Abstract
We aimed to evaluate the role of genetic polymorphisms in IL-1β, IL-8 and IL-10 in the risk of coronary artery disease (CAD). We identified 325 patients with CAD and 342 control subjects without CAD between January 2013 and December 2014. Genotyping of IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A, IL-10-1082A/G and IL-10-819C/T was performed in a 384-well plate format on the Sequenom MassARRAY platform (Sequenom, San Diego, USA). By Multivariate logistic regression analysis, the GG and AG+GG genotypes of IL-10-1082A/G were significantly associated with an increased risk of CAD. The ORs (95% CI) for GG and AG+GG genotypes were 2.12 (1.32-3.43) and 1.56 (1.14-2.14), respectively. Patients carrying the AG+GG genotype of IL-10-1082A/G was associated with an increased risk of CAD in those with hypertension, diabetes mellitus and smokers, and the ORs (95% CI) were 1.41 (0.93-2.14), 7.13 (2.28-23.56) and 2.12 (1.17-3.89), respectively. Our study found that IL-10-1082A/G polymorphism is associated with an increased risk of CAD, especially in hypertension, diabetes mellitus and smokers.
Keywords: IL-1β, IL-8, IL-10, polymorphism, coronary artery disease
Introduction
Cardiovascular disease is one of the major causes of death worldwide, including China, and coronary artery disease (CAD) is the common heart disease for atherosclerosis [1,2]. It is well known that CAD is caused by multiple factors, including genetic factors and environmental factors and their interactions [2,3]. The main environmental factors for CAD included hypertension, hypercholesterolemia, diabetes, obesity and smoking as well as drinking [4]. However, the traditional factors cannot well predict the CAD, and thus genetic factors may contribute to the underlying pathogenesis of CAD [5-9].
Inflammatory mechanism contributes to the process of CAD, and cytokines are the main mediators for the inflammatory response. Inflammation plays a critical role in the inflammatory response, immune regulation and development of CAD through promotion of atherosclerosis [10]. Several previous epidemiology studies have showed that polymorphisms of several functional interleukin genes, such as IL-1β, IL-6, IL-10, IL-17 and IL-18, are genetically correlated with the pathogenesis of CAD [5-9,11].
Identification of novel genetic variants for screening early risk of CAD has potentially important clinical implications, such as identifying high-risk individuals and adapting therapeutic management to the individual’s genetic make-up. Polymorphisms in functional interleukin factors could influence the plasma levels and biological activity of the corresponding proteins. Therefore, we conducted this case-control study to investigate the genetic role of IL-1β, IL-8 and IL-10 in the pathogenesis of CAD in a Chinese population.
Methods and materials
Subjects
A hospital-based case-control study was conducted. 325 patients with CAD were included in this study between January 2013 and December 2014. The criterion for enrolment of CAD case were ≥70% stenosis of one major coronary artery, or ≥50% stenosis of the left main coronary artery, as identified by coronary angiography. Individuals who had experienced myocardial spasms or a myocardial bridge, autoimmune disease, congenital heart disease, childhood hypertension, type 1 diabetes mellitus, severe kidney or liver disease and malignancy were excluded from our study.
342 control subjects were collected from subjects who sought a health examination in the physical examination center of our hospital during the same period. The control subjects who had a history of CAD or arteriosclerotic lesions by angiography were excluded from this study.
The demographic and clinical characteristics of all the patients and controls were collected using a structured questionnaire and medical records. The disease status of hypertension and diabetes mellitus, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c) and triglyceride (TG) were measured according guideline and collected from medical records. Data on sex, age, smoking and drinking habits, diabetes and hypertension were obtained from self-designed questionnaire. This study was approved by the ethics committee of the Second Hospital of Chongqing Medical University.
