Abstract
A neonatal rat model with hypoxic ischemic brain damage (HIBD) was established. Forty 7-day-old neonatal Wistar rats were randomly divided into four groups: sham operation, model, progesterone and Akt inhibitor. Electron microscopy revealed that the neonatal rats with HIBD showed neuronal changes. The protein expression levels of pAkt, Nuclear factor κB (NF-κB) and Bcl-2 in the hippocampus were detected by immunohistochemistry and Western blot. The neuronal structure was normal in the sham operation group after HIBD for 24 h. Cavitation change due to hypoxic ischemic brain damage was observed in the neurons of the model group. Progesterone treatment improved neuronal damage and cavitation. Neuronal cavitation was clearly changed in the Akt inhibitor group. The protein expression levels of hippocampal pAkt and Bcl-2 did not significantly change after HIBD, whereas that of NF-κB increased. Progesterone pre-treatment increased the expression levels of pAkt and Bcl-2 but decreased that of NF-κB. The protein expression levels of pAkt and Bcl-2 decreased in the Akt inhibitor group, whereas that of NF-κB increased. This result indicates that progesterone can decrease inflammation in HIBD, inhibit apoptosis and protect the brain by activating the Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signalling pathway.
Keywords: Progesterone, hypoxic-ischemic brain damage, PI3K/Akt, NF-κB, Bcl-2
Introduction
Hypoxic ischemic brain damage (HIBD) induced by perinatal asphyxia generally leads to neonatal death and developmental disorders in the nervous system. This disease not only seriously threatens neonatal life but also causes disability among children beyond the neonatal period. Effective treatment for HIBD remains lacking to date [1]. PI3K/Akt is a key link in cell growth, metabolism, inflammation, cell survival and other signal transmissions. When PI3K conformation is changed, Ser473 and Thr308 phosphorylation yields phosphorylated Akt (pAkt). pAkt can phosphorylate many downstream proteins. Nuclear factor κB (NF-κB) coordinates the expression and regulation of pro-inflammatory genes, quickly reacts to different inflammatory stimuli, activates the transcription of downstream inflammatory genes and promotes inflammation [2]. pAkt can also activate the downstream factors Bcl-2, bad and Bax, which regulate anti-cell apoptosis and promote cell survival [3,4]. Bcl-2 is the most important anti-apoptosis gene among the members of the anti-apoptotic family.
Progesterone protects the brain against HIBD [5,6]. A laboratory study revealed that progesterone can alleviate cerebral edema after brain injury, inhibit nerve cell apoptosis and relieve calcium overload and inflammatory reactions [7,8]. The present study aims to determine whether or not progesterone can reduce inflammation, inhibit apoptosis and protect the brain through the PI3K/Akt pathway using neonatal rats with HIBD.
Materials and methods
Animal sources and grouping
Forty 7-day-old Wistar rats weighing 13.5 ± 1.8 g were provided by the Experimental Animal Centre of Xinxiang Medical University. These rats were randomly divided into four groups (n = 10 each): sham operation (only a neck incision was conducted without hypoxic ischemic treatment), model (hypoxic ischemic treatment was conducted in accordance with the animal model method), progesterone (the animals were treated with hypoxia ischemia; intraperitoneal injection with 8 mg/kg progesterone solution was conducted 30 min before hypoxia treatment [8]) and wortmannin, a Akt inhibitor was injected (the left hippocampus was positioned using a stereotaxic apparatus 30 min before the neonatal rat HIBD model was established) at 16 µg/kg [9]. Progesterone and wortmannin were purchased from Sigma-Aldrich (St. Louis, Mo, USA). The solution was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Eighth edition, 2011). The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Xinxiang Medical University (Xinxiang, China).
Establishment of the animal model
Relevant literature [9] states that the left common carotid artery of neonatal Wistar rats was separated and ligated with a silk thread after these rats anaesthetised with isoflurane (5%). The rats that did not exhibit rotary movement to the left side were eliminated after recovery and feeding for 2 h. The rats were then placed in a sealed container at a constant temperature of 37°C. The mixed gas of 8% O2 and 92% N2 was ventilated for 2.5 h at a speed of 1.5 L/min. The hypoxic ischemic animal model was then prepared.
