Abstract
This study aimed to assess the effects of thoracic anodal and cathodal transcutaneous spinal direct current stimulation (tsDCS) on upper and lower limb corticospinal excitability. Although there have been studies assessing how thoracic tsDCS influences the spinal ascending tract and reflexes, none has assessed the effects of this technique over upper and lower limb corticomotor neuronal connections. In 14 healthy subjects we recorded motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) from abductor hallucis (AH) and hand abductor digiti minimi (ADM) muscles before (baseline) and at different time points (0 and 30 min) after anodal or cathodal tsDCS (2.5 mA, 20 min, T9–T11 level). In 8 of the 14 subjects we also tested the soleus H reflex and the F waves from AH and ADM before and after tsDCS. Both anodal and cathodal tsDCS left the upper limb MEPs and F wave unchanged. Conversely, while leaving lower limb H reflex unchanged, they oppositely affected lower limb MEPs: whereas anodal tsDCS increased resting motor threshold [(mean ± SE) 107.33 ± 3.3% increase immediately after tsDCS and 108.37 ± 3.2% increase 30 min after tsDCS compared with baseline] and had no effects on MEP area and latency, cathodal tsDCS increased MEP area (139.71 ± 12.9% increase immediately after tsDCS and 132.74 ± 22.0% increase 30 min after tsDCS compared with baseline) without affecting resting motor threshold and MEP latency. Our results show that tsDCS induces polarity-specific changes in corticospinal excitability that last for >30 min after tsDCS offset and selectively affect responses in lower limb muscles innervated by lumbar and sacral motor neurons.
Keywords: direct current stimulation, spinal cord stimulation, spinal cord, corticospinal system, transcutaneous spinal direct current stimulation, transcranial direct current stimulation, transcranial magnetic stimulation, motor potentials
transcutaneous spinal direct current stimulation (tsDCS) is a simple, painless, and noninvasive technique for modulating spinal cord function in humans (Cogiamanian et al. 2011, 2012; Lamy and Boakye 2013b; Priori et al. 2014). tsDCS consists in delivering a constant direct current (DC) at 1.5–2.5 mA over the spinal cord through a pair of sponge electrodes. The technique induces effects lasting from minutes to hours (Cogiamanian et al. 2008; Lamy et al. 2012; Lim and Shin 2011; Winkler et al. 2010) and is well tolerated by subjects. After the first reports (Cogiamanian et al. 2008; Winkler et al. 2010), this noninvasive method for spinal neuromodulation has come into increasingly widespread use (Cogiamanian et al. 2011; Hubli et al. 2013; Lamy and Boakye 2013b). Although different settings and stimulation parameters have been used, the “monopolar” montage (active electrode over the lower thoracic spinal cord, return electrode over the right arm) reduces the spread of the current toward the higher spinal cord levels or to the brain stem (Parazzini et al. 2014). Equally important, longitudinal electrical fields, such as those induced by this montage, may have important implications for rehabilitation, as they promote axonal regrowth and prevent fiber degeneration (Hernandez-Labrado et al. 2011).
In earlier research in our laboratory (Cogiamanian et al. 2008), we found that thoracic anodal tsDCS depresses the cervico-medullary somatosensory evoked potential (SEP) component (P30). tsDCS also modulates postactivation H-reflex dynamics (Lamy et al. 2012; Winkler et al. 2010) and the flexion reflex in the human lower limb (Cogiamanian et al. 2011). Further experiments reported that tsDCS impairs conduction in the ascending nociceptive spinal pathways, thus increasing pain tolerance in healthy subjects (Truini et al. 2011). Cervical spinal DC stimulation increased upper limb muscle motor evoked potential (MEP) amplitudes (Lim and Shin 2011). The effects induced by tsDCS could arise from the influence of electric field on impulse conduction, membrane excitability, and γ-aminobutyric acid (GABA)ergic and glutamatergic transmission (Priori et al. 2014). Whatever the mechanisms, by modulating spinal cord function, tsDCS could provide a novel therapeutic tool complementary to drugs and invasive spinal cord stimulation in managing various pathological conditions, including pain, spasticity, and movement disorders.
Having more information on the underlying mechanisms is an essential prerequisite for designing future clinical applications, and, more specifically, to understand whether and how tsDCS influences the corticospinal system would be relevant. Although interesting, the work of Lim and Shin (2011) on upper limb motor responses cannot rule out possible nonspecific effects. Hence, to expand the knowledge about tsDCS effects on the human corticospinal pathways, we tested upper and lower limb MEPs elicited by transcranial magnetic stimulation (TMS) in a group of healthy subjects before and after tsDCS. To evaluate possible motor neuronal or reflex excitability changes induced by tsDCS we also tested the F wave from the lower limb abductor hallucis (AH) and upper limb abductor digiti minimi (ADM) muscles and the H reflex from soleus muscle.
MATERIALS AND METHODS
Transcutaneous spinal DC stimulation.
