Abstract
Current data regarding the association between the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and the risk of developing gastric cancer are insufficient to draw definite conclusions. Therefore, the present meta-analysis was conducted to achieve a more precise estimation of the association. MEDLINE, EMBASE and Wanfang database searches resulted in the identification of 28 eligible studies describing 5,757 cases and 8,501 controls. The strength of the association between the MTHFR C677T polymorphism and gastric cancer risk were evaluated using crude odds ratios (ORs), with 95% confidence intervals (CIs). The pooled ORs were determined using homozygous (TT vs. CC), heterozygous (CT vs. CC), dominant (TT+CT vs. CC) and recessive (TT vs. CC+CT) models. When all studies were pooled into the meta-analysis, significant associations were identified between the MTHFR C677T polymorphism and the risk of gastric cancer (homozygous model: OR, 1.39; 95% CI, 1.20–1.62; heterozygous model: OR, 1.18; 95% CI, 1.05–1.32; dominant model: OR, 1.23; 95% CI, 1.10–1.38; recessive model: OR, 1.26; 95% CI, 1.12–1.42). Stratification of the data by ethnicity identified a statistically significantly elevated risk of gastric cancer in Asian MTHFR C677T polymorphism populations (homozygous model: OR, 1.64; 95% CI, 1.43–1.90; heterozygous model: OR, 1.30; 95% CI, 1.16–1.45; dominant model: OR, 1.39; 95% CI, 1.25–1.54; recessive model: OR, 1.41; 95% CI, 1.25–1.51), but not in Caucasian populations (homozygous model: OR, 1.15; 95% CI, 0.89–1.48; heterozygous model: OR, 1.03; 95% CI, 0.84–1.25; dominant model: OR, 1.05; 95% CI, 0.86–1.28; recessive model: OR, 1.09; 95% CI, 0.91–1.31). Following adjustment for heterogeneity, the current meta-analysis demonstrated that the MTHFR C677T polymorphism was not associated with the risk of gastric cancer in Caucasian individuals. Furthermore, no evidence of publication bias was observed. Thus, the current meta-analysis indicates that the MTHFR C677T allele may be a low-penetrant risk factor for the development of gastric cancer in Asian populations.
Keywords: gastric cancer, case-control, meta-analysis, methylenetetrahydrofolate reductase, polymorphism
Introduction
Gastric cancer is the second most common cause of cancer-associated mortality in the world. In particular, it is one of the predominant cancer types in Korean and East Asian populations (1–4). Gastric cancer is a multifactorial malignant disorder caused by a wide range of risk factors, including genetic predisposition, the environment and Helicobacter pylori infection (1). Persistent H. pylori infection in the human stomach elicits a chronic inflammatory response, the extent of which may vary between individuals depending on the genetic makeup of the host. This phenomenon may aid in explaining the diverse range of outcomes observed in individuals infected with H. pylori. Therefore, polymorphisms in genes that are important in the host inflammatory response to this infection may alter an individual's susceptibility to gastric cancer (2). Notably, associations have been identified between gastric cancer and the expression of various genes involved in folate metabolism, such as methionine synthase (MTR), methylenetetrahydrofolate reductase (MTHFR) and MTR reductase (MTRR) (3).
MTHFR is an essential component of folate metabolism that has been indicated to be involved in DNA methylation and synthesis (4). The common MTHFR C677T polymorphism results in the substitution of alanine by valine, producing of a thermolabile variant that retains only ~30% of the activity of the wild-type MTHFR enzyme (5). The association between this gene polymorphism and the risk of gastric cancer has drawn increasing attention in the scientific community and has been investigated extensively, with 27 original studies (3,6–32) examining the role of the MTHFR C667T polymorphism in the development of gastric cancer. However, these studies have yielded conflicting results, partially due to the small effect of the gene polymorphism on the risk of gastric cancer and the relatively small sample sizes used. Therefore, the aim of the current meta-analysis was to determine a more precise estimation of the association between the MTHFR C677T polymorphism and gastric cancer risk.
