Abstract
Empathic impairment is one of the hallmarks of psychopathy, a personality dimension associated with poverty in affective reactions, lack of attachment to others, and a callous disregard for the feelings, rights, and welfare of others. Neuroscience research on the relation between empathy and psychopathy has predominately focused on the affective sharing and cognitive components of empathy in forensic populations, and much less on empathic concern. The current study used high-density electroencephalography in a community sample to examine the spatiotemporal neurodynamic responses when viewing people in physical distress under two subjective contexts: one evoking affective sharing, the other, empathic concern. Results indicate that early automatic (175–275 ms) and later controlled responses (LPP 400–1,000 ms) were differentially modulated by engagement in affective sharing or empathic concern. Importantly, the late event-related potentials (ERP) component was significantly impacted by dispositional empathy and psychopathy, but the early component was not. Individual differences in dispositional empathic concern directly predicted gamma coherence (25–40 Hz), whereas psychopathy was inversely modulatory. Interestingly, significant suppression in the mu/alpha band (8–13 Hz) when perceiving others in distress was positively associated with higher trait psychopathy, which argues against the assumption that sensorimotor resonance underpins empathy. Greater scores on trait psychopathy were inversely related to subjective ratings of both empathic concern and affective sharing. Overall, the study demonstrates that neural markers of affective sharing and empathic concern to the same cues of another's distress can be distinguished at an electrophysiological level, and that psychopathy alters later time-locked differentiations and spectral coherence associated with empathic concern.
Keywords: affective sharing, empathy, empathic concern, event-related potentials, gamma coherence, mu suppression, high-density EEG, perception of distress, psychopathy
psychopathy is considered by many scholars as a disorder of the moral brain, although it is not included as such in the DSM V. Psychopathic individuals seem to have the cognitive capacity to appreciate right from wrong but do not care, and do exhibit a flagrant disregard for social and moral norms (Aharoni et al. 2012; Cleckley 1941; Hare 1999; Kiehl 2014; Maibom 2005). The notion of a moral brain is used here to encompass the integration of cognitive, emotional, and motivational mechanisms, shaped through evolution, development, and culture, to facilitate how people should treat one another (Decety and Wheatley 2015). Psychopathy is usually understood as a constellation of personality dispositions that comprise affective and interpersonal deficits, as well as social deviance and poor behavioral control (Hare 1999; Hare and Neumann 2008). Antisociality, and its inclusion as a defining component of psychopathy, remains highly contentious (Skeem and Cooke 2010; Skeem et al. 2011). The interpersonal/affective component of psychopathy is much less controversial. It is largely characterized by a lack of empathy, poor attachment to others and an incapacity for love, poverty in affective reactions, and a callous disregard for others' well-being (Dadds et al. 2009; Hare and Neumann 2008; Kiehl 2014; Skeem and Cooke 2010). This deficit in empathy is indeed acknowledged to be at the core of the syndrome based on clinical descriptions, and appears to account for psychopaths' lack of insight for the impacts of their harmful actions on others (Cleckley 1941; Hare et al. 1993; Kiehl 2014; but see Domes et al. 2013).
The vast majority of empirical research on psychopathy has been conducted on forensic populations, mainly incarcerated male offenders. This has led to some dispute regarding the generalizability of the findings to community samples (Hall et al. 2004). Yet, studies with samples from the general population have increased in popularity, as the dimensional approach to personality disorders has gained support in both the clinical and research domains (Lilienfeld and Andrews 1996). Psychopathy can thus be viewed as either a dichotomous or a continuous variable, and there is now solid evidence that psychopathic traits are continuously distributed in the general population (Kotov et al. 2011; Lilienfeld et al. 2014; Seara-Cardoso et al. 2012; Skeem et al. 2003). This is important because it suggests that information about psychopathy can be drawn from both clinical populations and community samples (Malterer et al. 2010).