Genetic analysis
The patients and control subjects provided a 5 mL peripheral venous blood sample after participating in our study. Genomic DNA was isolated from peripheral blood using a TIANamp Blood DNA kit (Tiangen Biotech Co., Ltd., Beijing, China). Genotyping of IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A, IL-10-1082A/G and IL-10-819C/T was performed in a 384-well plate format on the Sequenom MassARRAY platform (Sequenom, San Diego, USA). The primers and probes for IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A, IL-10-1082A/G and IL-10-819C/T were designed using Assay Design 3.1 software (Sequenom Inc., San Diego, CA, USA). The primers for IL-1β +3954 C/T were 5’-GCCTGCCCTTCTGATTTTATACC-3’ and 5’-CATCGTGCACATAAGCCTCGTTA-3’; for IL-1β -511 C/T, the primers were 5’-TTGAGGGTGTGGGTCTCTACCT-3’ and 5’-AGGAGCCTGAACCCTGCATAC-3’; for IL-8-251T/A, the primers were 5’-TAAAATACTGAAGCTCCACAATTTGG-3’ and 5’-ATCTTGTTCTAACACCTGCCACTCT-3’; for IL-10-1082A/G, the primers were 5’-GATAGGAGGTCCCTTACTTTCCTCTTA-3’ and 5’-CACACACAAATCCAAGACAACACTAC-3’; for IL-10-819C/T, 5’-ATGGTGTACAGTAGGGTGAG-3’ and 5’-TTTCCACCTCTTCAGCTGTC-3’. Briefly PCR was performed in a final volume of 5 μL reaction solution with 50 ng genomic DNA template using, GeneAmp® PCR System 9700 with Dual 384-Well Sample Block Module (Applied Biosystems, Carlsbad, USA). The MassARRAY Analyzer Compact with ACQUAIRE Module (Sequenom) acquired spectra from the SpectroCHIP, and spectral data were automatically processed and saved to the MassARRAY database (Figure 1).
Figure 1.
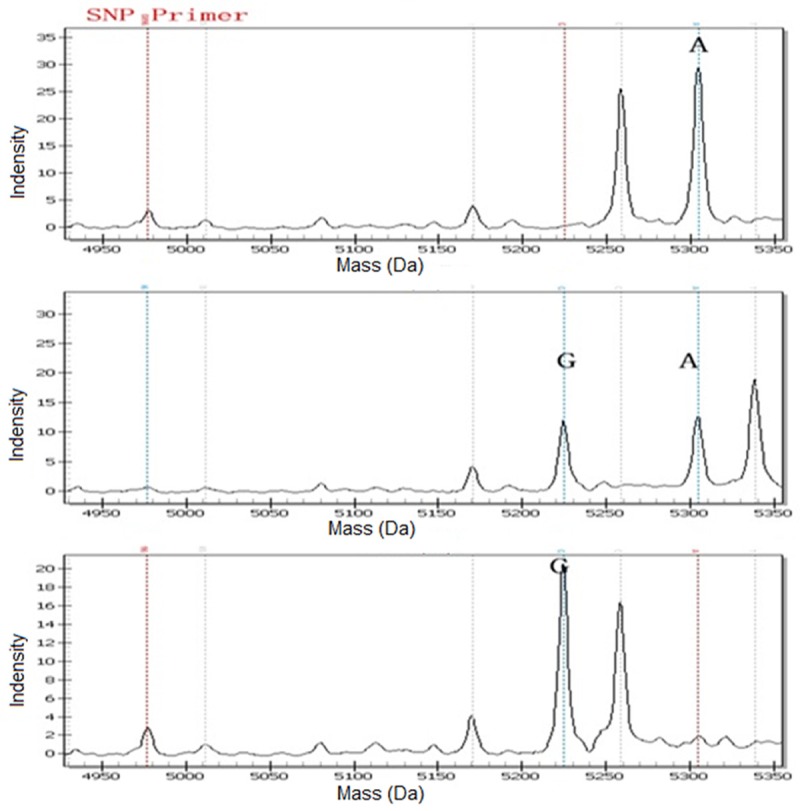
Spectra for three genotypes of IL-10-1082A/G polymorphism of an anonymous DNA samples: A for AA, B for AG, and C for GG.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (SD), and categorical variables were expressed as frequencies and percentage (%). Student’s t-test or X2-test was used to compare continuous variables and categorical variables between case and control groups. The Hardy-Weinberg equilibrium (HWE) for IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A, IL-10-1082A/G and IL-10-819C/T in controls was tested by Fisher’s exact test. A multivariate logistic model was performed to analyze the association between IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A, IL-10-1082A/G and IL-10-819C/T gene polymorphisms and risk of CAD, and the results were expressed by odds ratios (OR) and its corresponding 95% confidence intervals (CIs). Homozygotes of the most frequent genotype were used to be reference group of all genes. All statistical analyses were performed using SPSS 18.0 software (SPSS, Chicago, IL, USA). A P value equal to or less than 0.05 was considered as statistically significant.