Materials and methods
Identification and eligibility of relevant studies
The current meta-analysis was performed according to the guidelines for systematic reviews of genetic association studies (33). Two investigators independently searched the MEDLINE, EMBASE and Wanfang electronic databases for studies published from inception to May 2013. Combining text words and Medical Subject Headings (MESH) terms, the following keywords were used to perform the literature search: ‘MTHFR’ or ‘methylenetetrahydrofolate reductase’ to search for MTHFR; ‘gastric cancer’ or ‘stomach cancer’ to search for gastric cancer; and ‘gene’, or ‘polymorphism’ or ‘genetic variation’ to search for genetic variations. The aforementioned search terms were used in conjunction with the ‘explode’ feature where applicable. Full studies published in the English and Chinese languages were considered for inclusion in the present study. In addition, the reference lists of all primary studies and reviews were manually searched. All case-control studies that investigated the association between the MTHFR 677C>T polymorphism and gastric cancer were included. Furthermore, when the same series was used in more than one case-control study, the study with the largest cohort was selected.
Data extraction
The following data was extracted from each of the selected studies: First author, year of publication, ethnicity of study population, and the number of cases and controls for each C677T genotype.
Statistical analysis
Crude odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to evaluate the association between the MTHFR 677C>T polymorphism and the risk of gastric cancer. The pooled ORs were obtained for homozygous (TT vs. CC), heterozygous (CT vs. CC), dominant (TT+CT vs. CC) and recessive (TT vs. CC +CT) models. Heterogeneity assumption was examined using the χ2-based Q test (34), with P≤0.01 considered to indicate heterogeneity among studies. Subsequently, the pooled OR estimate was calculated for each study using the fixed-effects model (Mantel-Haenszel method) (35). Otherwise, the random-effects model (DerSimonian and Laird method) was used (36). To evaluate the source of between-study heterogeneity, Galbraith plots was constructed to identify outliers that may be acting as major sources of between-study heterogeneity. In addition, subgroup analyses by ethnicity were performed. The potential publication bias of the present study was estimated by constructing a funnel plot in which the standard error of log(OR) was plotted against log(OR), for each study. Funnel plot asymmetry, which indicates a possible publication bias, was evaluated using Egger's linear regression test. Furthermore, the significance of the intercept was determined by performing a t-test, as proposed by Egger (37). P<0.05 was considered to indicate a statistically significant publication bias (38). All statistical analyses were performed using Stata software (version 10.0; Stata Corporation, College Station, TX, USA).
Results
Study characteristics
A total of 27 publications met the inclusion criteria of the current meta-analysis (3,6–32), thus, a total of 5,757 cases and 8,501 controls were used in the pooled analyses. Tables I and II list the included studies and their major characteristics. In the 27 studies, the sample sizes ranged between 72 and 1,230 individuals. Furthermore, the studies included 12 European and 17 Asian populations, and the majority of controls were matched for gender and age.
Table I.
Major characteristics of all studies included in the current meta-analysis.
| First author (ref.) | Year of publication | Country | Ethnicity | Cases, n | Controls, n |
|---|---|---|---|---|---|
| Miao et al (8) | 2002 | China | Asian | 217 | 468 |
| Gao et al (7) | 2002 | China | Asian | 107 | 200 |
| Gao et al (32) | 2001 | China | Asian | 107 | 200 |
| Stolzenberg-Solomon et al (21) | 2003 | China | Asian | 90 | 398 |
| Bi et al (9) | 2005 | China | Asian | 309 | 188 |
| Shen et al (10) | 2005 | China | Asian | 320 | 313 |
| Sarbia et al (14) | 2005 | Germany | Caucasian | 332 | 255 |
| Wang et al (20) | 2005 | China | Asian | 129 | 315 |
| Si et al (18) | 2005 | China | Asian | 122 | 101 |
| Kim et al (19) | 2005 | Korea | Asian | 133 | 445 |
| Li et al (30) | 2006 | China | Asian | 170 | 140 |
| Graziano et al (13) | 2006 | Italy | Caucasian | 162 | 164 |
| Lacasaña-Navarro et al (24) | 2006 | Mexico | Caucasian | 201 | 427 |
| Weng et al (17) | 2006 | China | Asian | 38 | 34 |
| Zeybek et al (26) | 2007 | Turky | Caucasian | 35 | 144 |
| Wang et al (16) | 2007 | China | Asian | 467 | 540 |
| Götze et al (12) | 2007 | Germany | Caucasian | 103 | 106 |
| Zhang et al (3) | 2007 | USA | Caucasian | 295 | 399 |
| Mu et al (6) | 2007 | China | Asian | 194 | 391 |
| Boccia et al (11) | 2007 | Italy | Caucasian | 102 | 254 |
| Vollset et al (25) | 2007 | Europe | Caucasian | 295 | 399 |
| Li et al (15) | 2007 | China | Asian | 170 | 140 |
| Zúñiga-Noriega et al (23) | 2008 | Mexico | Caucasian | 51 | 83 |
| Galván-Portillo et al (22) | 2009 | Mexico | Caucasian | 248 | 478 |
| Yang et al (31) | 2010 | China | Asian | 139 | 165 |
| De Re et al (27) | 2010 | Italy | Caucasian | 57 | 454 |
| Saberi et al (29) | 2012 | Iran | Caucasian | 450 | 780 |
| Gao et al (28) | 2013 | China | Asian | 264 | 535 |
Table II.