With participants lying supine on a comfortable couch, tsDCS (2.5 mA, 20 min) was delivered by a constant-current programmable electrical stimulator (HDCStim, Newronika) connected to a pair of electrodes, one centered over the spinous process of the 10th thoracic vertebra with the major axis longitudinally placed so that it spanned from 9th to 11th thoracic vertebrae and the other above the right shoulder on the deltoid muscle (Cogiamanian et al. 2008, 2011). Because the tibial nerve arises from L4 to S3 spinal levels that correspond to the 9th to 12th vertebral levels, the active tsDCS electrode was placed over lumbar and sacral motor neurons. tsDCS electrodes were thick (6 mm) rectangular pieces of saline-soaked synthetic sponge (7 × 5 cm, 35 cm2). We applied current at a density of 0.071 mA/cm2 and delivered a total charge density of 85.7 mC/cm2, which is below the threshold values for tissue damage (Liebetanz et al. 2009; McCreery et al. 1990). The wide electrode surface avoided the possible harmful effects of high current density. Apart from occasional, transient, and short-lasting tingling and burning sensations below the electrodes, tsDCS remained below the conscious sensory threshold throughout the experimental session. tsDCS polarity (cathodal or anodal) refers to the electrode over the spinal cord.
Motor evoked potentials.
TMS was delivered by a Novametrix Magstim 200 stimulator (Magstim, Whitland, UK) through a flat coil (outer diameter 13.5 cm) in which current flows clockwise (viewed from above). The coil was kept in a constant position centered over the vertex for both the upper and lower limbs; for the upper limb one edge of the coil was slightly tilted toward the hemisphere to be stimulated (Groppa et al. 2012). MEPs were recorded at rest by two standard nonpolarizable Ag-AgCl surface electrodes (diameter 10 mm; Technomed Europe), for upper limbs one placed over the belly of the ADM muscle and the other on the skin overlying the first metacarpophalangeal joint of the fifth finger of the left hand; for lower limbs, MEPs were recorded through one electrode placed over the belly of the AH muscle and the other on the first metatarsophalangeal joint of the left toe. Because both ADM and AH have been used in many TMS studies in normal subjects and in patients (Chen et al. 1998; Nakanishi et al. 2006; Osei-Lah and Mills 2004) and our laboratory uses both muscles in routine TMS studies, we selected these two muscles for our experiments.
Stimulation intensity was set at 120% of resting motor threshold (RMT) defined as the minimum stimulator output that evoked MEPs higher than 50 μV in at least 5 of 10 trials when muscle was completely relaxed (Di Lazzaro et al. 1999; Ni et al. 2007). The threshold was set differently for each muscle and was analyzed at each time point [baseline (B) and immediately (T0) and 30 min (T30) after tsDCS offset]. However, to compare MEP modifications across time we used the same stimulation intensity (baseline). A total of 10 MEPs were collected at ∼10-s intervals and averaged for each time point. MEPs were amplified and filtered (bandwidth 3 Hz–3 kHz; Nicolet Viking IV P). Three different variables were measured: RMT (% of stimulator output), onset latency (ms), and area under the curve (mVms) of motor response. RMT was measured before and after tsDCS; MEP area and latency were measured off-line on the MEPs averaged from 10 sweeps.
H reflex.
H reflexes were elicited in eight subjects by delivering 1-ms rectangular pulses through Ag-AgCl electrodes (10-mm diameter) placed over the left tibial nerve at the popliteal fossa (interelectrode distance 20 mm) and recorded from the soleus muscle through Ag-AgCl electrodes (10-mm diameter) placed 2 cm apart over the muscle belly. The leg was fixed, with the hip semiflexed (∼110°), the knee slightly flexed (∼150°), and the ankle in ∼10° plantar flexion. The current intensity was progressively increased to obtain H-reflex threshold (defined as the minimum stimulation intensity that evoked reproducible response higher than 50 μV), maximal H reflex (Hmax), and maximal compound muscle action potential (CMAPmax). To avoid postactivation effects, the tibial nerve was stimulated at intervals randomly varying between 10 and 20 s.
To define threshold and maximum size of H reflex, stimulation began at 0-mA intensity and increased in 1-mA steps up to the intensity eliciting the maximal H reflex. Signals were amplified and band-pass filtered (3 Hz–3 kHz). We measured the H-reflex size (peak-to-peak amplitude, mV), and we calculated the Hmax-to-CMAPmax ratio.
F wave.
F waves were elicited in eight subjects with a 25% supramaximal stimulation applied to the tibial nerve and recorded from the AH muscle or applied to the ulnar nerve and recorded from the ADM muscle through a pair of 10-mm surface Ag-AgCl electrodes in a belly-to-tendon configuration. Tibial nerve-evoked F waves from the AH muscle were obtained by 20 stimuli delivered to the left ankle with an interstimulus interval of 1 s (1 Hz). Similarly, ulnar F waves from ADM muscles were elicited by 20 stimuli delivered to the ulnar nerve at the left wrist with an interstimulus interval of 1 s (1 Hz). Subjects were asked to fully relax. To ensure the absence of muscular activity, we recorded the audio EMG feedback from the same muscles used for MEP recording at all time points. F-wave mean latency (ms), minimal latency (ms), mean amplitude (mV), and mean temporal dispersion (ms) were collected and analyzed. The filter setting was 15-1,500 Hz, and skin temperature at the ankle and wrist was kept above 32°C.