Results
Characteristics of included patients and controls
The demographic and clinical characteristics of study subjects are shown in Table 1. In this study, the mean age of CAD patients and controls were 58.7±11.4 years and 56.5±10.8 years, respectively. There were 194 males and 131 females in CAD patients, and 194 males and 148 females in control subjects. Compared with control subjects, some risk factors were found more prevalent in CAD patients, such as hypertension, diabetes mellitus and tobacco smoking. Moreover, CAD patients were more likely to have higher levels of TC, LDL-c and TG and lower level of HDL-c when compared with the control subjects.
Table 1.
Demographic and clinical characteristics of included CAD patients and control subjects
| CAD cases N=325 | % | Controls N=342 | % | χ2 test or t test | P value | |
|---|---|---|---|---|---|---|
| Age | ||||||
| <55 | 147 | 45.23 | 165 | 48.25 | ||
| ≥55 | 178 | 54.77 | 177 | 51.75 | 0.61 | 0.44 |
| Sex | ||||||
| Male | 194 | 59.69 | 194 | 56.73 | ||
| Female | 131 | 40.31 | 148 | 43.27 | 0.60 | 0.43 |
| Hypertension | ||||||
| No | 161 | 49.54 | 254 | 74.27 | ||
| Yes | 164 | 50.46 | 88 | 25.73 | 43.36 | <0.05 |
| Diabetes mellitus | ||||||
| No | 267 | 82.15 | 316 | 92.40 | ||
| Yes | 58 | 17.85 | 26 | 7.60 | 15.89 | <0.05 |
| Tobacco smoking | ||||||
| Current or ever | 73 | 22.46 | 185 | 54.09 | ||
| Never | 252 | 77.54 | 157 | 45.91 | 70.30 | <0.05 |
| Alcohol drinking | ||||||
| Current or ever | 123 | 37.85 | 114 | 33.33 | ||
| Never | 202 | 62.15 | 228 | 66.67 | 1.48 | 0.22 |
| TC | 192.6±37.6 | 168.7±25.5 | 9.65 | <0.05 | ||
| LDL-c | 110.5±26.3 | 91.8±12.6 | 11.80 | <0.05 | ||
| HDL-c | 36.2±7.4 | 44.6±8.3 | 13.77 | <0.05 | ||
| TG | 138.6±45.7 | 114.2±23.4 | 8.74 | <0.05 |
Analysis of association between IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A, IL-10-1082A/G and IL-10-819C/T polymorphisms and risk of CAD
Genotype distributions of IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A, IL-10-1082A/G and IL-10-819C/T are shown in Table 2. Genotype distributions of IL-1β -511 C/T, IL-8-251T/A and IL-10-819C/T in control subjects were in Hardy-Weinberg equilibrium, however the genotype distribution of IL-1β +3954 C/T and IL-10-1082A/G was not. We found that the frequencies of the GG and AG+GG genotypes of IL-10-1082A/G were significantly higher in CAD cases than them in controls. By Multivariate logistic regression analysis, the GG and AG+GG genotypes of IL-10-1082A/G were associated with an increased risk of CAD. The ORs (95% CI) for GG and AG+GG genotypes were 2.12 (1.32-3.43) and 1.56 (1.14-2.14), respectively. However, we did not find significant association of IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A and IL-10-819C/T polymorphisms with the risk of CAD.