Genotypes of the methylenetetrahydrofolate reductase C677T polymorphism included in the meta-analysis.
| Cases, n | Controls, n | ||||||
|---|---|---|---|---|---|---|---|
| First author (ref.) | Year of publication | CC | CT | TT | CC | CT | TT |
| Gao et al (32) | 2001 | 22 | 61 | 24 | 63 | 99 | 38 |
| Miao et al (8) | 2002 | 47 | 107 | 63 | 151 | 217 | 100 |
| Gao et al (7) | 2002 | 22 | 61 | 24 | 63 | 99 | 38 |
| Stolzenberg-Solomon et al (21) | 2003 | 17 | 36 | 37 | 65 | 209 | 124 |
| Bi et al (9) | 2005 | 139 | 150 | 20 | 97 | 76 | 15 |
| Shen et al (10) | 2005 | 105 | 171 | 44 | 113 | 172 | 28 |
| Sarbia et al (14) | 2005 | 138 | 153 | 41 | 107 | 115 | 33 |
| Wang et al (20) | 2005 | 25 | 45 | 59 | 74 | 143 | 98 |
| Si et al (18) | 2005 | 58 | 48 | 16 | 49 | 43 | 9 |
| Kim et al (19) | 2005 | 42 | 64 | 27 | 143 | 239 | 63 |
| Li et al (30) | 2006 | 61 | 78 | 31 | 67 | 56 | 17 |
| Graziano et al (13) | 2006 | 34 | 86 | 42 | 67 | 68 | 29 |
| Lacasaña-Navarro et al (24) | 2006 | 56 | 85 | 60 | 144 | 179 | 104 |
| Weng et al (17) | 2006 | 14 | 19 | 5 | 15 | 11 | 8 |
| Zeybek et al (26) | 2007 | 18 | 12 | 5 | 64 | 65 | 15 |
| Wang et al (16) | 2007 | 74 | 203 | 190 | 119 | 234 | 187 |
| Götze et al (12) | 2007 | 46 | 45 | 12 | 41 | 49 | 16 |
| Zhang et al (3) | 2007 | 146 | 116 | 33 | 185 | 178 | 36 |
| Mu et al (6) | 2007 | 50 | 106 | 38 | 135 | 199 | 57 |
| Boccia et al (11) | 2007 | 29 | 51 | 22 | 98 | 115 | 41 |
| Vollset et al (25) | 2007 | 109 | 104 | 32 | 248 | 277 | 94 |
| Li et al (15) | 2007 | 61 | 78 | 31 | 67 | 56 | 17 |
| Zúñiga-Noriega et al (23) | 2008 | 16 | 23 | 12 | 17 | 49 | 17 |
| Galván-Portillo et al (22) | 2009 | 37 | 132 | 79 | 89 | 217 | 172 |
| Yang et al (31) | 2010 | 44 | 80 | 15 | 62 | 75 | 28 |
| De Re et al (27) | 2010 | 18 | 25 | 14 | 152 | 238 | 64 |
| Saberi et al (29) | 2012 | 422 | 308 | 50 | 198 | 172 | 35 |
| Gao et al (28) | 2013 | 115 | 105 | 44 | 277 | 207 | 51 |
Meta-analysis of the MTHFR C677T polymorphism
Table III indicates the major results of the current meta-analysis. When all the studies were pooled into the meta-analysis, the MTHFR T allele was determined to be associated with a significantly increased risk of developing gastric cancer (homozygous model: OR, 1.39; 95% CI, 1.20–1.62; heterozygous model: OR, 1.18; 95% CI, 1.05–1.32; dominant model: OR, 1.23; 95% CI, 1.10–1.38; recessive model: OR, 1.26; 95% CI, 1.12–1.42) (P<0.001). In the subgroup analysis by ethnicity, no significantly increased risk of gastric cancer was identified in Caucasians with the MTHFR C677T polymorphism [homozygous model: OR, 1.15; 95% CI, 0.89–1.48 (Fig. 1A); heterozygous model: OR, 1.03; 95% CI, 0.84–1.25; dominant model: OR, 1.05; 95% CI, 0.86–1.28 (Fig. 1B); recessive model: OR, 1.09; 95% CI, 0.91–1.31]; however, significantly increased risks were identified in Asian populations (homozygous model: OR, 1.64; 95% CI, 1.43–1.90; heterozygous model: OR, 1.30; 95% CI, 1.16–1.45; dominant model: OR, 1.39; 95% CI, 1.25–1.54; recessive model: OR, 1.41; 95% CI, 1.25–1.51).