Subjects and experimental procedure.
A group of 14 healthy right-handed volunteers (9 women and 5 men, mean ± SD age 25.6 ± 4.3 yr) participated in the study, which was approved by the institutional review board. Before enrollment, the study protocol was explained to each subject and informed written consent was obtained. Experimental procedures were conducted in accordance with the Declaration of Helsinki.
Subjects were studied before and after anodal and cathodal tsDCS. Cathodal and anodal tsDCS in each subject were tested in random order, and at least 1 wk elapsed between sessions. The subjects were blinded as to tsDCS polarity. Because preliminary experiments showed that anodal and cathodal tsDCS elicited MEP changes in opposite directions and subjects were unable to discriminate stimulation polarity [as for brain transcranial direct current stimulation (tDCS)], as in previous works (Cogiamanian et al. 2008; Truini et al. 2011), we avoided using sham stimulation.
Soleus H reflex, lower (AH) and upper (ADM) limb MEPs, and F wave were recorded before tsDCS [baseline (B)], immediately after tsDCS offset (T0), and at 30 min after tsDCS offset (T30); all tests were performed in the same order as listed above. For all electrophysiological recordings we chose the left side to avoid any possible modification of motor response on the right side due to the current flowing through the reference tsDCS electrode possibly acting on peripheral nerves. During each tsDCS session, subjects were interviewed to assess the general tolerability of the procedure and were asked to report any adverse effect, particularly itching, tingling, burning, and pain sensations.
Data analysis.
We analyzed statistically significant changes for the following variables: MEP threshold (% of maximum stimulator output), MEP area (mVms), MEP latency (ms); H-reflex threshold, latency (ms), amplitude (μV), and Hmax-to-CMAPmax amplitude ratio; F-wave mean latency (ms), minimal latency (ms), amplitude (μV), and temporal dispersion (ms). All the neurophysiological measures were considered as independent variables and were analyzed separately. Each variable is expressed throughout the text as a percentage of baseline values (= 100%), after anodal and cathodal tsDCS, at T0 and T30.
First, to verify the absence of biases due to intrasubject changes across tsDCS sessions, a t-test was run to compare baseline data for anodal and cathodal tsDCS. Values of P < 0.05 were considered to indicate statistical significance.
Then tsDCS-induced changes in each variable were tested with a two-way repeated-measure analysis of variance (ANOVA) (STATISTICA 5.5, StatSoft) with main factors of stimulation [2 levels (anodal and cathodal)] and time [3 levels (B, T0, and T30)]. Bonferroni-corrected t-tests were used for post hoc comparison (P < 0.025). Values are expressed as means ± SE.
RESULTS
All participants tolerated the procedure well, and none of them reported adverse effects. Participants occasionally reported a slight tingling or itching sensation below the stimulating electrodes (not distinguishable between the 2 polarities) that disappeared within a few seconds or after wetting the electrode sponges.
tsDCS effects in lower limb muscles.
No differences were found between anodal and cathodal tsDCS in baseline values for any of the measured variables in the lower limb muscles. Neither anodal nor cathodal tsDCS induced changes in H-reflex and F-wave variables (Table 1).
Table 1.
