Abstract
The development of bioactive surgical additives to regulate the inflammation and increase the speed of healing process is one of the great challenges in clinical research. In this sense, platelet rich fibrin (PRF) appears as a natural and satisfactory alternative with favorable results and low risks. The following review attempts to summarize the relevant literature regarding the technique of using PRF, focusing on its preparation, advantages, and disadvantages of using it in clinical applications. PRF alone or in combination with other biomaterials seems to have several advantages and indications both for medicine and dentistry, due it is a minimally invasive technique with low risks and satisfactory clinical results.
Keywords: Blood platelet, fibrin, platelet-rich fibrin, bone regeneration, oral surgery
Introduction
The development of bioactive surgical additives, which are being used to regulate the inflammation and increase the speed of healing process [1], is one of the great challenges in clinical research. In this sense, healing is a complex process, which involves cellular organization, chemical signals, and the extracellular matrix for tissue repair [2]. The understanding of healing process is still incomplete, but it is well known that platelets play an important role in both hemostasis and wound healing processes [3].
Platelets’ regenerative potential was introduced in the 70’s [4], when it was observed that they contain growth factors that are responsible for increase collagen production, cell mitosis, blood vessels growth, recruitment of other cells that migrate to the site of injury, and cell differentiation induction, among others [5].
One of the latest innovations in oral surgery is the use of platelet concentrates for in vivo tissue engineering applications: 1) platelet-rich plasma (PRP) and 2) platelet-rich fibrin (PRF). Platelet concentrates are a concentrated suspension of growth factors found in platelets, which act as bioactive surgical additives that are applied locally to induce wound healing [5].
Whitman et al [6], in 1997, were the first to introduce the use of platelet-rich plasma in oral surgical procedures, reporting great advantages because it enhances osteoprogenitor cells in the host bone and bone graft. However, using it also presents risk because bovine thrombin, which is used to handle PRP, may generate antibodies to factors V, XI, and thrombin that could cause coagulopathies that may endanger life [5].
On the other hand, PRF was first used in 2001 by Choukroun et al. [7], specifically in oral and maxillofacial surgery, and is currently considered as a new generation of platelet concentrate. It consists of a matrix of autologous fibrin [1] and has several advantages over PRP, including easier preparation and not requiring chemical manipulation of the blood, which makes it strictly an autologous preparation [5].
The following review attempts to summarize the relevant literature regarding the technique of using platelet rich fibrin (PRF), focusing on its preparation, advantages, and disadvantages of using it in clinical applications.
Platelets and fibrin
Platelets are the second-most numerous corpuscles in the blood [8]. They are cytoplasmic fragments that lack core derived from megakaryocytes [9,10]. Their lifetime is between 7 and 10 days [10], and the normal peripheral blood concentration is 150-450 × 109/L [8]. These unactivated platelets are biconvex discoid structures shaped like a lens with dimensions of approximately 2.0-4.0 by 0.5 µm and a mean volume of 7-11 fl [8].
Platelets are formed by a peripheral area, which corresponds to a phospholipid membrane, a series of microtubules, and a very extensive canalicular system connecting the surface to the cytoplasm. Glycogen granules, mitochondria, lysosomes, peroxisomes, and various types of inclusions, including alpha and dense granules, can be identified within the cytoplasm [11]. Alpha granules are large macromolecules that constitute 15% of the total platelet volume [10]. They contain platelet-specific and non-platelet-specific proteins (fibrinogen, fibronectin, thrombospondin, growth factors, etc.) [11]. Additionally, the dense granules have high content of calcium, inorganic phosphorus, ADP, ATP, and serotonin [11]. However, the most important elements of platelets to achieve the healing and repair processes are the leukocytes and growth factors, being the latter, polypeptides that participate in differentiation, proliferation, migration, and cell metabolism [12].
Growth factors stimulate and attract stem cells to the site of injury, promoting cell mitosis and inducing angiogenesis and osteogenesis [13]. These growth factors, after activation from the platelets trapped within fibrin matrix, have demonstrated stimulating a mitogenic response of periosteum cells to achieve bone healing [14]. Cytokines are also released from the platelets, being responsible in modulating platelet activation and the proliferation and differentiation of leukocytes, playing an important role in immunology, specifically, in inflammation mechanism [13].