Table III.
Pooled OR data obtained in the current meta-analysis.
| Contrast model | Studies, n | OR (95% CI) | P-value | Model | I2, % | P-value |
|---|---|---|---|---|---|---|
| Total studies | ||||||
| Homozygous | 27 | 1.39 (1.20–1.62) | <0.001 | Random | 41.5 | 0.011 |
| Heterozygous | 27 | 1.18 (1.05–1.32) | 0.006 | Random | 47.3 | 0.003 |
| Recessive | 27 | 1.26 (1.12–1.42) | <0.001 | Random | 34.1 | 0.039 |
| Dominant | 27 | 1.23 (1.10–1.38) | <0.001 | Random | 52.8 | 0.016 |
| Caucasian | ||||||
| Homozygous | 12 | 1.15 (0.89–1.48) | 0.791 | Random | 58.2 | 0.006 |
| Homozygous (adjusted for heterogeneity) | 10 | 1.13 (0.93–1.36) | 0.215 | Fixed | 10.6 | 0.345 |
| Heterozygous | 12 | 1.03 (0.84–1.25) | <0.001 | Fixed | 0.0 | 0.674 |
| Recessive | 12 | 1.09 (0.91–1.31) | 0.367 | Fixed | 32.1 | 0.134 |
| Dominant | 12 | 1.05 (0.86–1.28) | 0.609 | Random | 63.3 | 0.002 |
| Dominant (adjusted for heterogeneity) | 10 | 1.00 (0.88–1.14) | 0.968 | Fixed | 27.6 | 0.19 |
| Asian | ||||||
| Homozygous | 17 | 1.64 (1.43–1.90) | <0.001 | Fixed | 0.0 | 0.674 |
| Heterozygous | 17 | 1.30 (1.16–1.45) | <0.001 | Fixed | 2.6 | 0.423 |
| Recessive | 17 | 1.41 (1.25–1.61) | <0.001 | Fixed | 8.1 | 0.361 |
| Dominant | 17 | 1.39 (1.25–1.54) | <0.001 | Fixed | 0.0 | 0.729 |
OR, odds ratio; CI, confidence interval; I2, index of heterogeneity.
Figure 1.