Empathy is not a unitary concept (Batson 2009; Decety 2011a). A complete taxonomy of empathy-related phenomena is outside the scope of this paper (Coplan 2011; Cuff et al. 2014, Decety and Jackson 2004; Roskies 2011). Recent theoretical and empirical work from developmental science, clinical neuroscience, and social neuroscience converge to view empathy as a construct broadly reflecting a natural capacity to share and understand the affective states of others, comprising emotional, cognitive, and motivational facets (Decety 2015). These facets or components interact with one another, but can be dissociated, and rely on specific cognitive and neurobiological mechanisms. Given the importance of empathy in interpersonal interactions, moral behavior, and in inhibiting antisocial behavior, studying individuals with various levels of psychopathy in the general population constitutes an important test case for understanding the mechanisms underpinning specific facets of empathy. This is critical when examining how each component contributes to processing the distress cues of others, pivotal in eliciting empathic concern and guiding moral behavior (Decety and Cowell 2014a).
The focus of most clinical neuroscience research on empathy has been on the emotional (affective sharing, or feeling the way another feels) and cognitive (perspective-taking, or putting oneself in someone else's place) components of empathy. In particular, it is well established that individuals with psychopathy do not experience distress cues as aversive. For instance, offenders with high levels of psychopathy show reduced autonomic arousal while viewing a confederate receiving electric shocks (Aniskiewicz 1979). They also show a lack of social threat avoidance tendencies (von Borries et al. 2012). Children with psychopathic tendencies have reduced electrodermal responses to distress signals and threatening stimuli (Blair 1999). Moreover, juvenile psychopaths with high scores on callous-unemotional traits exhibit atypical neural responses to visual stimuli depicting physical pain (Cheng et al. 2012). In that latter study, this abnormality was exemplified by a lack of an early electrophysiological response (N120 ms), thought to reflect an automatic attention to negative stimuli.
Functional MRI (fMRI) studies have documented a well-established pattern of brain activation when individuals are presented with, or even imagine, others in pain or in emotional distress (Lamm et al. 2011 for a meta-analysis). Adolescents with psychopathic traits or with conduct problems show reduced activity to stimuli depicting pain in this neural network including the anterior cingulate cortex (ACC), anterior insula (aINS), and amygdala (Lockwood et al. 2013; Marsh et al. 2013; but see Decety et al. 2009). However, in forensic psychopaths, relative to incarcerated controls, no reduction in neural response was detected in the ACC and aINS (i.e., regions involved in affective processing of pain) when they viewed pictures of physical pain, facial expressions of pain, or scenarios depicting interpersonal harm from a first-person perspective (Decety et al. 2013a,b; Decety et al. 2014; Decety et al. 2015). Yet, the participants from the aforementioned studies, who scored high on the psychopathy check list revised (PCL-R >30) exhibited significantly less activation in the ventromedial prefrontal cortex and lateral orbitofrontal cortex, regions critical to experiencing empathic concern and a motivation to care for others' well-being (Decety and Cowell 2014a,b; Parsons et al. 2013; Sobhani and Bechara 2011). Another fMRI study reported that while individuals with psychopathy demonstrated a typical response in pain-affective brain regions when taking an imagine-self perspective, they failed to recruit this neural circuit during an imagine-other perspective, and that diminished response may contribute to their lack of empathic concern (Decety et al. 2013a). Thus clinical neuroscience research with adult incarcerated psychopaths seems to suggest that when they pay attention to stimuli depicting harm, they activate the same regions that control participants would, with the notable exception of the ventromedial prefrontal cortex, a pivotal hub for empathic concern and moral decision-making (Decety and Cowell 2014a,b; Moll and de Oliveira-Souza 2007; Molenberghs et al. 2014; Parsons et al. 2013; Taber-Thomas et al. 2014; Young and Dungan 2012).