Furthermore, fibrin is a bridging molecule that allows a series of cell interactions and supplies a provisional matrix in which cells may proliferate, organize, and carry out their functions, mainly, in sites that suffered injury or inflammation [15]. Fibrin provides a matrix for migration of fibroblasts and endothelial cells, which are involved in the angiogenesis process and are responsible in the healing of new tissues [3]. The formation of a fibrin network is the result of the transformation of soluble fibrinogen - a large glycoprotein-into a soluble fibrin by the action of thrombin and factor XIIIa [16]. This process may be divided into three stages: 1) proteolysis of fibrinogen by thrombin; 2) polymerization of fibrin monomers; and 3) stabilization of fibrin by factor XIIIa [17].
Fibrin may suffer multiple variations in its structure due to different physiological situations, such as the concentration of calcium ions and fibrinogen and the quality of it. Moreover, fibrin may change its structure in patients with certain comorbidities, such as diabetes and nephrotic syndrome, among others [18].
Platelet-rich fibrin
PRF consists of an autologous leukocyte-platelet-rich fibrin matrix [1,13], composed of a tetra molecular structure, with cytokines, platelets, cytokines, and stem cells within it [11,19], which acts as a biodegradable scaffold [20] that favors the development of microvascularization and is able to guide epithelial cell migration to its surface [19,21]. Also, PRF may serve as a vehicle in carrying cells involved in tissue regeneration [22] and seems to have a sustained release of growth factors [23] in a period between 1 and 4 weeks, stimulating the environment for wound healing in a significant amount of time [24]. It has a complex architecture of strong fibrin matrix with favorable mechanical properties and is slowly remodeled, similar to blood clot [24]. Some studies [25-28] have demonstrated that PRF is a healing biomaterial with a great potential for bone and soft tissue regeneration, without inflammatory reactions and may be used alone or in combination with bone grafts, promoting hemostasis, bone growth, and maturation. This autologous matrix demonstrated in in vitro studies a great potential to increase cell attachment [24] and a stimulation to proliferate and differentiate osteoblasts [29]. Dohan et al. [21,30] stated that PRF has immunological and antibacterial properties, may lead to leukocyte degranulation, and has some cytokines that may induce angiogenesis and pro/anti-inflammatory reactions.
The difference between natural blood clot and PRF is that the latter is more homogeneous and stable and easy to handle and place in the indicated local [31].
In surgical procedures, PRF could serve as a resorbable membrane for guided bone regeneration (GBR) [22], preventing the migration of non-desirable cells into bone defect and providing a space that allows the immigration of osteogenic and angiogenic cells and permits the underlying blood clot to mineralize [32]. However, a normal PRF membrane has rapid degradability (1-2 weeks), but if fibers are cross-linked, it could provide resistance against enzymatic degradation and could be more stable during the healing time [23]. With the above, Kawase et al. [23], in an in vitro research using mice, suggested heat compression of PRF membrane in cases with indications of guided bone regeneration because this procedure is a less cytotoxic technique, which reduces the surface area and porosity, delaying its degradation until 4 weeks.
Simonpieri et al. [31] mentioned the concept of “natural bone Regeneration” (NBR), which includes regeneration through PRF membranes both the bone volume and gingival tissue. The same authors reported satisfactory clinical results related to reshaping the whole alveolar bone and the restoration of gingival volume and peri-implant bone, achieving adequate mechanical and aesthetic properties.
PRF membrane has exhibited favorable clinical results in the treatment of periodontal infrabony defects [22], protecting open wounds from oral environment when the suture cannot bind the mucosal margins [13,19,22,31], and accelerating hard and soft tissue healing [19,31,33]. Some clinical studies [34,35] used PRF membrane as a sole grafting material to achieve maxillary sinus floor augmentation, presenting promising results.
Other authors, including Tofler et al. [36], recommended the use of PRF membrane to seal an undetected sinus membrane perforation during a lateral window osteotomy in a maxillary sinus lift procedure.
PRF membrane helps in wound healing, protecting the surgical site [33,36], promoting soft tissue repair; when mixed with bone graft, it may act as a “biological connector”, which attracts stem cell, favors the migration of osteoprogenitor cells to the center of the graft, and provides a neo-angiogenesis [36].
Furthermore, Choukroun et al. [37] conducted a study in which they wanted to see the potential of using PRF in conjunction with freeze-dried bone allograft with (FDBA) to enhance bone regeneration in a maxillary sinus lift procedure. The results showed a decreased healing time prior to implant placement. From the histological point of view, this healing time was reduced by half - from 8 months to 4 months; however, large-scale studies are needed to validate these results [37]. Additionally, the addition of PRF to the bone graft can lead to a reduction of the volume of bone substitute used and seems to improve revascularization of the graft by supporting angiogenesis [37]. Simonpieri et al. [31] suggested using a mix of PRF with a bone graft, placing in bone defects, or, in cases of immediate implants, covering it with several PRF layers, noting good clinical results.