Changes in F-wave and H-reflex variables over time in both experimental conditions (anodal and cathodal stimulation)
| F Wave |
H Reflex Soleus |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AH |
ADM |
|||||||||
| Minimal latency, ms | Mean latency, ms | Dispersion, ms | Minimal latency, ms | Mean latency, ms | Dispersion, ms | Threshold, ms | Latency, ms | Area, mVms | Ratio H/M | |
| Anodal | ||||||||||
| B | 46.4 ± 1.3 | 48.2 ± 1.5 | 4.3 ± 0.6 | 25.8 ± 0.6 | 27.9 ± 0.8 | 2.9 ± 0.5 | 14.7 ± 3.5 | 28.9 ± 1.0 | 7.4 ± 1.0 | 0.4 ± 0.1 |
| T0 | 47.0 ± 1.5 | 49.3 ± 1.4 | 4.3 ± 0.5 | 26.2 ± 1.0 | 27.8 ± 0.8 | 2.8 ± 0.7 | 13.9 ± 2.8 | 28.3 ± 1.3 | 7.1 ± 0.6 | 0.4 ± 0.05 |
| T30 | 47.2 ± 1.4 | 50.5 ± 2.5 | 4.4 ± 0.7 | 26.3 ± 0.9 | 28.3 ± 1.9 | 3.0 ± 0.4 | 13.9 ± 1.8 | 29.3 ± 1.4 | 7.7 ± 0.9 | 0.4 ± 0.13 |
| Cathodal | ||||||||||
| B | 46.3 ± 1.2 | 48.6 ± 1.2 | 4.4 ± 0.5 | 25.3 ± 1.2 | 27.8 ± 1.5 | 3.0 ± 0.7 | 14.1 ± 3.0 | 28.0 ± 1.2 | 7.7 ± 0.6 | 0.4 ± 0.04 |
| T0 | 46.3 ± 1.4 | 48.8 ± 1.7 | 4.7 ± 0.3 | 25.5 ± 1.5 | 28.0 ± 1.0 | 3.1 ± 0.4 | 14.1 ± 2.5 | 27.8 ± 1.3 | 7.6 ± 1.1 | 0.4 ± 0.1 |
| T30 | 47.2 ± 1.6 | 49.1 ± 1.5 | 4.8 ± 0.7 | 26.0 ± 1.1 | 28.3 ± 1.0 | 2.8 ± 0.6 | 13.8 ± 2.7 | 28.9 ± 0.8 | 7.9 ± 1.4 | 0.3 ± 0.3 |
| P value | 0.6 | 0.2 | 0.8 | 0.3 | 0.9 | 0.3 | 0.8 | 0.7 | 0.8 | 0.3 |
Data are means ± SE. We found no significant changes in F-wave and H-reflex variables across the different time points [baseline (B), immediately after transcutaneous spinal direct current stimulation (tsDCS) offset (T0), and at 30 min after tsDCS offset (T30)].
AH, abductor hallucis; ADM, abductor digiti minimi; H/M, maximal H reflex/maximal compound muscle action potential.
P values refer to 2-way repeated-measures ANOVA with stimulation and time as factors (interaction effects).
Although MEP latency showed a nonspecific effect of time (2-way ANOVA, factor time: P = 0.009) [baseline latency values were in general shorter than latency values at T30 (post hoc analysis: baseline vs. T30 103.53 ± 0.95%, P = 0.008)], MEP latency was not affected by tsDCS (2-way ANOVA, factor stimulation: P = 0.83) or by the interaction between tsDCS and time (2-way ANOVA, interaction stimulation × time: P = 0.85).
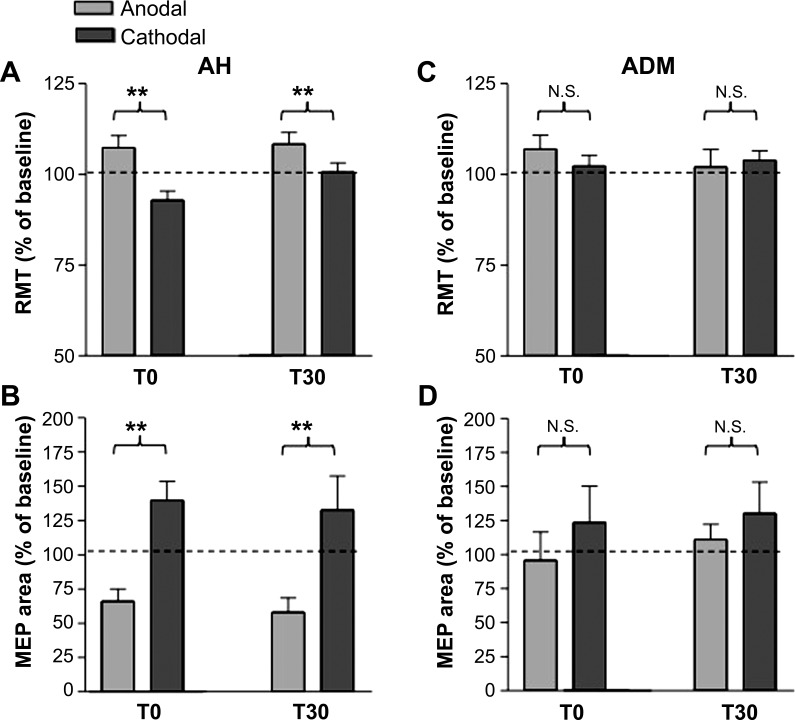
RMT was affected by tsDCS (2-way ANOVA, factor stimulation: P = 0.012, factor time: P = 0.11; interaction stimulation × time: P = 0.011). More specifically, after anodal tsDCS (Fig. 1A) RMT increased (post hoc analysis anodal tsDCS: T0A vs. BA: 107.33 ± 3.3%, P = 0.006; T30A vs. BA: 108.37 ± 3.2%, P = 0.002) whereas after cathodal tsDCS (Fig. 1B and Fig. 2A) it remained unchanged (post hoc analysis, cathodal tsDCS: T0C vs. BC: 96.83 ± 2.3%, P = 0.15; T30C vs. BC: 100.60 ± 2.1%, P = 0.8).