Cognitive empathy, often described as perspective taking, has also been well studied in both typical and atypical populations with fMRI and electrophysiology. As documented by several neuroimaging studies, when participants are asked to adopt the perspective of another person and imagine what she feels, increased hemodynamic signal is detected in the medial and ventromedial prefrontal cortex in conjunction with the posterior superior temporal sulcus. These regions enable an emotional understanding of others' subjective states by maintaining two concurrent sets of competing information, thus facilitating consideration of self and other perspectives (Behrens et al. 2009; Decety and Jackson 2004; Decety and Porges 2011; Decety and Sommerville 2003; Jackson et al. 2006; Lamm et al. 2007, 2010; Mazzarella et al. 2013; Ruby and Decety 2004, 2003; van der Heiden et al. 2013). In high-density EEG research, modulation of the mu/alpha range (8–12 Hz) has been reported when taking the perspective of another person in distress (Perry et al. 2010). Another study found that perspective taking modulates the late but not the early components of the ERP response to the pain of others (Li and Han 2010).
Psychopaths are usually considered to have intact cognitive empathy, that is, they are able to describe what and why other people feel a certain way, even if they do not share or care about those feelings (Book et al. 2007; Decety et al. 2015; Maibom 2009; Shamay-Tsoory et al. 2010, but see Brook and Kosson 2013; Lishner et al. 2012). Thus, examining the neural responses associated with each of these facets and their relations to individual dispositions can provide theoretical and empirical clarity to the link between psychopathy and empathy.
In comparison with the accumulated neurobiological knowledge of the emotional and cognitive components of empathy described above, and a few personality studies that have documented that, in the general population, trait psychopathy is associated with lower propensity to feel empathic concern and less difficulty to make decision in moral dilemmas (Decety and Yoder 2015; Seara-Cardoso et al. 2012), there are fewer functional neuroimaging studies that have examined the neural computations underpinning empathic concern. Empathic concern (or sympathy) reflects an other-oriented motivation elicited by the perceived welfare of someone in need. The desire to care for others is vital from an evolutionary perspective across many species (Batson 2012; Bell and Richard 2000). Empathic concern is an important element of human empathy due to its role in driving prosocial and caregiving behaviors, even when the behavior is costly to the individual. This motivation emerges very early in development without necessitating advanced theory of mind or verbal abilities (Davidov et al. 2013; Zahn-Waxler and Radke-Yarrow 1990). Empathic concern stems from the neural circuits implicated in parental behavior, selected through evolution to protect and nurture offspring and kin, which are highly conserved across species, and are modulated by intrinsic and environmental factors (Decety 2011b). Regions of this circuit include the periaqueductal gray, ventral tegmental area, specific nuclei of the hypothalamus, basal ganglia, and ventromedial prefrontal cortex (Noriuchi et al. 2008; Swain et al. 2012). One recent neuroimaging study conducted by Feldman Hall and colleagues (2015) found that trait empathic concern motivates costly altruism, and this relationship was supported by neural activity in the ventral tegmental area, caudate, and ventromedial prefrontal cortex, which are key regions for promoting social attachment and caregiving (Moll and Schulkin 2009; Parsons et al. 2013). It is also worth noting that item eight of the PCL-R (the primary assessment tool used to diagnose psychopathy in forensic populations) specifically indexes the lack of concern and callous disregard for the feelings, rights, and welfare of others and is separate from item seven that assesses shallow affect (Hare 1998). Thus there is general agreement on a severe empathy deficit in psychopathy. However, one study found little evidence in both college undergraduates and forensic inpatients linking psychopathy with impairment in the experience of either emotional contagion or empathic concern (Lishner et al. 2012). Studying affective sharing and empathic concern as separate phenomena or components is a sound approach, as each uniquely influences moral cognition and predicts differential outcomes in moral behavior (Decety and Cowell 2014b). In addition, individual trait differences in empathy and psychopathy have yet to be linked to online neural dynamics in empathic concern.
In addition, there is burgeoning work in social neuroscience with functional MRI and high-density electroencephalography (EEG) in nonforensic populations showing that cognitive empathy and empathic concern predict motivation for justice and moral decision-making (Yoder and Decety 2014a,b) and that trait psychopathy is associated with a diminished disposition in empathic concern and lower sensitivity to injustices towards others (Decety and Yoder 2015).