Yilmaz et al. [38] histologically and stereologically compared the healing effects of β-TCP and PRF, alone and in combination, in standardized bone defects in pig’s tibiae. The results showed that when β-TCP and PRF were used together, the newly formed bone was significantly greater than when used both separately. In addition, PRF may act as a biologic adhesive to hold the particles together, facilitating the manipulation of the bone grafts [38].
Protocol for PRF preparation
The protocol tries to accumulate platelets and the released cytokines in a fibrin clot [35].
PRF protocol requires only centrifuged blood without any addition of anticoagulant and bovine thrombin [1]. Then, a blood sample is taken without anticoagulant in 10-mL tubes in a glass or glass-coated plastic tube [1,11,39], then immediately centrifuged at 3,000 rpm for 10 minutes [1,3,39].
The resultant product consists of the following three layers [1]:
● Top-most layer consisting of a cellular plasma.
● PRF clot in the middle.
● Red corpuscle base at the bottom.
After this, it is necessary to put the PRF clot in a sterile cup for approximately 10 minutes to allow the release of the proper serum contained within [1]. Mazor et al. [35] reported that clot could be transformed into a membrane through the compression between two sterile gauzes or in a specific tool.
At the beginning, fibrinogen is concentrated in the upper part of the tube before the proper thrombin transforms it into fibrin [1]. Due to the lack of an anticoagulant, blood begins to coagulate as soon as it comes in contact with the glass surface. The contact with a silica surface is required to activate the clot polymerization process [40]. In this sense, PRF may be obtained only in dry glass tubes or glass-coated plastic tubes. Moreover, the silica particles do not represent a risk of cytotoxicity compared with, for example, the bovine thrombin used for PRP preparation [40].
Finally, platelets seem to be trapped massively in the fibrin meshes [1]. Therefore, for successful preparation of PRF, speedy blood collection and immediate centrifugation before the initiation clotting cascade is absolutely essential [41]. Quick handling is the key to achieve a clinically usable PRF clot [1].
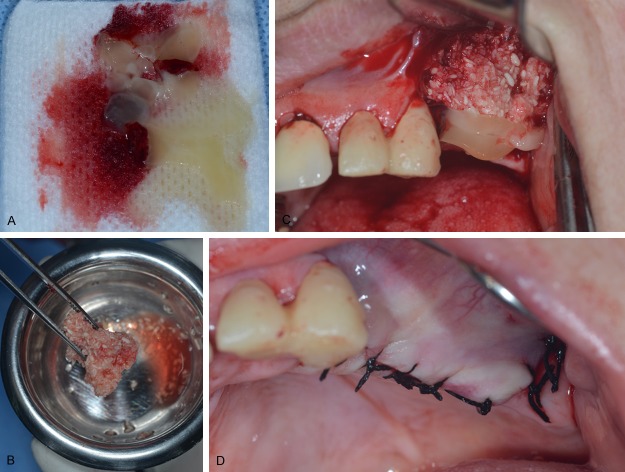
Figure 1 shows a horizontal bone augmentation in maxillary ridge with platelet-rich fibrin mixed with an allograft (Puros®, Cortico-Cancellous Particulate Allograft, Zimmer Dental Inc, San Diego, CA, USA).
Figure 1.
Clinical use during a horizontal bone augmentation surgery. A. PRF clot in sterile gauze for serum release. B. Allograft mixed with PRF clot. C. Location of PRF + allograft in the alveolar ridge to augment in width. Note the PRF layer over the bone graft. D. Final suture. It was not necessary to expose the PRF membrane layer because in this case it was possible to bind the mucosal margins.
Advantages of using PRF
Some advantages are reported in the literature related to the use of PRF, such as the following:
● Its preparation is a simplified and efficient technique, with centrifugation in a single step, free and openly accessible for all clinicians [31,40].
● It is obtained by autologous blood sample [19].
● Minimized blood manipulation [42].
● It does not require the addition of external thrombin because polymerization is a completely natural process, without any risk of suffering from an immunological reaction [1,42].
● It has a natural fibrin framework with growth factors within that may keep their activity for a relatively longer period and stimulate tissue regeneration effectively [24].