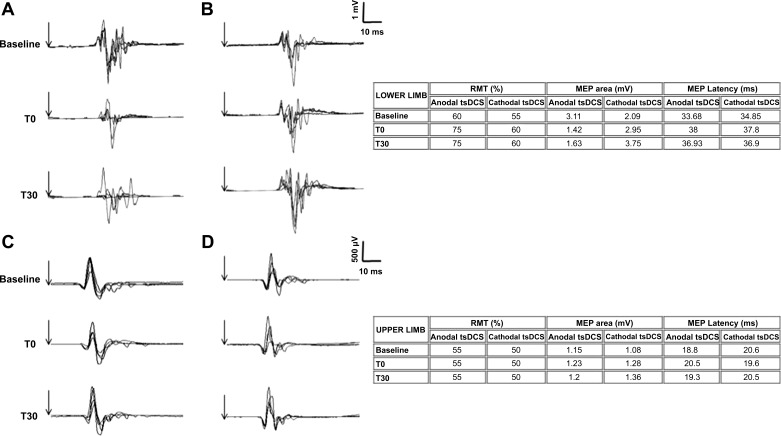
Fig. 1.
A and B: lower limb motor evoked potentials (MEPs) before (baseline), immediately after (T0), and at 30 min after (T30) anodal (A) or cathodal (B) transcutaneous spinal direct current stimulation (tsDCS) in a representative subject. Each trace is the superimposition of 5 sweeps. Table on right reports average values of resting motor threshold (RMT), MEP area, and MEP latency for the represented subject. Note that whereas anodal tsDCS decreased the MEP area at T0 and T30, cathodal tsDCS increased the MEP area. Vertical arrows represent the stimuli. C and D: upper limb MEPs before (baseline), immediately after (T0), and at 30 min after (T30) anodal (C) or cathodal (D) tsDCS in a representative subject. Each trace is the superimposition of 5 sweeps. Table on right reports average values of RMT, MEP area, and MEP latency for the represented subject. Note that neither anodal nor cathodal tsDCS affected MEPs. Vertical arrows represent the stimuli.
Fig. 2.
A and B: effects of tsDCS on RMT (A) and MEP area (B) when responses were recorded from abductor hallucis muscle (AH; data are expressed as % of baseline). Group data are presented as mean ± SE changes induced by anodal or cathodal tsDCS immediately after current offset (T0) and 30 min later (T30) (error lines are SE). Note that anodal and cathodal tsDCS induced significantly different, opposite changes in RMT and MEP area. C and D: effects induced by tsDCS on RMT (C) and MEP area (D) when responses were recorded from abductor digiti minimi (ADM) muscles. Group data are presented as mean ± SE changes induced by anodal or cathodal tsDCS immediately after current offset (T0) and 30 min later (T30) (error lines are SE). Neither anodal nor cathodal tsDCS significantly changed RMT or MEP area. **P < 0.05. N.S., not significant.
MEP area was also modulated after tsDCS (2-way ANOVA, factor stimulation: P = 0.96, factor time: P = 0.78; interaction stimulation × time: P = 0.008) (Fig. 2B): whereas after anodal tsDCS MEP area failed to change (post hoc analysis anodal tsDCS: T0A vs. BA: 66.09 ± 9.1%, P = 0.24; T30A vs. BA: 58.12 ± 10.48%, P = 0.13), after cathodal tsDCS MEP area increased (post hoc analysis cathodal tsDCS: T0C vs. BC: 139.71 ± 12.9%, P = 0.018; T30C vs. BC: 132.74 ± 22.0%, P = 0.02).
In conclusion, anodal tsDCS increases RMT whereas cathodal tsDCS increases the MEP area in lower limb muscles.
tsDCS effects in upper limb muscles.
No differences were found between anodal and cathodal tsDCS in baseline values for any of the measured variables in the upper limb muscles. Neither anodal nor cathodal tsDCS induced changes in F-wave variables (Table 1).
The two-way ANOVA disclosed no anodal or cathodal tsDCS-induced effect on MEP variables (Fig. 1, C and D, Fig. 2, C and D). RMT did not change over time and across sections (Fig. 2C; 2-way ANOVA, factor stimulation: P = 0.54, factor time: P = 0.14; interaction stimulation × time: P = 0.48), nor did MEP area (Fig. 2D; 2-way ANOVA, factor stimulation: P = 0.58, factor time: P = 0.63; interaction stimulation × time: P = 0.23). MEP latency was not affected by stimulation (2-way ANOVA, factor stimulation: P = 0.26; interaction stimulation × time: P = 0.29) but showed a nonspecific time-related increase at T30 compared with baseline (2-way ANOVA, time P = 0.013; post hoc analysis: baseline vs. T30 103.53 ± 0.95%, P = 0.011).
DISCUSSION
Whereas tsDCS leaves H reflex, F wave, and upper limb RMT and MEP size unchanged, it modulates the excitability in corticospinal projections to lower limb muscles for at least 30 min after stimulation offset, inducing polarity-dependent excitability changes: anodal tsDCS significantly increases RMT, whereas cathodal stimulation increases MEP area.