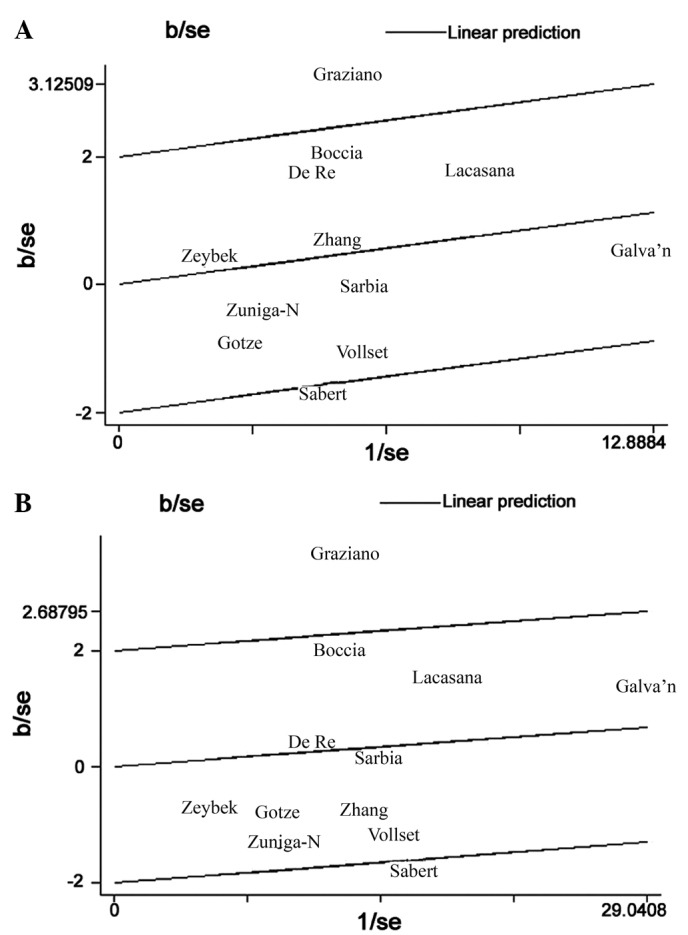
Galbraith plots of the association between the methylenetetrahydrofolate reductase T677C polymorphism and gastric cancer risk in Caucasian populations, using a (A) homozygous and (B) dominant model. Each author name represents a respective and separate study included in the current meta-analysis. b, bias; se, standard error.
Publication bias
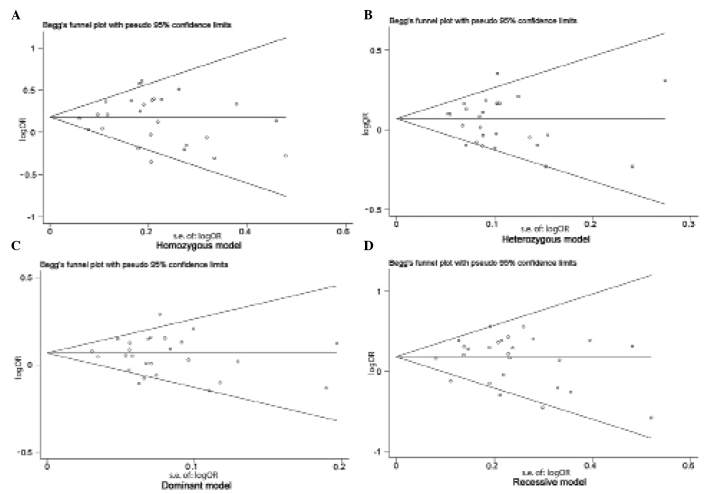
Begg's funnel plot and Egger's test were performed to assess the publication bias of studies included in the current meta-analysis. No evidence of marked asymmetry was observed in the funnel plot (Fig. 2). Furthermore, Egger's test did not indicate any statistical evidence of asymmetry and therefore, publication bias (homozygous model, P=0.866; heterozygous model, P=0.940; dominant model, P=0.851; recessive model, P=0.358).
Figure 2.
Begg's funnel plots for assessing the publication bias risk, using (A) homozygous (P=0.866), (B) heterozygous (P=0.940) (C) dominant (P=0.851) and (D) recessive (P=0.358) models. s.e., standard error; OR, odds ratio.
Discussion
It is well documented that individual susceptibility to the development of cancer can vary, even when exposed to the same environmental carcinogens (2,33). This difference in susceptibility may be associated with genetic variations, such as polymorphisms, in genes involved in carcinogenesis. Therefore, genetic susceptibility to the development of cancer has been the focus of considerable scientific research. Recently, extensive investigation of genetic variants of the MTHFR gene has taken place to determine its role in the etiology of gastric cancer. Numerous studies have examined the role of the MTHFR C677T polymorphism in gastric cancer risk, however, the data is contradictory. Therefore, to improve understanding of the association between the MTHFR C677T polymorphism and the risk of gastric cancer, the present meta-analysis of pooled data from a large sample was conducted. To the best of our knowledge, this is the first meta-analysis regarding the association between the MTHFR C677T polymorphism and the risk of gastric cancer to be conducted. In addition, subgroup analysis and heterogeneity evaluations were performed. The results indicated that the MTHFR 677 T allele is associated with a significantly increased risk of developing gastric cancer. Furthermore, significant associations were identified in Asian individuals, but not in Caucasian individuals, indicating a possible role of ethnicity in the risk of gastric cancer, due to differences in genetic backgrounds, geography and environment (37). However, it is possible that the effect of the MTHFR 677 C allele is masked by the expression of thus far unidentified causal genes involved in the development of gastric cancer in Caucasian individuals. In addition, the ethnic differences observed in the present study may be due to chance, as studies with small sample sizes typically lack the statistical power to detect marginal effects and may generate a fluctuated risk estimate (39). Considering the limited number of studies included in the present meta-analysis and the small Caucasian populations, the current results should be interpreted with caution.