Changes in hippocampal ultrastructure via electron microscopy
Three rats from each group were rapidly decapitated after hypoxia ischemia for 24 h. The brain was removed, pre-fixed in 2.5% glutaraldehyde solution at 4°C, post-fixed with 1% osmic acid, dehydrated with ethanol and acetone gradient series and then embedded with epoxy resin. Ultrathin sections were obtained. The double staining of acetic acid lead nitrate was performed. These sections were observed under a transmission electron microscope and then photographed.
Immunohistochemistry staining
The brain tissue was rapidly removed and fixed overnight in 4% paraformaldehyde after hypoxia for 24 h. Conventional dehydration and paraffin embedding were performed. Continuous coronal sections approximately 4 µm thick were obtained on the thalamic level, dewaxed, dried and then stored at normal temperature for hippocampal pAkt, NF-κB and Bcl-2 immunohistochemical staining.
Immunohistochemical technique was adopted in accordance with the instructions in its kit (BoAoSen Company, Beijing, China). A phosphate buffer solution was used to replace the primary antibody as the negative control. Brownish yellow cells in the cytoplasm and cell membrane were considered positive. The dyeing results were analysed using an HMIAS-200 colour image analysis system (Champion images technology Co., Ltd., Wuhan, China).
Western blot analysis
Protein was extracted from the left hippocampal tissue. The isometric 6×SDS sample buffer was added to an Eppendorf tube and boiled for 5 min to fully degenerate the protein. The sample was added for electrophoresis at 30 µg/hole. The sample protein was transferred onto a nitrocellulose membrane. Rabbit anti-rat p-Akt (Ser 473), rabbit anti-rat NF-κB, rabbit anti-rat Bcl-2 or anti-β-actin antibodies were added as the primary antibodies after sealing, and the kit used was provided by Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China). The secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was added and shaken for 1 h at 37°C. A 5 ml aliquot of a chromogenic agent (prepared on the spot) was used for colouration away from light for 3 min to 5 min until the desired band appeared. The colouration was terminated with double distilled water. The water was soaked up, dried away from light, preserved and then photographed. The integrated optical density of Western blot-positive bands was measured by a gel image analysis system (Tocan, Shanghai, China). The ratio of pAkt, NF-κB and Bcl-2 IOD and the corresponding IOD of b-actin represented the relative expressions of two proteins.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS Inc, Chicago, IL, USA). Data were expressed as x̅ ± s. Single-factor ANOVA was used to compare the groups. Statistical significance was considered at P < 0.05.
Results
Changes in the hippocampal ultrastructure of rats in each group
The neuronal structure in the sham operation group was basically normal; neuronal nuclei were slightly oval, and the nuclear chromatin was evenly distributed. The mitochondria, rough endoplasmic reticulum and Golgi bodies were observed in the cytoplasm. The neuropil structure was normal, the neurons showed cavitation change in the model group, the nuclei were irregular and the nuclear matrix was cavitated. The mitochondria in the cytoplasm were cavitated and swollen, the cristae broke, the cytoplasmic matrix was cavitated and the neuropil showed cavitated changes. The fracture and dissolution of the axonal neurofilament were clear. The neuronal nuclei in the progesterone group were oval, the intranuclear chromatin was evenly distributed and the mitochondria occasionally showed cavitation and cristae fracture. The rough endoplasmic reticulum was abundant and arranged in appropriate order. The mild cavitation of neuropil improved, the neuronal cavitation was clear in the Akt inhibitor group, the intranuclear chromatin was unevenly distributed and the cristae clearly changed (Figure 1).
Figure 1.
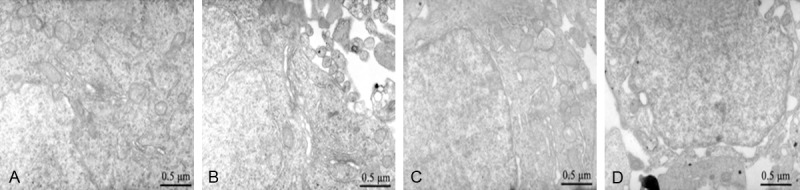
Ultrastructural changes in the hippocampus of the (A) sham, (B) model, (C) PROG and Akt inhibitor treated group. PROG, progesterone. Magnification, ×20, 000; uranyl acetate and lead nitrate double staining.