Table 2.
Genotype distributions of IL-1β +3954 C/T, IL-1β -511 C/T, IL-8-251T/A, IL-10-1082A/G and IL-10-819C/T in CAD patients and controls and their association with CAD risk
| CAD cases | % | Controls | % | OR (95% CI)1 (Multivariate analysis) | P value | |
|---|---|---|---|---|---|---|
| IL-1β +3954 C/T | ||||||
| CC | 217 | 66.77 | 244 | 71.35 | 1.0 (Ref.) | 1 |
| CT | 72 | 22.15 | 70 | 20.47 | 1.16 (0.78-1.71) | 0.45 |
| TT | 36 | 11.08 | 28 | 8.19 | 1.45 (0.83-2.55) | 0.17 |
| CT+TT | 108 | 33.23 | 98 | 28.65 | 1.24 (0.88-1.75) | 0.2 |
| IL-1β -511 C/T | ||||||
| GG | 95 | 29.23 | 114 | 33.33 | 1.0 (Ref.) | 1 |
| GA | 152 | 46.77 | 155 | 45.32 | 1.18 (0.81-1.70) | 0.37 |
| AA | 78 | 24.00 | 73 | 21.35 | 1.28 (0.82-1.99) | 0.24 |
| GA+AA | 230 | 70.77 | 228 | 66.67 | 1.21 (0.86-1.70) | 0.25 |
| IL-8-251T/A | ||||||
| TT | 85 | 26.15 | 108 | 31.58 | 1.0 (Ref.) | 1 |
| TA | 147 | 45.23 | 149 | 43.57 | 1.25 (0.86-1.84) | 0.22 |
| AA | 93 | 28.62 | 85 | 24.85 | 1.39 (0.90-2.14) | 0.11 |
| TA+AA | 240 | 73.85 | 234 | 68.42 | 1.30 (0.92-1.85) | 0.12 |
| IL-10-1082A/G | ||||||
| AA | 138 | 42.46 | 183 | 53.51 | 1.0 (Ref.) | 1 |
| AG | 123 | 37.85 | 119 | 34.80 | 1.37 (0.97-1.94) | 0.06 |
| GG | 64 | 19.69 | 40 | 11.70 | 2.12 (1.32-3.43) | 0.001 |
| AG+GG | 187 | 57.54 | 159 | 46.49 | 1.56 (1.14-2.14) | 0.004 |
| IL-10-819C/T | ||||||
| CC | 115 | 35.38 | 135 | 39.47 | 1.0 (Ref.) | 1 |
| CT | 144 | 44.31 | 143 | 41.81 | 1.18 (0.83-1.68) | 0.33 |
| TT | 66 | 20.31 | 64 | 18.71 | 1.21 (0.77-1.89) | 0.38 |
| CT+TT | 210 | 64.62 | 207 | 60.53 | 1.19 (0.86-1.65) | 0.28 |
Adjusted for sex, age, hypertension, diabetes, tobacco smoking and alcohol drinking.
Interaction between IL-10-1082A/G polymorphism and demographic and clinical characteristics
By interaction analysis, we found patients carrying the AG+GG genotype of IL-10-1082A/G were associated with an increased risk of CAD in those with hypertension, diabetes mellitus and smokers, and the ORs (95% CI) were 1.41 (0.93-2.14), 7.13 (2.28-23.56) and 2.12 (1.17-3.89), respectively (Table 3). A significant interaction was found between the AG+GG genotype of IL-10-1082A/G and hypertension, diabetes mellitus and smoking in the CAD risk. However, we did not find significant interaction between IL-10-1082A/G polymorphism and age, sex and alcohol drinking. Moreover, we did not find significant association between IL-10-1082A/G polymorphism and TC, LDL-c, HDL-c and TG in CAD risk.
Table 3.