Heterogeneity is a potential problem that may affect the interpretation of the results of all meta-analyses. In the present meta-analysis, significant between-study heterogeneity for OR was identified in the overall comparisons (homozygous model, P=0.011; heterozygous model, P=0.003; dominant model, P=0.016; recessive model, P=0.039). However, subgroup analysis by ethnicity demonstrated that heterogeneity was only evident between studies involving Caucasian populations (homozygous model, P=0.006; recessive model, P=0.002) but not for those involving Asian populations (Table III). Heterogeneity may also occur in poorly-designed studies that do not exclude biases, as these biases may affect the estimation of the real effects and cause incorrect conclusions to be drawn (40,41). Therefore, Galbraith plots were used to identify the outlier studies with poor quality designs. Following subgroup analysis of Caucasian studies, the Galbraith plot identified two studies that appeared to be major sources of heterogeneity (Fig. 1), with no between-study heterogeneity observed among the remaining 10 studies (homozygous model, P=0.345; recessive model, P=0.190). As a result, the fixed-effects model was used to pool the ORs from the two outlier studies, effectively removing heterogeneity from the current meta-analysis and thus confirming that the two excluded studies contributed the heterogeneity. Following adjustment for heterogeneity, the current data demonstrated that the MTHFR MTHFR C677T polymorphism was significantly associated with an increased risk of gastric cancer in Asian individuals, but not in Caucasian individuals.
A number of limitations should be taken into consideration when interpreting the findings of the current meta-analysis. First, the controls were not uniformly defined. Although the majority of the control subjects were recruited from healthy populations, certain individuals exhibited benign medical disorders. As a number of studies in the present meta-analysis included control groups that may have different risks of developing gastric cancer, non-differential misclassification bias may have occurred. Second, the current results were based on unadjusted estimates. If individual data is made available, future studies should consider using it to perform more precise analyses, as individual data would allow for the adjustment for additional co-variates, such as age, smoking status, environmental factors and lifestyle. Despite the aforementioned limitations, the current meta-analysis exhibited high statistical power, as a large number of cases and controls were pooled from different studies. In addition, no publication bias was detected, indicating that the overall pooled effects were unbiased.
In conclusion, the current meta-analysis indicated that the MTHFR T allele is a low-penetrant genetic risk factor for the development of gastric cancer. However, well-matched case-control studies with homogeneous cancer patients of multi-ethnic groups using standardized unbiased genotyping methods are warranted in the future. Furthermore, it is recommended that investigations should be conducted into the effects of gene-gene and gene-environment interactions on the development of gastric cancer.