Immunohistochemical staining of hippocampal pAkt, NF-κB and Bcl-2
p-Akt was expressed in the hippocampal neural cytoplasm of rats in the sham operation group. p-Akt protein was weakly positive in the sham operation group. The expression of positive neurons was lower in the model group than in the sham operation group. However, the difference was not statistically significant. The expression of positive neurons was significantly higher in the progesterone group than in the model group (P < 0.05). The expression of p-Akt was significantly lower in the Akt inhibitor group than in the progesterone group (P < 0.05) (Figure 2A-D; Table 1).
Figure 2.
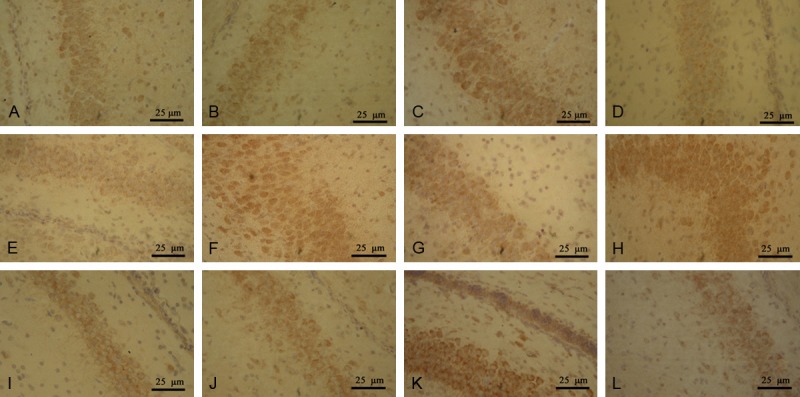
Rat hippocampal tissue expression levels of pAkt in the (A) sham, (B) model, (C) PROG and (D) Akt inhibitor groups, NF-κB in the (E) sham, (F) model, (G) PROG and (H) Akt inhibitor groups and Bcl-2 in the (I) sham, (J) model, (K) PROG and (L) Akt inhibitor groups. PROG, progesterone. Magnification, ×400; streptavidin-biotin complex (SABC) staining.
Table 1.
MOD values of pAkt, NF-κB and Bcl-2 in the hippocampus (mean ± SD) (x̅ ± s)
| Group | pAkt | NF-κB | Bcl-2 |
|---|---|---|---|
| Sham | 0.415 ± 0.019 | 0.225 ± 0.030 | 0.287 ± 0.022 |
| Model | 0.335 ± 0.038 | 0.634 ± 0.041a | 0.346 ± 0.028 |
| PROG | 0.616 ± 0.029b | 0.358 ± 0.033b | 0.624 ± 0.035b |
| Akt inhibitor | 0.298 ± 0.032c | 0.628 ± 0.031c | 0.315 ± 0.026c |
P<0.05, vs. sham;
P<0.05, vs. model;
P<0.05, vs. PROG.
The calculation method of immunohistochemical positive cells was as follows: Three random hippocampal visual fields were observed under a light microscope (magnification, ×400) and digital images were captured and analyzed for staining. From these the MOD values were calculated. MOD, mean optical density; PROG, progesterone; NF-κB, nuclear factor-κB.
NF-κB was expressed in the hippocampal neural cytoplasm of rats in the sham operation group. The number of positive cells decreased. The number of positive cells was significantly higher in the model group than in the sham operation group (P < 0.05); these cells were also found in the cytoplasm and nuclei. The number of positive cells was significantly lower in the progesterone group than in the model group. The expression of NF-κB in the Akt inhibitor group was significantly higher than that in the progesterone group (P < 0.05) (Figure 2E-H; Table 1).
Bcl-2-positive cells showed brownish yellow granules that were mainly located in the cytoplasm. Bcl-2-positive cells were sparsely distributed in the sham operation group. The average optical density value was low, and the number of Bcl-2-positive cells in the model group decreased. The staining was shallow, and the distribution was sparse. The average optical density value was higher in the model group than in the sham operation group, but the two groups showed no statistical significance (P > 0.05). The highest expression of Bcl-2 was observed in the progesterone group. Immunoreactive particles were intensively distributed, and deep dyeing was observed. The average optical density was significantly higher in the progesterone group than in the hypoxic ischemic group (P < 0.05). Bcl-2 expression was significantly lower in the Akt inhibitor group than in the progesterone group (P < 0.05) (Figure 2I-L; Table 1).
pAkt, NF-κB and Bcl-2 protein expressions
The protein expression levels of pAkt and Bcl-2 were higher in the model group than in the sham operation group after hypoxia ischemia for 24 h, although the difference was not significant. The expression levels of pAkt and Bcl-2 were significantly higher in the progesterone group than in the model and Akt inhibitor groups (P < 0.05). The protein expression of NF-κB was significantly higher in the model group than in the sham operation and progesterone groups (P < 0.05). Meanwhile, the protein expression of NF-κB was significantly higher in the Akt inhibitor group than in the progesterone group (P < 0.05) (Figure 3; Table 2).
