damage (P=0.013 and P=0.038, respectively).
No statistically significant difference was observed between the PCT+NAC group and the PCT + LB group. A statistically significant difference was found between the PCT group and the PCT + LB group in terms of Grade I, II and III liver damage (P=0.003, P=0.038, and P=0.038, respectively).
The number of rats with hepatic damage in each group according to the histopathological grade is presented in Table 2.
Table 2.
Number of rats in each group according to histopathological grade
| Pathological Grade | Groups | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | PCT | LB | PCT + NAC | PCT + LB | PCT + NAC + LB | |
| (0) | 8 | 0 | 8 | 0 | 2 | 0 |
| (I) | 0 | 0a,b | 0 | 5 | 6 | 5 |
| (II) | 0 | 4a | 0 | 3 | 0 | 3 |
| (III) | 0 | 4a,b | 0 | 0 | 0 | 0 |
Significantly different compared with the PCT+LB group: For Grades I, II and III, P=0.003, P=0.038, and P=0.038, respectively.
Significantly different compared with the PCT+NAC and PCT+NAC+LB groups: For Grades I and III, P=0.013 and P=0.038, respectively.
The mean TAS level of the PCT group was significantly lower than those of the other groups. Additionally, the mean TOS, OSI, ALT and AST values were significantly higher in the PCT group.
Though the mean TAS level in the PCT + LB group was significantly higher than that in the PCT group, it was similar to the levels in the PCT + NAC and PCT + NAC + LB groups. In addition, the TOS, OSI, ALT, AST levels in PCT + LB group were significantly lower than those in PCT group 2, whereas they were similar to those in the PCT + NAC and PCT + NAC + LB groups.
A statistical comparison of the mean serum AST, ALT, TAS, TOS and OSI values of all groups in the study is presented in Table 3 along with the p values.
Table 3.
Statistical comparison of the groups according to AST, ALT, TAS, TOS and OSI values
| Groups | PCT | LB | PCT + NAC | PCT + LB | PCT + NAC + LB |
|---|---|---|---|---|---|
| Control | TAS*** | TAS | TAS | TAS | TAS |
| TOS** | TOS | TOS | TOS | TOS | |
| OSI*** | OSI | OSI | OSI | OSI | |
| AST*** | AST | AST | AST | AST | |
| ALT*** | ALT | ALT | ALT | ALT | |
| PCT | TAS*** | TAS*** | TAS*** | TAS*** | |
| TOS*** | TOS* | TOS*** | TOS** | ||
| OSI*** | OSI* | OSI*** | OSI** | ||
| AST*** | AST*** | AST*** | AST*** | ||
| ALT*** | ALT*** | ALT*** | ALT*** | ||
| LB | TAS | TAS | TAS | ||
| TOS | TOS | TOS | |||
| OSI | OSI | OSI | |||
| AST | AST | AST | |||
| ALT | ALT | ALT | |||
| PCT+NAC | TAS* | TAS | |||
| TOS | TOS | ||||
| OSI | OSI | ||||
| AST | AST | ||||
| ALT | ALT | ||||
| PCT+LB | TAS | ||||
| TOS | |||||
| OSI | |||||
| AST | |||||
| ALT |
P<0.05;
P<0.01;
P<0.001.
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TOS: Total oxidant status; TAS: Total antioxidant status; OSI: Oxidative stress index; PCT: Paracetamol; LB: Lycium barbarum; NAC: N-acetylcysteine.
Discussion
Our study shows the efficiency of LB extract in the prevention of liver damage due to PCT intoxication. This efficiency has been demonstrated with respect to histopathological evaluations, biochemical analyses and oxidative stress markers.
In the last 10 years, a number of studies focusing on the protective effects of various natural herbal extracts with antioxidant properties on organ damage associated with various causes have been published. Epigallocatechin gallate in green tea [22], the coprinus comatus fungus [23], and ginsan obtained from panax ginseng [24] can be mentioned among these substances.
LB extract has been profoundly investigated in recent years. These studies have suggested anti-oxidant, anti-aging, anti-tumor, and immune stimulating effects of LB extract. These effects are associated with various agents including beta carotene, riboflavin, ascorbic acid, thiamine cerebroside and betaine [25-29]. Additionally, the anti-oxidant protective effects of LB extract on the cellular damage that develops in the liver, eyes, small intestine and kidney have also been studied [30-33].
After ingestion, approximately 90% of PCT is transformed into its sulfate and glucuronide metabolites in the liver, which are excreted in the urine. The remaining 8% of PCT is transformed into the more toxic metabolite N-acetyl-p benzoquinone imine (NAPQI) by cytochrome p450. When the glutathione (GSH) stores are depleted [34], NAPQI bonds with cysteine in the hepatocytes, which leads to oxidative damage and hepatocellular centrilobular necrosis [35]. AST and ALT are released into the circulation from the damaged hepatocytes. Therefore, the classical laboratory evidence of hepatotoxicity is elevated AST and ALT levels [36].