References
- 1.Ahn DH, Rah H, Choi YK, et al. Association of the miR-146aC>G, miR-149T>C, miR-196a2T>C, and miR-499A>G polymorphisms with gastric cancer risk and survival in the Korean population. Mol Carcinog (Suppl 1) 2013;52:E39–E51. doi: 10.1002/mc.21962. [DOI] [PubMed] [Google Scholar]
- 2.Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259–270. doi: 10.1093/aje/kwq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang FF, Terry MB, Hou L, et al. Genetic polymorphisms in folate metabolism and the risk of stomach cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:115–121. doi: 10.1158/1055-9965.EPI-06-0513. [DOI] [PubMed] [Google Scholar]
- 4.Warshauer ME, Silverman DT, Schottenfeld D, Pollack ES. Stomach and colorectal cancers in Puerto Rican-born residents of New York City. J Natl Cancer Inst. 1986;76:591–595. doi: 10.1093/jnci/76.4.591. [DOI] [PubMed] [Google Scholar]
- 5.Jacques PF, Bostom AG, Williams RR, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.CIR.93.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Mu LN, Cao W, Zhang ZF, et al. Polymorphisms of 5, 10-methylenetetralydrofolate reductase (MTHFR), fruit and vegetable intake, and the risk of stomach cancer. Biomarkers. 2007;12:61–75. doi: 10.1080/13547500600945101. [DOI] [PubMed] [Google Scholar]
- 7.Gao C, Wu J, Ding J, et al. Polymorphisms of methylenetetrahydrofolate reductase C677T and the risk of stomach cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:289–292. (In Chinese) [PubMed] [Google Scholar]
- 8.Miao X, Xing D, Tan W, Qi J, Lu W, Lin D. Susceptibility to gastric cardia adenocarcinoma and genetic polymorphisms in methylenetetrahydrofolate reductase in an at-risk Chinese population. Cancer Epidemiol Biomarkers Prev. 2002;11:1454–1458. [PubMed] [Google Scholar]
- 9.Bi J, Cai L, Zheng Z. Study on C667T gene polymorphism and susceptibility to gastric cancer. Chin J Public. 2005;21:661. [Google Scholar]
- 10.Shen H, Newmann AS, Hu Z, et al. Methylenetetrahydrofolate reductase polymorphisms/haplotypes and risk of gastric cancer: A case-control analysis in China. Oncol Rep. 2005;13:355–360. [PubMed] [Google Scholar]
- 11.Boccia S, Gianfagna F, Persiani R, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and susceptibility to gastric adenocarcinoma in an Italian population. Biomarkers. 2007;12:635–644. doi: 10.1080/13547500701546766. [DOI] [PubMed] [Google Scholar]
- 12.Götze T, Röcken C, Röhl FW, et al. Gene polymorphisms of folate metabolizing enzymes and the risk of gastric cancer. Cancer Lett. 2007;251:228–236. doi: 10.1016/j.canlet.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Graziano F, Kawakami K, Ruzzo A, et al. Methylenetetrahydrofolate reductase 677C/T gene polymorphism, gastric cancer susceptibility and genomic DNA hypomethylation in an at-risk Italian population. Int J Cancer. 2006;118:628–632. doi: 10.1002/ijc.21397. [DOI] [PubMed] [Google Scholar]
- 14.Sarbia M, Geddert H, Kiel S, et al. Methylenetetrahydrofolate reductase C677T polymorphism and risk of adenocarcinoma of the upper gastrointestinal tract. Scand J Gastroenterol. 2005;40:109–111. doi: 10.1080/00365520410009500. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Ji M, He N, Lu Z. Application of microarray-based method for methylenetetrahydrofolate reductase (MTHFR) polymorphisms in the risk of gastric carcinoma in east China population. J Nanosci Nanotechnol. 2007;7:3245–3249. doi: 10.1166/jnn.2007.693. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Guo W, He Y, et al. Association of MTHFR C677T and SHMT1 C1420T with susceptibility to ESCC and GCA in a high incident region of Northern China. Cancer Causes Control. 2007;18:143–152. doi: 10.1007/s10552-006-0097-4. [DOI] [PubMed] [Google Scholar]
- 17.Weng YR, Sun DF, Fang JY, Gu WQ, Zhu HY. Folate levels in mucosal tissue but not methylenetetrahydrofolate reductase polymorphisms are associated with gastric carcinogenesis. World J Gastroenterol. 2006;12:7591–7597. doi: 10.3748/wjg.v12.i47.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Si PR, Fang DC, Zhang H, Yang LQ, Luo YH, Liao HY. The relationship between methylenetetrahydrofolate reductase gene polymorphism and microsatellite instability in gastric cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:794–799. (In Chinese) [PubMed] [Google Scholar]