Figure 3.
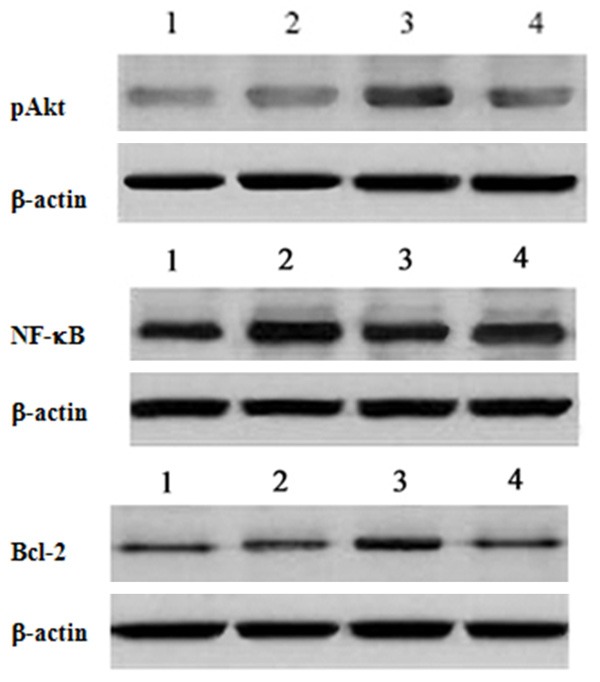
Expression of p-Akt, NF-κB and bcl-2 were observed by western blot analysis. Lane 1, sham group; lane 2, model group; lane 3, PROG group; lane 4, Akt inhibitor group.
Table 2.
Effect of PROG on the expression of p-Akt, NF-κB and bcl-2 in the brain tissue of neonatal rats
| Group | pAkt/b-actin | NF-κB/b-actin | Bcl-2/b-actin |
|---|---|---|---|
| Sham | 0.52 ± 0.04 | 0.45 ± 0.03 | 0.48 ± 0.06 |
| Model | 0.61 ± 0.05 | 0.89 ± 0.05a | 0.59 ± 0.05 |
| PROG | 1.22 ± 0.06b | 0.53 ± 0.03b | 1.19 ± 0.08b |
| Akt inhibitor | 0.63 ± 0.05c | 0.71 ± 0.04c | 0.65 ± 0.06c |
Note: Values are presented as the mean ± standard deviation. PROG, progesterone; p-Akt, phosphorylated Akt.
P<0.05 vs. the sham group;
P<0.05 vs. model group;
P<0.05 vs. the PROG group.
Discussion
Progesterone exerts a neuroprotective effect on HIBD by inhibiting macrophage infiltration and the secretions of tumour necrosis factor α, interleukin-1 and other cytokines. Several factors related to the inflammatory pathological process are also affected by progesterone regulation; these factors include complement factor, nuclear factor and NO synthetic enzyme. Progesterone can also decrease brain oedema, decrease lipid peroxidation, decrease neuronal apoptosis and abnormality, promote the stability of blood brain barrier and improve cognition after brain damage [10,11]. However, Tsuji et al. reported PROG and allopregnanolone aggravated hypoxic-ischemic brain damage in immature rats [12].
A study on different HIBD animal models showed that the hippocampus is highly sensitive to ischemic injury [13]. Changes in the hippocampal pathological structure of neonatal rats from all groups were observed under an electron microscope. Results showed that the neuronal structure in the sham operation group was basically normal. Cavitation appeared on neurons in the model group because of hypoxic ischemic damage. Progesterone treatment improved the damaged condition of the hypoxic ischemic neurons and decreased the cavitation. The protective effect of progesterone on hypoxic ischemic neuronal damage was blocked in the Akt inhibitor group.
