Abstract
Background: Patients with functional dyspepsia (FD) have increased risks for psychological dysfunction than healthy peoples. This study aimed to explore the roles of psychosocial factors and duodenal mast cells in the pathogenesis of FD. Material and methods: We prospectively included 48 FD patients and 21 age- and sex-match healthy volunteers. There were 23 patients with postprandial distress syndrome (PDS) and 25 patients with epigastric pain syndrome (EPS). The Hospital Anxiety Depression Scale (HADS) was administered to evaluate their psychosocial status. Upper endoscopy was performed with biopsy of the mucosa from the bulb of duodenum. Mast cells counts and degranulation rates were identified by toluidine blue staining. The relationship among the scores of HADS-A (anxiety) and HADS-D (depression) and the mast cell counts and degranulation rates were analyzed. Results: The scores of HADS-A and HADS-D were significantly higher in PDS and EPS patients than the normal controls (P<0.05). The mast cell counts and degranulation rates in the duodenum were significantly increased in PDS and EPS patients than the controls (P<0.05). In either PDS or EPS patients, the HADS-A and HADS-D scores were positively correlated with the mast cell counts and degranulation rate. Conclusion: FD patients had significantly higher risks for anxiety and depression, which may lead to FD through the increased mast cell counts and degranulation.
Keywords: Duodenum, gastrointestinal disease, psychological factor, immune
Introduction
Functional dyspepsia (FD) is the most prevalent functional gastrointestinal disorder, and consists of two subtypes of postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) [1]. However, the etiology and pathogenesis of FD are not completely clear yet. Patients with FD manifest decreased antral/duodenal motility, increased spontaneous duodenal acid exposure, increased sensitivity to intraduodenal lipid, and duodenal immune hyperactivity. It has been shown that the duodenum is a critical site where symptoms of FD originate [2]. The mast cells in the duodenum are important local immune effector cells and are found to be involved in the pathogenesis of FD [3-5].
Psychological factors have been shown to play important roles in the gastrointestinal sensorimotor functions and symptoms [6]. The state of anxiety is associated with gastric sensitivity and compliance in FD patients [7]. It has also been found that comorbidity of mood and anxiety disorders in functional gastrointestinal disorder patients is 50% higher than in the general population [6,8,9].
Psychological factors such as anxiety and depression may affect gastrointestinal target organ by the brain-gut axis, leading to changes in the gastrointestinal motor, sensory, secretion, and the immune functions. However, the relationship between psychological factors and mast cells in FD is not clear. The aim of this study was to investigate the relationship between anxiety and depression status and the activation of mast cells in the duodenum of FD patients.
Material and methods
Patients and controls
We prospectively recruited 48 newly diagnosed FD patients diagnosed according to the Rome III [10] criteria and 21 age- and sex-matched healthy volunteers. A complete medical history and physical examination was carried out to exclude history of surgery, Helicobacter pylori (HP) infection, gastroesophageal reflux, esophagitis, malignancy, and anaphylactic diseases in all participants. Informed consent was obtained from the participants. This study was approved by the Ethics Committee of Tai’an City Central Hospital.
Anxiety and depression scores
Two well-trained physicians interviewed all the participants and administered the Hospital Anxiety Depression Scale (HADS) [11]. The anxiety score (HADS-A) and depression score (HADS-D) were calculated.
Specimen collection
Gastroscopy (Olympus, Japan) was performed to obtain 2-3 pieces of mucous mucosa from the duodenal bulb from the FD patients and the controls. The samples were fixed in formaldehyde, imbedded in paraffin, and cut into 3-μm sections.
Toluidine blue staining
Toluidine blue staining was used to identify the mast cells. Sections were deparaffined, stained with 0.5% toluidine blue solution, washed with water, differentiated with 0.5% glacial acetic acid solution until showing clear nuclei and cytoplasm granules. The mast cells were counted under microscope (BX51T, Olympus, Japan). The percentage of mast cells with degranulation was calculated.
Statistical analysis
Continuous data were expressed as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 13.0 software (SPSS, US). Comparison was made using Kruskal-Wallis test. Correlation analysis was performed with Spearman coefficient. A P-value less than 0.05 was considered statistically significant.
Results
Patient information
There were 23 patients with postprandial distress syndrome (PDS) and 25 patients with epigastric pain syndrome (EPS). No significant difference in age and sex was found between PDS patients, EPS patients, and normal controls (Table 1).
Table 1.
Basic information of the patients and normal controls
| PDS (n=23) | EPS (n=25) | Controls (n=21) | P-value | |
|---|---|---|---|---|
| Mean age (year) | 44.6±7.3 | 47±15.7 | 45.5±10.6 | >0.05 |
| Sex (male/female) | 5/18 | 8/17 | 9/12 | >0.05 |
HADS scores
The scores of HADS-A and HADS-D were significantly higher in subjects with PDS (P=0.032, P=0.023) and EPS (P=0.018, P=0.035) in comparison with normal controls (Table 2). However, no significant difference was found in HADS scores between the PDS and EPS patients (P>0.05).
Table 2.