In various studies, acetaminophen has been demonstrated to cause tissue lipid peroxidation, enzymatic inactivation, and oxidative damage, which also targets the antioxidant defense systems of the hepatocytes. Additionally, the oxidant parameters have been shown to increase and the antioxidant parameters have been found to decrease subsequent to oxidative damage due to acetaminophen intoxication [37,38]. In our study, an increase in the serum TOS levels, a decrease in the serum TAS levels, and an associated significant increase in the OSI levels were observed in the PCT group.
NAC - a precursor of cysteine-is a standard therapeutic agent used to prevent or reduce the liver damage caused by PCT [39]. Due to its known antioxidant properties, NAC is also used for various types of liver injury [40]. In this study, the TOS levels were decreased, the TAS levels were increased, and the OSI levels were significantly increased in the PCT + NAC group compared to the PCT group. Additionally, the ALT and AST levels in the PCT + NAC group were significantly lower. Finally, the histopathological evaluation revealed that the liver damage in the PCT + NAC group was significantly milder than that in the PCT group. This is an indication of the efficacy of NAC treatment in our study. This study has histopathologically demonstrated the protective effect of the LB extract on PCT intoxication. This protective effect has been verified through oxidative parameters and the ALT-AST levels. The PCT + LB group was similar to the PCT + LB + NAC group with respect to all parameters. This result indicates the lack of a synergistic effect between the two antioxidants.
In conclusion, LB extract is an efficient agent for prevention of the hepatotoxic effects of PCT intoxication. Future clinical studies may provide stronger evidence for the practical uses of this agent for both protection and treatment purposes in daily practice.
Acknowledgements
This study was conducted at the Dicle University Medical Faculty Health Research Center with the approval of the ethics committee for animal studies. This study was not supported by any financial sources; it was financed by the authors’ own budgets.
Disclosure of conflict of interest
None.
References
- 1.Kalsi S, Wood DM, Waring WS, Dargan PI. Does cytochrome P450 liver isoenzyme induction increase the risk of liver toxicity after paracetamol overdose? Open Access Emerg Med. 2011;3:69–76. doi: 10.2147/OAEM.S24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessems JG, Vermeulen NP. Paracetamol-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- 3.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava A, Maggs JL, Antoine DJ, Williams DP, Smith DA, Park BK. Role of reactive metabolites in drug induced hepatotoxicity. Handb Exp Pharmacol. 2010;196:165–194. doi: 10.1007/978-3-642-00663-0_7. [DOI] [PubMed] [Google Scholar]
- 5.Buckley NA, Eddleston M, Li Y. IV versus oral acetylcysteine. Ann Emerg Med. 2010;55:393–394. doi: 10.1016/j.annemergmed.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Su MK, Greller HA, Lee DC, Chan GM. IV versus oral N-acetylcysteine. Ann Emerg Med. 2009;54:857–858. doi: 10.1016/j.annemergmed.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Klein-Schwartz W, Doyon S. Intravenous acetylcysteine for the treatment of acetaminophen overdose. Expert Opin Pharmacother. 2011;12:119–130. doi: 10.1517/14656566.2011.537261. [DOI] [PubMed] [Google Scholar]
- 8.Lopez DP. Emergency: acetaminophen poisoning. Am J Nurs. 2009;109:48–51. doi: 10.1097/01.NAJ.0000360312.23879.b9. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Miki K, He X, Killeen ME, Fink MP. Prolonged treatment with N-acetylcysteine delays liver recovery from acetaminophen hepatotoxicity. Crit Care. 2009;13:R55–R61. doi: 10.1186/cc7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potterat O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76:7–19. doi: 10.1055/s-0029-1186218. [DOI] [PubMed] [Google Scholar]
- 11.Zhu YP, Gou QZ. In: Chinese Materia Medica Chemistry, Pharmacology and Applications. Zhu YP, editor. Amsterdam Harwood Academic Publishers; 1998. pp. 642–646. [Google Scholar]
- 12.Ananthi R, Chandra N, Santhiya ST, Ramesh A. Genotoxic and antigenotoxic effects of Hemidesmus indicus R. Br. root extract in cultured lymphocytes. J Ethnopharmacol. 2010;127:558–560. doi: 10.1016/j.jep.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Mu M. Studies on extraction of polysaccharides from Lycium barbarum. J Anhui Agric Sci. 2007;35:3736–3737. [Google Scholar]
- 14.Meng LY, Qiu AS, Lan TF, Jiang CC. Optimization of ultrasound extraction technology of Lycium barbarum polysaccharide and study on its antioxidation ability. J Anhui Agric Sci. 2009;37:12168–12170. [Google Scholar]