HIBD is related to many factors, among which inflammation injury is a primary cause of neuronal death or apoptosis after HIBD [14]. The present study indicated that the PI3K/Akt pathway is a key pathway that serves a neuroprotective function. It can decrease the expression of pro-inflammatory cytokine and increase the expression of anti-apoptotic factors.
PI3K is an important member of the phosphoinositide-dependent kinase family and can transduce the signal from tyrosine kinase and G protein-coupled receptors. The tyrosine kinase of the receptor itself is activated after the growth factor or cytokine is combined with the receptor. PI3K conformation was changed, and Akt was activated into pAkt. The expression of pAkt in neurons increased after HIBD for 1 h to 3 h, reached a peak at 4 h to 6 h and then gradually normalised at approximately 12 h. This result suggests that the PI3K/Akt signalling pathway is involved in stress to the ischemic tissue at the early stage [15]. In the present study, HIBD was observed after 24 h. The results of immunohistochemistry and Western blot revealed that the protein expression of p-Akt was weak in the sham operation group. The number of p-Akt-positive cells decreased in the sham operation group, although the difference was not significant. The protein expression in the progesterone group was significantly higher than that in the model group. The protein in the Akt inhibitor group was clearly lower than that in the progesterone group.
Strong inflammation occurred in the brain tissues at the early stage after IBD [16]. NF-κB is a transcription factor with a multi-directional transcription regulatory effect. NF-κB is activated under ischemia and hypoxia, virus infection, mechanical damage, radiation and other stress conditions, which trigger the correlated target gene transcription involved in inflammation, immune response, cell apoptosis, free radical damage and other pathological and physiological processes [17,18]. The results of immunohistochemical staining and Western blot showed that the protein expression of NF-κB increased in brain tissues after HIBD for 24 h. However, progesterone pre-treatment decreased this expression. The protective effect of progesterone was blocked in the Akt inhibitor group. These data suggest that the inhibitory effect of progesterone on the expression of the inflammatory mediator NF-κB was related to the activation of the PI3K/Akt signalling pathway.
Apoptosis is a programmed and initiative cellular death regulated by genes. This condition is determined by the related signal transduction pathway activation and apoptosis gene. The PI3K/Akt pathway is an important anti-apoptotic signal pathway that has been studied in recent years. Bcl-2 is an important molecule that regulates apoptosis. Ischemia, hypoxia and other factors change the mitochondrial permeability to induce the release of cytochrome C. The down-regulation of Bcl-2 and Bcl-xL results in cell apoptosis [19-21]. In the present study, the expression level of the anti-apoptotic protein Bcl-2 was higher in the model group than in the sham operation group; however, the two groups showed no significant difference. The protein expression of Bcl-2 was significantly higher in the progesterone group than in the model and Akt inhibitor groups. These results suggest that progesterone exerted its anti-apoptotic effect by up-regulating Bcl-2 expression, which is also related to the activation of the PI3K/Akt signalling pathway.
The effects of HIBD on neonatal rats were obvious after 24 h. The expression of inflammatory mediators and apoptosis in the brain tissue increased. The prophylactic progesterone can increase pAkt level, inhibit NF-κB expression, promote Bcl-2 expression, relieve inflammation reaction and apoptosis after HIBD and protect the brain through the activation of the PI3K/Akt signalling pathway. The results of this study may serve as a guide for the clinical prevention and treatment of neonatal hypoxic ischemic encephalopathy.
Acknowledgements
This study was supported by grants from the Education Department of Henan Science Research Program (No. 15A310007), the Funding Program for Young Backbone Teachers in Colleges and Universities of Henan (No. 2012GGJS-134), the Open Projects in Key Research Areas of Xinxiang Medical University (No. ZD2011-2) and National Natural Science Foundation of China (No. 81371346).
Disclosure of conflict of interest
None.