● It can be used solely or in combination with bone grafts, depending on the purpose [31,37].
● Increases the healing rate of the grafted bone [37,42].
● It is an economical and quick option compared with recombinant growth factors when used in conjunction with bone grafts [43].
● Used as a membrane, it avoids a donor site surgical procedure and results in a reduction in patient discomfort during the early wound-healing period [44].
● The studies of PRF present it to be more efficient and with less controversies on its final clinical results when compared to PRP [31].
Disadvantages of using PRF
PRF may present some disadvantages as follows:
● The final amount available is low because it is autologous blood [19].
● The success of the PRF protocol depends directly on the handling, mainly, related to blood collection time and its transference for the centrifuge [1].
● Need of using a glass-coated tube to achieve clot polymerization [40].
● Possible refusal of treatment by the puncture required for blood collection (Wani 2014).
● Only needs a minimal experience of clinician for PRF manipulation [13,31].
Other clinical applications
The literature reports some other possible applications of PRF such as:
● Inperiodontal bone defects: achieving a probing depth reduction and a radiographic defect fills [22].
● In localized osteitis, 90% of osteitis reduction was found in surgical sites of the third molars [46].
● As an adjunct to palatal wound healing after harvesting a free gingival graft [47].
● As a potential scaffold in pulp revascularization procedures of necrotic immature permanent tooth: as it is rich in growth factors, it seems to enhance cellular proliferation and differentiation, augmenting angiogenesis, acting as a matrix for tissue growth, and regulating the inflammatory reaction [48].
● In multiple extractions to preserve the alveolar ridge height [31].
● Bone regeneration around immediate implants, inside the alveolar defect [31].
● Reconstruction of large bone defects after cancer surgery [49].
● In plastic surgery, PRF clots are often directly used to fill cavities [50] or mixed with an adipocyte graft in a lipostructure [51].
● In the membrane form, it could be useful for small otologic surgery [52,53].
Conclusion
In vitro and in vivo studies have demonstrated safe and promising results, without contradictory findings, related to the use of PRF alone or in combination with other biomaterials. It has several advantages and possible indications to be used both in medicine and dentistry. Currently, platelet-rich fibrin seems to be an accepted minimally invasive technique with low risks and satisfactory clinical results.
Disclosure of conflict of interest
None.
References
- 1.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Gassling VL, Açil Y, Springer IN, Hubert N, Wiltfang J. Platelet-rich plasma and platelet-rich fibrin in human cell culture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:48–55. doi: 10.1016/j.tripleo.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974;71:1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiran NK, Mukunda KS, Tilak Raj TN. Platelet concentrates: A promising innovation in dentistry. J Dent Sci Res. 2011;2:50–61. [Google Scholar]
- 6.Whitmann DH, Berry RL, Green DM. Platelet gel: an alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–1299. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 7.Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implantodontie. 2000;42:55–62. [Google Scholar]
- 8.Harrison P. Platelet function analysis. Blood Rev. 2005;19:111–123. doi: 10.1016/j.blre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Castro HC, Ferreira BLA, Nagashima T, Schueler A, Rueff C, Camisasca D, Moreira G, Scovino G, Borges L, Leal M, Filgueira M, Paschoal P, Bernardo V, Bourguinhon S, Rodrigues CR, Santos DO. Plaquetas: ainda um alvo terapêutico. J Bras Patol Med Lab. 2006;42:321–332. [Google Scholar]
- 10.Fernández-Delgado N, Hernández-Ramírez P, Forrellat-Barrios M. Platelet functional spectrum: from hemostasis to regenerative medicine. Rev Cubana Hematol Inmunol Hemoter. 2012;28:200–216. [Google Scholar]
- 11.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Garcia VG, Corral I, Bascones-Martínez A. Plasma rico en plaquetas y su utilización en implantología dental. Av Period. 2004;16:81–92. [Google Scholar]