- 15.Jin SM, Kil HR, Park K, Noh C. Gene expression in rat hearts following oral administration of a single hepatotoxic dose of acetaminophen. Yonsei Med J. 2012;53:172–180. doi: 10.3349/ymj.2012.53.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang NT, Lin HI, Yeh DY, Chou TY, Chen CF, Leu FC, Wang D, Hu RT. Effects of the antioxidants Lycium barbarum and ascorbic acid on reperfusion liver injury in rats. J Ethnopharmacol. 2012;139:462–470. doi: 10.1016/j.transproceed.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Qiao S, Lei S, Liu Y, Ng KF, Xu A, Lam KS, Irwin MG, Xia Z. N-acetylcysteine and allopurinol synergistically enhance cardiac adiponectin content and reduce myocardial reperfusion injury in diabetic rats. PLoS One. 2011;6:e23967. doi: 10.1371/journal.pone.0023967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesik V, Güven A, Vurucu S, Tunç T, Uysal B, Gündoğdu G, Özta E, Korkmaz A. Melatonin and 1400 W Ameliorate both intestinal and remote organ injury following mesenteric ischemia/reperfusion. J Surg Res. 2009;1:1–9. doi: 10.1016/j.jss.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Chan HC, Chang RC, Koon-Ching Ip A, Chiu K, Yuen WH, Zee SY, So KF. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203:269–273. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Popovic M, Vukmirovic S, Stilinovic N, Capo I, Jakovljevic V. Anti-oxidative activity of an aqueous suspension of commercial preparation of the mushroom Coprinus comatus. Molecules. 2010;15:4564–4571. doi: 10.3390/molecules15074564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shim JY, Kim MH, Kim HD, Ahn JY, Yun YS, Song JY. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response. Toxicol Appl Pharmacol. 2010;242:318–325. doi: 10.1016/j.taap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Gan L, Hua ZS, Liang YX, Bi XH. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int Immunopharmacol. 2004;4:563–569. doi: 10.1016/j.intimp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Deng HB, Cui DP, Jiang JM, Feng YC, Cai NS, Li DD. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on non-enzyme glycation in D-galactose induced mouse aging model. Biomed Environ Sci. 2003;16:267–275. [PubMed] [Google Scholar]
- 27.Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 28.Amagase H, Nance DM. A randomized, double-blind, placebo-controlled, clinical study of the general effects of a standardized Lycium barbarum (Goji) juice, GoChi. J Altern Complement Med. 2008;14:403–412. doi: 10.1089/acm.2008.0004. [DOI] [PubMed] [Google Scholar]
- 29.Seeram NP. Berry fruits: compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem. 2008;56:627–629. doi: 10.1021/jf071988k. [DOI] [PubMed] [Google Scholar]
- 30.Xiao J, Zhu Y, Liu Y, Tipoe GL, Xing F, So KF. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int J Biol Macromol. 2014;69:73–78. doi: 10.1016/j.ijbiomac.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Xiao J, Liong EC, Ching YP, Chang RC, Fung ML, Xu AM, So KF, Tipoe GL. Lycium barbarum polysaccharides protect rat liver from non- alcoholic steatohepatitis-induced injury. Nutr Diabetes. 2013;3:e81. doi: 10.1038/nutd.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Bai H, Cai W, Li J, Zhou Q, Wang Y, Han J, Zhu X, Dong M, Hu D. Lycium barbarum polysaccharides reduce intestinal ischemia/reperfusion injuries in rats. Chem Biol Interact. 2013;204:166–172. doi: 10.1016/j.cbi.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Yu MS, Leung SK, Lai SW, Che CM, Zee SY, So KF, Yuen WH, Chang RC. Neuroprotective effects of antiaging oriental medicine Lycium barbarum against beta-amyloid peptide neurotoxicity. Exp Gerontol. 2005;40:716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Bessems JG, Vermeulen NP. Paracetamol-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- 35.Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- 36.Hung OL, Nelson LS. Acetaminophen. In: Tintinalli JE, Kelen GD, Stapczynski JS, editors. Emergency Medicine: A Comprehensive Study Guide. New York: McGraw-Hill; 2004. pp. 1088–1094. [Google Scholar]
- 37.Isık B, Bayrak R, Akcay A, Sogut S. Erdosteine against acetaminophen induced renal toxicity. Mol Cell Biochem. 2006;287:185–191. doi: 10.1007/s11010-005-9110-6. [DOI] [PubMed] [Google Scholar]
- 38.Kuvandik G, Duru M, Nacar A, Yonden Z, Helvaci R, Koc A, Kozlu T, Kaya H, Sogut S. Effects of erdosteine on acetaminophen-induced hepatotoxicity in rats. Toxicol Pathol. 2008;36:714–719. doi: 10.1177/0192623308320800. [DOI] [PubMed] [Google Scholar]
- 39.Yağmurca M, Bas O, Mollaoglu H, Sahin O, Nacar A, Karaman O, Songur A. Protective effects of erdosteine on doxorubicin induced hepatotoxicity in rats. Arch Med Res. 2007;38:380–385. doi: 10.1016/j.arcmed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, Velayati AA. Protective effect of N-acetylcysteine on antituberculosis drug induced hepatotoxicity. Eur J Gastroenterol Hepatol. 2010;22:1235–1238. doi: 10.1097/MEG.0b013e32833aa11b. [DOI] [PubMed] [Google Scholar]