The absence of F wave changes in our experiments therefore argues against the occurrence of changes in postsynaptic motor neuronal excitability after tsDCS. The absence of Hmax-to-CMAPmax ratio changes in this and a previous study from our group (Cogiamanian et al. 2011) agrees with previous observations (Lamy et al. 2012; Winkler et al. 2010).
The tsDCS-induced corticospinal excitability changes at lower limb level are in line with our previous observation that tsDCS modulates conduction along human spinal ascending pathways (Cogiamanian et al. 2008; Truini et al. 2011) and are consistent with the effects of anodal spinal DC on motor potentials elicited by cortical stimulation in the mouse triceps surae (Ahmed 2011).
How tsDCS influences the corticospinal system remains hypothetical. First, because tDCS influences neurotransmitters in the brain (Rango et al. 2008), tsDCS could do the same in the human spinal cord, ultimately modulating the corticospinal output as in animals (Ahmed and Wieraszko 2012). As recently shown in animals (Ahmed 2013), cathodal tsDCS can amplify segmental responses to supraspinal drive by increasing glutamate release at the spinal level, although mice typically lack the monosynaptic corticomotor neuronal synapse of higher primates. Another possibility is that tsDCS could influence neural activity in ascending spinal pathways, ultimately modulating the excitability in their cortical targets including the motor areas, as changes in RMT suggest. Possible support for a cortical mechanism comes from a report that invasive spinal stimulation seems to modulate intracortical facilitation (Schlaier et al. 2007). Thus our data expand, rather than being simply contradictory, previous knowledge on putative tsDCS targets, including supraspinal, and possibly polarity-specific, effects of spinal current polarization; this possibility also agrees with recent evidence in rats that noninvasive spinal stimulation modulates the activity of gracile nucleus and primary somatosensory cortex (Aguilar et al. 2011).
Finally, tsDCS could influence the conductive properties of the corticospinal tract, for example, by decreasing/increasing the number of axons conducting an action potential. For instance, anodal tsDCS can induce a hyperpolarizing conduction block, thus blocking action potentials along the pyramidal tract (Bhadra and Kilgore 2004). However, given that we used low current intensities, anodal block could not be the sole explanation for the effects of anodal tsDCS; moreover, it is known that also in routinary electrodiagnostic testing geometry and tissue distribution of electrical fields are additional critical parameters for inducing a hyperpolarizing block (Dreyer et al. 1993; Kirshblum et al. 1998).
Although the mechanisms underlying the tsDCS-induced changes in the corticospinal system remain speculative, our finding that anodal tsDCS seems mainly to affect the RMT whereas cathodal tsDCS predominantly influences MEP area is intriguing. A possible explanation is that the mechanisms underlying cathodal and anodal tsDCS differ and could have different putative circuit(s)-pathway(s)-neurotransmitters, i.e., anodal current might preferentially act on one target system, whereas cathodal current acts on another. This possibility agrees with evidence that anodal and cathodal brain tDCS act through different brain neurotransmitters: for instance, whereas anodal tDCS reduces GABA, cathodal tDCS decreases glutamate (Stagg et al. 2009). Increase in MEP area after cathodal tsDCS is in line with previous reports (Aguilar et al. 2011; Ahmed 2011, 2013; Alanis 1953; Eccles et al. 1962) and agrees with data in animals showing an increased recruitment of larger motor units after cathodal polarization (Ahmed and Wieraszko 2012); as suggested in a recent work by our group, cathodal, but not anodal, stimulation could have a transsynaptic effect mediated by spinal interneurons likely involving Renshaw cell network (Bocci et al. 2014).
Among methodological issues related to our study the first is that to avoid subjecting participants to another experimental session, we decided not to test them under sham conditions. Sham testing was also unnecessary given that even though changes induced by cathodal tsDCS and anodal tsDCS go in opposite directions, subjects cannot distinguish between the two polarities. We therefore considered each polarity as the best possible control for the other, as previously reported (Cogiamanian et al. 2008; Lamy and Boakye 2013a; Truini et al. 2011). Besides, as recently shown by Kessler and colleagues (Kessler et al. 2012), sham stimulation may be an inappropriate control condition for some studies, because sensory side effects seem to be more frequent and severe in active than in sham tDCS.
A second methodological issue concerns possible pitfalls concerning the use of the H reflex and the F wave to assess spinal motor neuron excitability. The absence of H-reflex changes suggests no modification to small motor neurons, and the lack of F-wave effects rules out changes in large motor neurons (McNeil et al. 2013). However, the classical view that the H reflex and the F wave represent separate and complementary events at spinal level, i.e., presynaptic inhibition versus changes in intrinsic motor neuron excitability (Fisher 1992; Leis et al. 1995), is questionable. In fact, while the H reflex could be also affected by postactivation depression and changes in axonal excitability itself (McNeil et al. 2013; Pierrot-Deseilligny et al. 1981), many reports have suggested that F waves offer only a flawed measure of motor neuron excitability (Hultborn and Nielsen 1995).