Interaction between IL-10-1082A/G polymorphism and clinical characteristics in CAD risk
| Variables | Cases | Controls | OR (95% CI) (Multivariate analysis) | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| AA | AG+GG | AA | AG+GG | |||
| Age | ||||||
| <55 | 65 | 90 | 82 | 75 | 1.51 (0.94-2.43) | 0.07 |
| ≥55 | 73 | 97 | 105 | 80 | 1.65 (0.96-2.61) | 0.06 |
| Sex | ||||||
| Male | 83 | 102 | 111 | 92 | 1.47 (0.97-2.26) | 0.06 |
| Female | 55 | 85 | 76 | 63 | 1.76 (0.95-2.66) | 0.08 |
| Hypertension | ||||||
| No | 73 | 137 | 88 | 117 | 1.41 (0.93-2.14) | 0.09 |
| Yes | 65 | 50 | 99 | 38 | 2.01 (1.16-3.59) | <0.05 |
| Diabetes mellitus | ||||||
| No | 122 | 168 | 145 | 148 | 1.36 (0.95-1.90) | 0.07 |
| Yes | 16 | 19 | 42 | 7 | 7.13 (2.28-23.56) | <0.05 |
| Smoking status | ||||||
| Current or ever | 25 | 97 | 48 | 88 | 2.12 (1.17-3.89) | <0.05 |
| Never | 113 | 90 | 139 | 67 | 1.52 (0.96-2.40) | 0.08 |
| Drinking status | ||||||
| Current or ever | 46 | 59 | 77 | 55 | 1.72 (0.96-3.02) | 0.09 |
| Never | 92 | 128 | 110 | 100 | 1.42 (0.91-2.11) | 0.10 |
Discussion
It is well known that cytokines are modulators for immune responses, and the balance between proinflammatory and anti-inflammatory stimuli has a critical role in the development of atherosclerosis. It is reported that locally higher secretion and concentrations of proinflammatory cytokines can cause severely damages in the epithelium of blood vessels and surrounding tissues, and cytokines from lymphocytes with the inflamed site can induce more damages [12]. Therefore, genetic polymorphisms of the function cytokines are associated with increased risk of vascular lesions [13,14]. Previous studies have reported the role of promoter polymorphisms of cytokines in the development of CAD [15-19], but previous case-control studies on the association between cytokines genes and CAD risk have shown conflicting results. Our study showed an association between IL-10-1082A/G polymorphism and risk of CAD in a multivariate analysis, even after adjusting confounding variables.
IL-10 is located at chromosome 1q31-32, and IL-10-1082G/A, -819C/T and -592C/A are three key gene locus mutations in the upstream of the transcription start site [20,21]. Previous cytology experimental or epidemiological studies have reported that IL-10 production can be regulated by genes [21-24], but the results are controversial. Turner et al. conducted an experimental study to investigate the polymorphism in the interleukin-10 gene promoter, and found that GG genotype of IL-10-1082G/A could down regulation of its expression [21]. In another experimental study, Eskdale et al. reported that the secreting IL-10 can vary in individuals according to the genetic composition of the IL-10 locus [24]. In epidemiologic study, Lio et al. reported that the AA genotype of IL-10-1082G/A was correlated with reduced production of IL-10-1082G/A and reduced risk of coronary heart disease [23]. However, Koch et al. found that IL-10 gene polymorphisms were not associated with an increased risk of CAD or myocardial infarction in angiographically examined patients [22]. In a recent meta-analysis with 16 case-control studies (7779 cases and 7271 controls), the AA genotype of interleukin-10-1082 was associated with an increased risk of atherosclerosis, and the AA genotype was associated with susceptible to coronary artery disease and stroke [19]. The discrepancy of results in previous studies may be caused by differences in populations, study design and sample size.
Our study also suggested that IL-10-1082A/G polymorphism had interaction with hypertension, diabetes mellitus and smoking in the risk of CAD. Tobacco smoking has an important role in the pathology of vascular systems, and previous studies have reported direct effects between tobacco smoking and cardiac remodeling and function [25,26]. Tobacco smoking can induce left atrium and ventricle enlargement, myocyte hypertrophy and systolic dysfunction [27,28]. Moreover, previous studies reported that IL-10-1082A/G polymorphism could influence the risk of diabetes and hypertension [29,30].