- 19.Kim JK, Kim S, Han JH, et al. Polymorphisms of 5,10-methylenetetrahydrofolate reductase and risk of stomach cancer in a Korean population. Anticancer Res. 2005;25:2249–2252. [PubMed] [Google Scholar]
- 20.Wang LD, Guo RF, Fan ZM, et al. Association of methylenetetrahydrofolate reductase and thymidylate synthase promoter polymorphisms with genetic susceptibility to esophageal and cardia cancer in a Chinese high-risk population. Dis Esophagus. 2005;18:177–184. doi: 10.1111/j.1442-2050.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 21.Stolzenberg-Solomon RZ, Qiao YL, Abnet CC, et al. Esophageal and gastric cardia cancer risk and folate- and vitamin B(12)-related polymorphisms in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2003;12:1222–1226. [PubMed] [Google Scholar]
- 22.Galván-Portillo MV, Cantoral A, Oñate-Ocaña LF, et al. Gastric cancer in relation to the intake of nutrients involved in one-carbon metabolism among MTHFR 677 TT carriers. Eur J Nutr. 2009;48:269–276. doi: 10.1007/s00394-009-0010-5. [DOI] [PubMed] [Google Scholar]
- 23.Zúñiga-Noriega JR, Velazco-Campos Mdel R, Aguirre-Rodríguez A, et al. C677T polymorphism of the MTHFR gene and the risk of developing distal gastric cancer in a Mexican population. Rev Gastroenterol Mex. 2007;72:355–358. (In Spanish) [PubMed] [Google Scholar]
- 24.Lacasaña-Navarro M, Galván-Portillo M, Chen J, López-Cervantes M, López-Carrillo L. Methylenetetrahydrofolate reductase 677C>T polymorphism and gastric cancer susceptibility in Mexico. Eur J Cancer. 2006;42:528–533. doi: 10.1016/j.ejca.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Vollset SE, Igland J, Jenab M, et al. The association of gastric cancer risk with plasma folate, cobalamin, and methylenetetrahydrofolate reductase polymorphisms in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16:2416–2424. doi: 10.1158/1055-9965.EPI-07-0256. [DOI] [PubMed] [Google Scholar]
- 26.Zeybek U, Yaylim I, Yilmaz H, et al. Methylenetetrahydrofolate reductase C677T polymorphism in patients with gastric and colorectal cancer. Cell Biochem Funct. 2007;25:419–422. doi: 10.1002/cbf.1317. [DOI] [PubMed] [Google Scholar]
- 27.De Re V, Cannizzaro R, Canzonieri V, et al. MTHFR polymorphisms in gastric cancer and in first-degree relatives of patients with gastric cancer. Tumour Biol. 2010;31:23–32. doi: 10.1007/s13277-009-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S, Ding LH, Wang JW, Li CB, Wang ZY. Diet folate, DNA methylation and polymorphisms in methylenetetrahydrofolate reductase in association with the susceptibility to gastric cancer. Asian Pac J Cancer Prev. 2013;14:299–302. doi: 10.7314/APJCP.2013.14.1.299. [DOI] [PubMed] [Google Scholar]
- 29.Saberi S, Zendehdel K, Jahangiri S, et al. Impact of methylenetetrahydrofolate reductase C677T polymorphism on the risk of gastric cancer and its interaction with Helicobacter pylori infection. Iran Biomed J. 2012;16:179–184. doi: 10.6091/ibj.1102.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Cai MJ, Hou P, He NY. Single nucleotide polymorphisms(C677T and A1298C) in methylenetetrahy-drofolate reductase gene and susceptibility to gastric carcinoma. J Southeast Univ. 2006;25:321–324. [Google Scholar]
- 31.Yang XX, Li FX, Yi JP, Li X, Sun JZ, Hu NY. Impact of methylenetetrahydrofolate reductase C677T polymorphism on the risk of gastric cancer, colorectal cancer and lung cancer. Guangdong Med J. 2010;31:2375–2378. [Google Scholar]
- 32.Gao CM, Wu JZ, Ding JH, et al. MTHFR C677T genotypes, lifestyle and the risk of stomach cancer. China J Cancer Prev Treat. 2001;8:187–190. [Google Scholar]
- 33.Sagoo GS, Little J, Higgins JP. Human Genome Epidemiology Network: Systematic reviews of genetic association studies. PLoS Med. 2009;6:e28. doi: 10.1371/journal.pmed.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 35.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7:889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 39.Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: An approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorlund K, Imberger G, Johnston BC, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS One. 2012;7:e39471. doi: 10.1371/journal.pone.0039471. [DOI] [PMC free article] [PubMed] [Google Scholar]