A further important point is to compare our data with those of Lim and Shim (2011), who found that cervical spinal DC stimulation influences upper limb MEPs. However, they did not report polarity-specific effects. Their results cannot be compared with ours because they studied a different anatomical region, with a different recording montage and stimulation intensity, and used smaller electrodes. Finally, there are known features in how corticospinal excitability differs between the upper and lower body, which may account, at least in part, for differences between our results and data obtained by Lim and Shin. In particular, monosynaptic corticomotor neuronal projections, with fast-conducting motor units, are more prominent for hand than for proximal arm or lower limb muscles (Brouwer and Ashby 1992; Dalpozzo et al. 2002; Palmer and Ashby 1992); in this view, the lack of changes in MEP amplitude after anodal tsDCS may be caused by the greater desynchronization of corticomotor neuronal input compared with upper limb muscles, related to the involvement of fibers with different sizes or activation of polysynaptic descending pathways.
Overall, our data support the conclusion that tsDCS induces changes in corticospinal tract excitability. The first clinical observations with tsDCS are encouraging: Hubli et al. (2013) found that anodal tsDCS can improve gait in patients with spinal cord injury. Together with a previous report describing how tsDCS influences ascending spinal pathways (Aguilar et al. 2011), the present experiments suggest that the spinal cord could act as a “highway” for conveying tsDCS-induced changes to the brain, thereby inducing suprasegmental effects in the brain and brain stem. Because tsDCS is simple, safe, and noninvasive, our observation opens the way to new approaches using this technique in widely ranging neurological conditions characterized by corticospinal and spinal cord dysfunction and possibly even in brain disorders.
GRANTS
This work was supported by FISM (Fondazione Italiana Sclerosi Multipla)-2009/R21.
DISCLOSURES
M. Vergari, S. Marceglia, F. Cogiamanian, and A. Priori are founders and shareholders of Newronika srl, Milan, Italy, a spin-off company of the University of Milan and of the Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico.
AUTHOR CONTRIBUTIONS
Author contributions: T.B., F.C., F.S., and A.P. conception and design of research; T.B., M.V., V.C., and F.C. performed experiments; T.B., S.M., M.V., F.C., F.S., and A.P. interpreted results of experiments; T.B. and S.M. drafted manuscript; T.B., S.M., M.V., V.C., and A.P. edited and revised manuscript; T.B., S.M., M.V., V.C., F.C., F.S., and A.P. approved final version of manuscript; S.M. analyzed data; M.V. and V.C. prepared figures.
ACKNOWLEDGMENTS
We gratefully acknowledge the participation of all subjects.
REFERENCES
- Aguilar J, Pulecchi F, Dilena R, Oliviero A, Priori A, Foffani G. Spinal direct current stimulation modulates the activity of gracile nucleus and primary somatosensory cortex in anaesthetized rats. J Physiol 589: 4981–4996, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Trans-spinal direct current stimulation modulates motor cortex-induced muscle contraction in mice. J Appl Physiol (1985) 110: 1414–1424, 2011. [DOI] [PubMed] [Google Scholar]
- Ahmed Z. Effects of cathodal trans-spinal direct current stimulation on mouse spinal network and complex multijoint movements. J Neurosci 33: 14949–14957, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Wieraszko A. Trans-spinal direct current enhances corticospinal output and stimulation-evoked release of glutamate analog, d-2,3-3H-aspartic acid. J Appl Physiol (1985) 112: 1576–1592, 2012. [DOI] [PubMed] [Google Scholar]
- Alanis J. Effects of direct current on motor neurones. J Physiol 120: 569–578, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N, Kilgore KL. Direct current electrical conduction block of peripheral nerve. IEEE Trans Neural Syst Rehabil Eng 12: 313–324, 2004. [DOI] [PubMed] [Google Scholar]
- Bocci T, Vannini B, Torzini A, Mazzatenta A, Vergari M, Cogiamanian F, Priori A, Sartucci F. Cathodal transcutaneous spinal direct current stimulation (tsDCS) improves motor unit recruitment in healthy subjects. Neurosci Lett 578: 75–79, 2014. [DOI] [PubMed] [Google Scholar]
- Brouwer B, Ashby P. Corticospinal projections to lower limb motoneurons in man. Exp Brain Res 89: 649–654, 1992. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 80: 2870–2881, 1998. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Ardolino G, Vergari M, Ferrucci R, Ciocca M, Scelzo E, Barbieri S, Priori A. Transcutaneous spinal direct current stimulation. Front Psychiatry 3: 63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Pulecchi F, Marceglia S, Priori A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol 119: 2636–2640, 2008. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Schiaffi E, Marceglia S, Ardolino G, Barbieri S, Priori A. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain 152: 370–375, 2011. [DOI] [PubMed] [Google Scholar]
- Dalpozzo F, Gerard P, De Pasqua V, Wang F, Maertens de Noordhout A. Single motor axon conduction velocities of human upper and lower limb motor units. A study with transcranial electrical stimulation. Clin Neurophysiol 113: 284–291, 2002. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial electrical stimulation over the motor cortex hand area in conscious humans. Exp Brain Res 124: 525–528, 1999. [DOI] [PubMed] [Google Scholar]