Two limitations should be considered in the present study. First, the patients and controls were enrolled from one hospital, which may not be representative of the general population. Moreover, genetic distributions of IL-1β +3954 C/T and IL-10-1082A/G were not in Hardy-Weinberg equilibrium, which indicates that selection bias may exist in our study. Second, the sample size in our study is relatively small, especially in the case subjects, which may limit the statistical power to detect differences between the patients and controls. Therefore, further studies using a large sample size are greatly required to verify the association between IL-1β, IL-8 and IL-10 polymorphisms and the risk of CAD.
In conclusion, our study suggests that IL-10-1082A/G polymorphism has association with an increased risk of CAD, especially in hypertension, diabetes mellitus and smokers. Further well designed and large sample size studies are greatly required to demonstrate the role of IL-1β, IL-8 and IL-10 polymorphisms in the risk of CAD.
Acknowledgements
This study was supported by the Key Topic Funding in Inner Mongolia Education Authorities (NJ100139).
Disclosure of conflict of interest
None.
References
- 1.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 5.Guven M, Ismailoglu Z, Batar B, Unal S, Onaran I, Karadag B, Ongen Z. The effect of genetic polymorphisms of TLR2 and TLR4 in Turkish patients with coronary artery disease. Gene. 2015;558:99–102. doi: 10.1016/j.gene.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Rai H, Parveen F, Kumar S, Kapoor A, Sinha N. Association of endothelial nitric oxide synthase gene polymorphisms with coronary artery disease: an updated meta-analysis and systematic review. PLoS One. 2014;9:e113363. doi: 10.1371/journal.pone.0113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foroughmand AM, Shahbazi Z, Galehdari H, Purmahdi Borujeni M, Dinarvand P, Golabgirkhademi K. Association of MEF2A gene polymorphisms with coronary artery disease. Iran Red Crescent Med J. 2014;16:e13533. doi: 10.5812/ircmj.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qintao C, Yan L, Changhong D, Xiaoliang G, Xiaochen L. Genetic polymorphism of matrix metalloproteinase-1 and coronary artery disease susceptibility: a case-control study in a han chinese population. Genet Test Mol Biomarkers. 2014;18:826–831. doi: 10.1089/gtmb.2014.0222. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Chen HX, Yang JG, Li W, Du R, Tian L. MEF2A gene mutations and susceptibility to coronary artery disease in the Chinese population. Genet Mol Res. 2014;13:8396–8402. doi: 10.4238/2014.October.20.15. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee I, Pandey U, Hasan OM, Parihar R, Tripathi V, Ganesh S. Association between inflammatory gene polymorphisms and coronary artery disease in an Indian population. J Thromb Thrombolysis. 2009;27:88–94. doi: 10.1007/s11239-007-0184-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Zhong M, Liang L, Gu F, Peng H. Interleukin-17 induces angiogenesis in human choroidal endothelial cells in vitro. Invest Ophthalmol Vis Sci. 2014;55:6968–6975. doi: 10.1167/iovs.14-15029. [DOI] [PubMed] [Google Scholar]
- 13.Weng KP, Hsieh KS, Hwang YT, Huang SH, Lai TJ, Yuh YS, Hou YY, Lin CC, Huang SC, Chang CK, Lin MW, Ger LP. IL-10 polymorphisms are associated with coronary artery lesions in acute stage of Kawasaki disease. Circ J. 2010;74:983–989. doi: 10.1253/circj.cj-09-0801. [DOI] [PubMed] [Google Scholar]