- 13.Gupta V, Bains BK, Singh GP, Mathur A, Bains R. Regenerative potential of platelet rich fibrin in dentistry: Literature review. Asian J Oral Health Allied Sci. 2011;1:22–28. [Google Scholar]
- 14.Gassling V, Douglas T, Warnke PH, Açil Y, Wiltfang J, Becker ST. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21:543–549. doi: 10.1111/j.1600-0501.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 15.Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost. 2006;4:932–939. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 16.Silver FH, Wang MC, Pins GD. Preparation and use of fibrin glue in surgery. Biomaterials. 1995;16:891–903. doi: 10.1016/0142-9612(95)93113-r. [DOI] [PubMed] [Google Scholar]
- 17.Pratt KP, Côté HC, Chung DW, Stenkamp RE, Davie EW. The primary fibrin polymerization pocket: three-dimensional structure of a 30-kDa C-terminal gamma chain fragment complexed with the peptide Gly-Pro-Arg-Pro. Proc Natl Acad Sci U S A. 1997;94:7176–7181. doi: 10.1073/pnas.94.14.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauricella AM. Fibrin network variability. Acta Bioquím Clín Latinoam. 2007;41:7–19. [Google Scholar]
- 19.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Pan S, Dangaria SJ, Gopinathan G, Kolokythas A, Chu S, Geng Y, Zhou Y, Luan X. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. Biomed Res Int. 2013;2013:638043. doi: 10.1155/2013/638043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51–55. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Chang YC, Zhao JH. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dent J. 2011;56:365–371. doi: 10.1111/j.1834-7819.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 23.Kawase T, Kamiya M, Kobayashi M, Tanaka T, Okuda K, Wolff LF, Yoshie H. The heat-compression technique for the conversion of platelet-rich fibrin preparation to a barrier membrane with a reduced rate of biodegradation. J Biomed Mater Res B Appl Biomater. 2015;103:825–31. doi: 10.1002/jbm.b.33262. [DOI] [PubMed] [Google Scholar]
- 24.Wu CL, Lee SS, Tsai CH, Lu KH, Zhao JH, Chang YC. Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust Dent J. 2012;57:207–212. doi: 10.1111/j.1834-7819.2012.01686.x. [DOI] [PubMed] [Google Scholar]
- 25.Saluja H, Dehane V, Mahindra U. Platelet-Rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann Maxillofac Surg. 2011;1:53–57. doi: 10.4103/2231-0746.83158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bölükbaşı N, Ersanlı S, Keklikoğlu N, Başeğmez C, Ozdemir T. Sinus augmentation with platelet-rich fibrin in combination with bovine bone graft versus bovine bone graft in combination with collagen membrane. J Oral Implantol. 2013 doi: 10.1563/AAID-JOI-D-13-00129. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Joseph VR, Sam G, Amol NV. Clinical evaluation of autologous platelet rich fibrin in horizontal alveolar bony defects. J Clin Diagn Res. 2014;8:ZC43–47. doi: 10.7860/JCDR/2014/9948.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TH, Kim SH, Sándor GK, Kim YD. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF) in rabbit-skull defect healing. Arch Oral Biol. 2014;59:550–558. doi: 10.1016/j.archoralbio.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Dohan Ehrenfest DM, Diss A, Odin G, Doglioli P, Hippolyte MP, Charrier JB. In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:341–352. doi: 10.1016/j.tripleo.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a choukroun’s platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546–555. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 31.Simonpieri A, Del Corso M, Vervelle A, Jimbo R, Inchingolo F, Sammartino G, Dohan Ehrenfest DM. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012;13:1231–1256. doi: 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]
- 32.Molly L, Quirynen M, Michiels K, Van Steenberghe D. Comparison between jaw bone augmentation by means of a stiff occlusive titanium membrane or an autologous hip graft: a retrospective clinical assessment. Clin Oral Implants Res. 2006;17:481–487. doi: 10.1111/j.1600-0501.2006.01286.x. [DOI] [PubMed] [Google Scholar]
- 33.Del Corso M, Toffler M, Dohan Ehrenfest DM. Use of an autologous leukocyte and platelet-rich fibrin (L-PRF) membrane in post-avulsion sites: an overview of Choukroun’s PRF. J Implant Adv Clin Dent. 2010;1:27–35. [Google Scholar]
- 34.Simonpieri A, Choukroun J, Del Corso M, Sammartino G, Dohan Ehrenfest DM. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent. 2011;20:2–12. doi: 10.1097/ID.0b013e3181faa8af. [DOI] [PubMed] [Google Scholar]