- Dreyer SJ, Dumitru D, King JC. Anodal block V anodal stimulation. Fact or fiction. Am J Phys Med Rehabil 72: 10–18, 1993. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Kostyuk PG, Schmidt RF. The effect of electric polarization of the spinal cord on central afferent fibres and on their excitatory synaptic action. J Physiol 162: 138–150, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA. AAEM Minimonograph #13: H reflexes and F waves: physiology and clinical indications. Muscle Nerve 15: 1223–1233, 1992. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Solé J, Siebner HR. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 123: 858–882, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Labrado GR, Polo JL, Lopez-Dolado E, Collazos-Castro JE. Spinal cord direct current stimulation: finite element analysis of the electric field and current density. Med Biol Eng Comput 49: 417–429, 2011. [DOI] [PubMed] [Google Scholar]
- Hubli M, Dietz V, Schrafl-Altermatt M, Bolliger M. Modulation of spinal neuronal excitability by spinal direct currents and locomotion after spinal cord injury. Clin Neurophysiol 124: 1187–1195, 2013. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Nielsen JB. H-reflexes and F-responses are not equally sensitive to changes in motoneuronal excitability. Muscle Nerve 18: 1471–1474, 1995. [DOI] [PubMed] [Google Scholar]
- Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul 5: 155–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshblum S, Cai P, Johnston MV, Shah V, O'Connor K. Anodal block in F-wave studies. Arch Phys Med Rehabil 79: 1059–1061, 1998. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Boakye M. BDNF Val66Met polymorphism alters spinal DC stimulation-induced plasticity in humans. J Neurophysiol 110: 109–116, 2013a. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Boakye M. Seeking significance of transcutaneous spinal DC stimulation. Clin Neurophysiol 124: 1049–1050, 2013b. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Ho C, Badel A, Arrigo RT, Boakye M. Modulation of soleus H reflex by spinal DC stimulation in humans. J Neurophysiol 108: 906–914, 2012. [DOI] [PubMed] [Google Scholar]
- Leis AA, Stetkarova I, Beric A, Stokic DS. Spinal motor neuron excitability during the cutaneous silent period. Muscle Nerve 18: 1464–1470, 1995. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol 120: 1161–1167, 2009. [DOI] [PubMed] [Google Scholar]
- Lim CY, Shin HI. Noninvasive DC stimulation on neck changes MEP. Neuroreport 22: 819–823, 2011. [DOI] [PubMed] [Google Scholar]
- McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng 37: 996–1001, 1990. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Butler JE, Taylor JL, Gandevia SC. Testing the excitability of human motoneurons. Front Hum Neurosci 7: 152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Tanaka N, Fujiwara Y, Kamei N, Ochi M. Corticospinal tract conduction block results in the prolongation of central motor conduction time in compressive cervical myelopathy. Clin Neurophysiol 117: 623–627, 2006. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol 583: 971–982, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Lah AD, Mills KR. Optimising the detection of upper motor neuron function dysfunction in amyotrophic lateral sclerosis—a transcranial magnetic stimulation study. J Neurol 251: 1364–1369, 2004. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol 448: 397–412, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini M, Fiocchi S, Liorni I, Rossi E, Cogiamanian F, Vergari M, Priori A, Ravazzani P. Modeling the current density generated by transcutaneous spinal direct current stimulation (tsDCS). Clin Neurophysiol 22: 222–223, 2014. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res 42: 337–350, 1981. [DOI] [PubMed] [Google Scholar]
- Priori A, Ciocca M, Parazzini M, Vergari M, Ferrucci R. Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J Physiol 592: 3345–3369, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rango M, Cogiamanian F, Marceglia S, Barberis B, Arighi A, Biondetti P, Priori A. Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a 1H-MRS study. Magn Reson Med 60: 782–789, 2008. [DOI] [PubMed] [Google Scholar]
- Schlaier JR, Eichhammer P, Langguth B, Doenitz C, Binder H, Hajak G, Brawanski A. Effects of spinal cord stimulation on cortical excitability in patients with chronic neuropathic pain: a pilot study. Eur J Pain 11: 863–868, 2007. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29: 5202–5206, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Vergari M, Biasiotta A, La Cesa S, Gabriele M, Di Stefano G, Cambieri C, Cruccu G, Inghilleri M, Priori A. Transcutaneous spinal direct current stimulation inhibits nociceptive spinal pathway conduction and increases pain tolerance in humans. Eur J Pain 15: 1023–1027, 2011. [DOI] [PubMed] [Google Scholar]
- Winkler T, Hering P, Straube A. Spinal DC stimulation in humans modulates post-activation depression of the H-reflex depending on current polarity. Clin Neurophysiol 121: 957–961, 2010. [DOI] [PubMed] [Google Scholar]