The scores of HADS-A and HADS-D in PDS patients, EPS patients, and normal controls (mean ± SD)
| PDS (n=23) | EPS (n=25) | Controls (n=21) | P-value | |
|---|---|---|---|---|
| HADS-A | 7.0±5.4* | 7.4±5.4* | 4.0±2.3 | 0.032 |
| HADS-D | 7.3±5.3* | 7.1±5.1* | 4.1±3.0 | 0.023 |
vs normal controls.
Duodenal mast cells counts and degranulation
Mast cells without degranulation showed even cytoplasm and clear membrane, while mast cells with degranulation were irregular with ruptured membrane, releasing particles around the membrane (Figure 1). Mast cells counts and the percentage of mast cells with degranulation were significantly higher in PDS and EPS patients than normal controls (P<0.001), but did not differ significantly between the PDS and EPS patients (Table 3).
Figure 1.
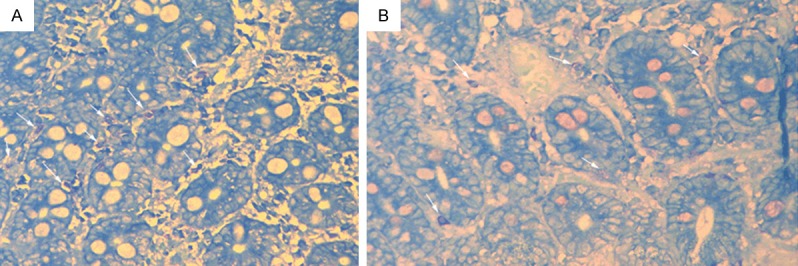
A. Duodenal mast cells in subjects with FD, showing significantly increased number and degranulation (arrow). B. Mast cells in the duodenal membrane of normal control (arrow) (40×).
Table 3.
Mast cells counts and degranulation rates in PDS patients, EPS patients, and normal controls
| PDS (n=23) | EPS (n=25) | Controls (n=21) | P-value | |
|---|---|---|---|---|
| Mast cells counts | 121±13.8* | 120.9±13.1* | 104.3±20.8 | <0.001 |
| Degranulation rate (%) | 59.8±4.5* | 60.6±5.7* | 25.4±2.3 | <0.001 |
vs normal controls.
Correlation analysis
In PDS patients, both HADS-A and HADS-D scores were positively correlated with the mast cell counts (r=0.843, P<0.001; r=0.654, P<0.001) and degranulation rates (r=0.714, P<0.001; r=0.461, P<0.001). Similarly, in EPS patients, both HADS-A and HADS-D scores were positively correlated with the mast cell counts (r=0.794, P<0.001; r=0.545, P<0.001) and degranulation rates (r=0.757, P<0.001; r=0.585, P<0.001).
Discussion
The prevalence of FD are estimated to be approximately 3.5%-27.0% in children/adolescents and 20%-30% in adults [12,13]. This disorder manifests persistent or recurrent pain or discomfort in the upper abdomen regardless the changes in stool frequency or form. FD in adults can be categorized into two subtypes, PDS and EPS. PDS is defined as bothersome postprandial fullness occurring after ordinary sized meals and/or early satiation that prevents finishing a regular meal. EPS is defined as intermittent pain or burning of at least moderate severity localized to the epigastrium. Psychological factors have been shown to be closely related to FD [6-9]. Our study investigated the psychological factors in FD patients using HADS, a validated score for the assessment of anxiety and depression. It was found that in patients with FD, either PDS or EPS, the anxiety and depression scores were significantly higher than normal controls, suggesting that psychological problems are common in FD patients.
Recently, the importance of inflammation as a contributing factor to FD is being increasingly appreciated. We speculate inflammation may serve as a mediator process between the psychological factors and gastrointestinal disorders. Our study found that the counts and degranulation rate of mast cells in the bulb of duodenum are significantly increased in FD patients. Mast cells are rich in the gastrointestinal tract and play important roles in the enteric nervous system [14-18]. It is thought that various stress factors such as infection, psychological stress, and food allergies may act on the stress response system of the brain, then affect the gastrointestinal target organs through the brain-gut axis, leading to changes in the gastrointestinal motor, sensory, secretion, and the immune functions, and finally leading to FD. However, the specific molecular mechanisms that link the psychological factors and master cells still need further investigation.
The pathological roles of mast cells in gastrointestinal diseases have been well studied in irritable bowel syndrome, which shows increased cell counts in the ileum and colon [19,20]. In addition, it also has been found that the number of degranulation mast cells and those close to intestinal nerves is associated with the symptom severity of irritable bowel syndrome [21]. Previous studies showed that FD patients had increased mucosal mast cell density in the stomach [3], and that patients with irritable bowel syndrome had increased mast cell density in the duodenum [22]. In our study, we found that the mast cell counts and degranulation were significantly increased in FD patients, regardless of PDS or EPS. Our findings suggest that the mast cells may also play a role in the pathogenesis FD in the duodenum.