- 35.Mazor Z, Horowitz RA, Del Corso M, Prasad HS, Rohrer MD, Dohan Ehrenfest DM. Sinus floor augmentation with simultaneous implant placement using Choukroun’s platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J Periodontol. 2009;80:2056–2064. doi: 10.1902/jop.2009.090252. [DOI] [PubMed] [Google Scholar]
- 36.Toffler M, Toscano N, Holtzclaw D, Corso MD, Dohan Ehrenfest DM. Introducing Choukroun’s platelet rich fibrin (PRF) to the reconstructive surgery milieu. J Implant Clin Adv Dent. 2009;1:21–30. [Google Scholar]
- 37.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz D, Dogan N, Ozkan A, Sencimen M, Ora BE, Mutlu I. Effect of platelet rich fibrin and beta tricalcium phosphate on bone healing. A histological study in pigs. Acta Cir Bras. 2014;29:59–65. doi: 10.1590/S0102-86502014000100009. [DOI] [PubMed] [Google Scholar]
- 39.Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36:1628–1632. doi: 10.1016/j.joen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Dohan DM, Del Corso M, Charrier J-B. Cytotoxicity analyses of Choukroun’s platelet-rich fibrin (PRF) on a wide range of human cells: The answer to a commercial controversy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:587–583. [Google Scholar]
- 41.Anilkumar K, Geetha A, Umasudhakar , Ramakrishnan T, Vijayalakshmi R, Pameela E. Platelet-rich-fibrin: A novel root coverage approach. J Indian Soc Periodontol. 2009;13:50–4. doi: 10.4103/0972-124X.51897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang YH, Jeon SH, Park JY, Chung JH, Choung YH, Choung HW, Kim ES, Choung PH. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A. 2011;17:349–359. doi: 10.1089/ten.TEA.2010.0327. [DOI] [PubMed] [Google Scholar]
- 43.Girish Rao S, Bhat P, Nagesh KS, Rao GH, Mirle B, Kharbhari L, Gangaprasad B. Bone regeneration in extraction sockets with autologous platelet rich fibrin gel. J Maxillofac Oral Surg. 2013;12:11–16. doi: 10.1007/s12663-012-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, Kenney EB, Camargo P. Use of platelet-rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. Int J Periodontics Restorative Dent. 2012;32:e41–50. [PubMed] [Google Scholar]
- 45.Wani AL, Ara A, Bhat SA. Blood injury and injection phobia: the neglected one. Behav Neurol. 2014;2014:471340. doi: 10.1155/2014/471340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoaglin DR, Lines GK. Prevention of localized osteitis in mandibular third-molar sites using platelet-rich fibrin. Int J Dent. 2013;2013:875380. doi: 10.1155/2013/875380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulkarni MR, Thomas BS, Varghese JM, Bhat GS. Platelet-rich fibrin as an adjunct to palatal wound healing after harvesting a free gingival graft: A case series. J Indian Soc Periodontol. 2014;18:399–402. doi: 10.4103/0972-124X.134591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keswani D, Pandey RK. Revascularization of an immature tooth with a necrotic pulp using platelet-rich fibrin: a case report. Int Endod J. 2013;46:1096–1104. doi: 10.1111/iej.12107. [DOI] [PubMed] [Google Scholar]
- 49.Reyes M, Montero S, Cifuentes J, Zarzar E. Extraction technique and surgical use of the plasma rich in growth factors (P. R.G.F.): Update. Rev Dent Chile. 2002;93:25–28. [Google Scholar]
- 50.Charrier JB, Monteil JP, Albert S, Collon S, Bobin S, Dohan Ehrenfest DM. Relevance of Choukroun’s Platelet-Rich Fibrin (PRF) and SMAS flap in primary reconstruction after superficial or subtotal parotidectomy in patients with focal pleiomorphic adenoma: a new technique. Rev Laryngol Otol Rhinol (Bord) 2008;129:313–318. [PubMed] [Google Scholar]
- 51.Braccini F, Dohan DM. The relevance of Choukroun’s platelet rich fibrin (PRF) during facial aesthetic lipostructure (Coleman’s technique): preliminary results. Rev Laryngol Otol Rhinol (Bord) 2007;128:255–260. [PubMed] [Google Scholar]
- 52.Choukroun JI, Braccini F, Diss A, Giordano G, Doglioli P, Dohan DM. Influence of platelet rich fibrin (PRF) on proliferation of human preadipocytes and tympanic keratinocytes: A new opportunity in facial lipostructure (Coleman’s technique) and tympanoplasty. Rev Laryngol Otol Rhinol (Bord) 2007;128:27–32. [PubMed] [Google Scholar]
- 53.Braccini F, Tardivet L, Dohan Ehrenfest DM. The relevance of Choukroun’s Platelet-Rich Fibrin (PRF) during middle ear surgery: preliminary results. Rev Laryngol Otol Rhinol (Bord) 2009;130:175–180. [PubMed] [Google Scholar]