References
- 1.Boggio PS, Coutinho de Macedo E, Pascual-Leone A, Tormos Muñoz JM, Schwartzman JS, Fregni F. Neuromodulation in hypoxic-ischemic injury. Brain Stimul. 2009;2:179–81. doi: 10.1016/j.brs.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. A dual role of the NF-kappa B pathway in neonatal hypoxic-ischemic brain damage. Stroke. 2008;39:2578–86. doi: 10.1161/STROKEAHA.108.516401. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Shravah J, Luo H, Raedschelders K, Chen DD, Ansley DM. Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem Biophys Res Commun. 2009;389:105–11. doi: 10.1016/j.bbrc.2009.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song JQ, Teng X, Cai Y, Tang CS, Qi YF. Activation of Akt/GSK-3 signaling pathway is involved in intermedin1-53 protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis. 2009;14:1299–307. doi: 10.1007/s10495-009-0398-7. [DOI] [PubMed] [Google Scholar]
- 5.Singh M, Su C. Progesterone-induced neuroprotection: factors that may predict therapeutic efficacy. Brain Res. 2013;1514:98–106. doi: 10.1016/j.brainres.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baudry M, Bi X, Aguirre C. Progesterone-estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2013;239:280–94. doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Zhang J, Yang Y, Dong W, Wang F, Wang L, Li X. Progesterone attenuates cerebral edema in neonatal rats with hypoxic-ischemic brain damage by inhibiting the expression of matrix metalloproteinase-9 and aquaporin-4. Exp Ther Med. 2013;6:263–267. doi: 10.3892/etm.2013.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Zhang J, Zhu X, Hou R, Li X, Dong X, Wang X, Lu C. Effects of progesterone on hippocampal ultrastructure and expression of inflammatory mediators in neonatal rats with hypoxic-ischemic brain injury. Exp Ther Med. 2014;7:1311–1316. doi: 10.3892/etm.2014.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang HC, Zhong RL, Xia Z, Song J, Feng L. Neuroprotective Effects of Rhynchophylline Against Ischemic Brain Injury via Regulation of the Akt/mTOR and TLRs Signaling Pathways. Molecules. 2014;19:11196–210. doi: 10.3390/molecules190811196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkaki AR, Khaksari Haddad M, Soltani Z, Shahrokhi N, Mahmoodi M. Time- and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J Neurotrauma. 2013;30:47–54. doi: 10.1089/neu.2010.1686. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal R, Medhi B, Pathak A, Dhawan V, Chakrabarti A. Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. J Pharm Pharmacol. 2008;60:731–7. doi: 10.1211/jpp.60.6.0008. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Ikeda T. Progesterone and allopregnanolone exacerbate hypoxic-ischemic brain injury in immature rats. Exp Neurol. 2012;233:214–20. doi: 10.1016/j.expneurol.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, Iams JD, Wapner RJ, Sorokin Y, Alexander JM, Harper M, Thorp JM Jr, Ramin SM, Malone FD, Carpenter M, Miodovnik M, Moawad A, O’Sullivan MJ, Peaceman AM, Hankins GD, Langer O, Caritis SN, Roberts JM. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, McCullough LD. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol Sin. 2013;34:1121–30. doi: 10.1038/aps.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu XL, Xiong LZ, Wang Q, Liu ZG, Ma X, Zhu ZH, Hu S, Gong G, Chen SY. Therapeutic time window and mechanism of tetramethylpyrazine on transient focal cerebral ischemia/reperfusion injury in rats. Neurosci Lett. 2009;449:24–7. doi: 10.1016/j.neulet.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Cordeau P, Kriz J. Real-time imaging after cerebral ischemia: model systems for visualization of inflammation and neuronal repair. Methods Enzymol. 2012;506:117–33. doi: 10.1016/B978-0-12-391856-7.00031-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Oh TH, Yoon WJ, Kang GJ, Yang EJ, Park SS, Lee NH, Kang HK, Yoo ES. Eutigoside C inhibits the production of inflammatory mediators (NO, PGE (2), IL-6) by down-regulating NF-kappaB and MAP kinase activity in LPS-stimulated RAW 264.7 cells. J Pharm Pharmacol. 2008;60:917–24. doi: 10.1211/jpp.60.7.0014. [DOI] [PubMed] [Google Scholar]
- 18.Brown KD, Claudio E, Siebenlist U. The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res Ther. 2008;10:212. doi: 10.1186/ar2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A, Fernandez-Salguero PM. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 2005;115:74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 20.Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, Zuo Z, Gozal Y. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J Pharmacol Exp Ther. 2006;318:186–94. doi: 10.1124/jpet.105.100537. [DOI] [PubMed] [Google Scholar]
- 21.Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience. 2012;210:442–50. doi: 10.1016/j.neuroscience.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