This study is with limitations. We recruited a small sample size and therefore the subgroups of PDS and EPS patients are even small in number. This may compromise the statistical power. Second, we excluded patients with HP infection. Although the potential role of HP infection in FD pathogenesis is still controversial [23], there is still research showing that HP infection may induce mast cell migration and activation [24]. Lack of HP infection status in our patients may introduce bias into the mast cell results.
Conclusions
In summary, FD patients have increased number of mast cells and degranulation in the duodenal bulb, and the patient anxiety and depression is associated with mast cell density and degranulation, suggesting a mechanism of psychological factors in the pathogenesis of FD.
Disclosure of conflict of interest
None.
References
- 1.Geeraerts B, Tack J. Functional dyspepsia: past, present, and future. J Gastroenterol. 2008;43:251–5. doi: 10.1007/s00535-008-2167-8. [DOI] [PubMed] [Google Scholar]
- 2.Walker MM, Warwick A, Ung C, Talley NJ. The role of eosinophils and mast cells in intestinal functional disease. Curr Gastroenterol Rep. 2011;13:323–30. doi: 10.1007/s11894-011-0197-5. [DOI] [PubMed] [Google Scholar]
- 3.Hall W, Buckley M, Crotty P, O’Morain CA. Gastric mucosal mast cells are increased in Helicobacter pylori-negative functional dyspepsia. Clin Gastroenterol Hepatol. 2003;1:363–9. doi: 10.1053/s1542-3565(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 4.Schappi MG, Borrelli O, Knafelz D, Williams S, Smith VV, Milla PJ, Lindley KJ. Mast cell-nerve interactions in children with functional dyspepsia. J Pediatr Gastroenterol Nutr. 2008;47:472–80. doi: 10.1097/MPG.0b013e318186008e. [DOI] [PubMed] [Google Scholar]
- 5.Song S, Song Y, Zhang H, Li G, Li X, Wang X, Liu Z. Increased counts and degranulation of duodenal mast cells and eosinophils in functional dyspepsia-a clinical study. Med Glas (Zenica) 2014;11:276–82. [PubMed] [Google Scholar]
- 6.Van Oudenhove L, Demyttenaere K, Tack J, Aziz Q. Central nervous system involvement in functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18:663–80. doi: 10.1016/j.bpg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Van Oudenhove L, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Demyttenaere K, Fischler B, Tack J. Relationship between anxiety and gastric sensorimotor function in functional dyspepsia. Psychosom Med. 2007;69:455–63. doi: 10.1097/PSY.0b013e3180600a4a. [DOI] [PubMed] [Google Scholar]
- 8.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65:528–33. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 9.Lydiard RB. Increased prevalence of functional gastrointestinal disorders in panic disorder: clinical and theoretical implications. CNS Spectr. 2005;10:899–908. doi: 10.1017/s1092852900019878. [DOI] [PubMed] [Google Scholar]
- 10.Shih DQ, Kwan LY. All Roads Lead to Rome: Update on Rome III Criteria and New Treatment Options. Gastroenterol Rep. 2007;1:56–65. [PMC free article] [PubMed] [Google Scholar]
- 11.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 12.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–37. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 14.Bassotti G, Villanacci V. Mast cells in intestinal motility disorders: please also look beyond IBS. Dig Dis Sci. 2012;57:2475–6. doi: 10.1007/s10620-012-2303-4. [DOI] [PubMed] [Google Scholar]
- 15.Nasser Y, Boeckxstaens GE, Wouters MM, Schemann M, Vanner S. Using human intestinal biopsies to study the pathogenesis of irritable bowel syndrome. Neurogastroenterol Motil. 2014;26:455–69. doi: 10.1111/nmo.12316. [DOI] [PubMed] [Google Scholar]
- 16.Schaeffer DF, Kirsch R, Riddell RH. Mast cells and intestinal motility disorders (mastocytic enteritis/colitis) Dig Dis Sci. 2012;57:1118–21. doi: 10.1007/s10620-012-2123-6. [DOI] [PubMed] [Google Scholar]
- 17.Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133:1521–34. doi: 10.1016/j.jaci.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winston JH, Chen J, Shi XZ, Sarna SK. Inflammation induced by mast cell deficiency rather than the loss of interstitial cells of Cajal causes smooth muscle dysfunction in W/W(v) mice. Front Physiol. 2014;5:22. doi: 10.3389/fphys.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weston AP, Biddle WL, Bhatia PS, Miner PB Jr. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590–5. doi: 10.1007/BF01303164. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O'Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–57. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 21.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 22.Walker MM, Talley NJ, Prabhakar M, Pennaneac'h CJ, Aro P, Ronkainen J, Storskrubb T, Harmsen WS, Zinsmeister AR, Agreus L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765–73. doi: 10.1111/j.1365-2036.2009.03937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32:671–6. doi: 10.1002/1521-4141(200203)32:3<671::aid-immu671>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 24.Sarnelli G, Cuomo R, Janssens J, Tack J. Symptom patterns and pathophysiological mechanisms in dyspeptic patients with and without Helicobacter pylori. Dig Dis Sci. 2003;48:2229–36. doi: 10.1023/b:ddas.0000007856.71462.6c. [DOI] [PubMed] [Google Scholar]