- 14.Khankhanian P, Baranzini SE, Johnson BA, Madireddy L, Nickles D, Croen LA, Wu YW. Sequencing of the IL6 gene in a case-control study of cerebral palsy in children. BMC Med Genet. 2013;14:126. doi: 10.1186/1471-2350-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Cai ZR, Zhang B, Cai X, Li W, Guo Z, Ma L. Functional polymorphisms in interleukin-23 receptor and susceptibility to coronary artery disease. DNA Cell Biol. 2014;33:891–897. doi: 10.1089/dna.2014.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He F, Teng X, Gu H, Liu H, Zhou Z, Zhao Y, Hu S, Zheng Z. Interleukin-6 receptor rs7529229 T/C polymorphism is associated with left main coronary artery disease phenotype in a Chinese population. Int J Mol Sci. 2014;15:5623–5633. doi: 10.3390/ijms15045623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satti HS, Hussain S, Javed Q. Association of interleukin-6 gene promoter polymorphism with coronary artery disease in Pakistani families. Scientific World Journal. 2013;2013:538365. doi: 10.1155/2013/538365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsaid A, Abdel-Aziz AF, Elmougy R, Elwaseef AM. Association of polymorphisms G (-174)C in IL-6 gene and G(-1082)A in IL-10 gene with traditional cardiovascular risk factors in patients with coronary artery disease. Indian J Biochem Biophys. 2014;51:282–292. [PubMed] [Google Scholar]
- 19.Chao L, Lei H, Fei J. A meta-analysis of interleukin-10-1082 promoter genetic polymorphism associated with atherosclerotic risk. Neurol India. 2014;62:130–136. doi: 10.4103/0028-3886.132323. [DOI] [PubMed] [Google Scholar]
- 20.Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol. 1992;148:3618–3623. [PubMed] [Google Scholar]
- 21.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 22.Koch W, Kastrati A, Böttiger C, Mehilli J, von Beckerath N, Schömig A. Interleukin-10 and tumor necrosis factor gene polymorphisms and risk of coronary artery disease and myocardial infarction. Atherosclerosis. 2001;159:137–144. doi: 10.1016/s0021-9150(01)00467-1. [DOI] [PubMed] [Google Scholar]
- 23.Lio D, Candore G, Crivello A, Scola L, Colonna-Romano G, Cavallone L, Hoffmann E, Caruso M, Licastro F, Caldarera CM, Branzi A, Franceschi C, Caruso C. Opposite effects of interleukin 10 common gene polymorphisms in cardiovascular diseases and in successful ageing: Genetic background of male centenarians is protective against coronary heart disease. J Med Genet. 2004;41:790–794. doi: 10.1136/jmg.2004.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskdale J, Gallagher G, Verweij CL, Keijsers V, Westendorp RG, Huizinga TW. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci U S A. 1998;95:9465–9470. doi: 10.1073/pnas.95.16.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. 2015;71:24–34. doi: 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Farsalinos KE, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: comparison with the effects of regular cigarettes. BMC Cardiovasc Disord. 2014;14:78. doi: 10.1186/1471-2261-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castardeli E, Paiva SA, Matsubara BB, Matsubara LS, Minicucci MF, Azevedo PS, Campana AO, Zornoff LA. Chronic cigarette smoke exposure results in cardiac remodeling and impaired ventricular function in rats. Arq Bras Cardiol. 2005;84:320–324. doi: 10.1590/s0066-782x2005000400009. [DOI] [PubMed] [Google Scholar]
- 28.Castardeli E, Duarte DR, Minicucci MF, Azevedo PS, Matsubara BB, Matsubara LS, Campana AO, Paiva SA, Zornoff LA. Exposure time and ventricular remodeling induced by tobacco smoke exposure in rats. Med Sci Monit. 2008;14:BR62–BR66. [PubMed] [Google Scholar]
- 29.Kaur R, Matharoo K, Sharma R, Bhanwer AJ. C-reactive protein + 1059 G>C polymorphism in type 2 diabetes and coronary artery disease patients. Meta Gene. 2013;1:82–92. doi: 10.1016/j.mgene.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukic L, Lalic NM, Rajkovic N, Jotic A, Lalic K, Milicic T, Seferovic JP, Macesic M, Gajovic JS. Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: potential targets for an efficient preventive intervention. Int J Environ Res Public Health. 2014;11:3586–3598. doi: 10.3390/ijerph110403586. [DOI] [PMC free article] [PubMed] [Google Scholar]