Although most research on empathy, whether in cognitive neuroscience or social and personality psychology, posits the existence of two separate, but functionally related, constructs of affective sharing and empathic concern, no previous study has specifically examined whether the two can be distinguished at a neurophysiological level. High-density EEG is a valuable tool to characterize potential information processing differences between empathic concern and affective sharing. Both event-related potentials (ERPs) and spectral features of EEG (power density, coherence, etc.) can provide precisely timed measures of neural responses to events. The present study, therefore, was designed to disentangle these two facets of empathy, i.e., affective sharing and empathic concern, in a nonforensic sample of participants. They were presented with ecologically valid visual stimuli depicting individuals in physical distress, and requested to either focus on how much pain these individuals were experiencing (to tap into the affective sharing component of empathy) or to report how sorry they felt for these individuals (to elicit the experience of empathic concern).
Based on previous electrophysiological studies, we anticipated differences in early and late ERP components that have been documented in both children and adults when perceiving another person in physical distress or being intentionally harmed (Cheng et al. 2014; Cowell and Decety 2015; Decety and Cacioppo 2012; Escobar et al. 2014; Han et al. 2008). These components include an early (∼200–300 ms post-stimulus onset), relatively automatic response to pain perception or preferential attention towards negative stimuli, typically indexed by an anterior N200 or a posterior P200 (Cheng et al. 2012; Fan and Han 2008; Han et al. 2008; Ibanez et al. 2012; Olofsson et al. 2008). The negative-valence evoked posterior P200 has been found at both parietal and occipital electrodes (Carretie et al. 2001; Carretie et al. 2004; Fan and Han 2008). The early response is argued to index attention orientation toward emotional stimuli (Kanske et al. 2011). Additionally, a late positive potential (LPP) component (Decety et al. 2010; Ibanez et al. 2012; Li and Han 2010; Yoder and Decety 2014a), identified as a sustained positive potential that responds to emotional stimuli as well as reflects top-down processes (Hajcak et al. 2009; Schupp et al. 2000), is commonly identified at midline central and parietal locations (∼400 to 1,000 ms; Hajcak et al. 2011). Consistent with previous EEG studies documenting task-related changes in amplitudes of later, but not early components (Cheng et al. 2012; Han et al. 2008; Ibanez et al. 2011), feeling concerned for someone in pain versus focusing on the intensity of the pain was predicted to modulate the LPP differences for painful versus neutral stimuli.
Furthermore, examining variations of spectral density in the mu/alpha range (8–13 Hz) may be informative of the degree to which sensorimotor resonance or attentional allocation differences are produced in response to the distress of others, as shown in previous studies (Gutsell and Inzlicht 2010; Perry et al. 2010). If mu rhythm indexes sensorimotor resonance, then greater suppression for painful stimuli compared with neutral stimuli may be expected. A burgeoning literature also suggests the importance of online coherence in the gamma band (25–40 Hz) for multisensory information processing (Balconi and Pozzoli 2009; Betti et al. 2009), and understanding the subjective states of others (Cohen et al. 2009). Event-related local and distal gamma coherence have been implicated in the strengthening of neural connections, reflecting the integration of top-down and bottom-up processes during a task (Ozerdem et al. 2011; Phillips and Singer 1997). Empathic concern requires the processing of distress signals and subsequent generation of a motivation to care for another, whereas affective sharing is a more rudimentary process of vicariously experiencing the emotional states of a conspecific. It was thus predicted that individuals with higher scores on trait psychopathy (and lower scores in dispositional empathic concern) would show less coherence during the empathic concern task. Finally, dispositional measures of empathy and psychopathy were predicted to differentially relate to both behavioral ratings and neural indexes of affective sharing and empathic concern. Based on recent fMRI studies of empathy in forensic psychopaths (Decety et al. 2013b), it was predicted that individuals scoring high on trait psychopathy would show a similar neural response in the affective sharing condition but reduced amplitudes of the LPP during the empathic concern task.
MATERIALS AND METHODS
Participants
Thirty-nine young adults were recruited for the study (20 female; mean age = 19.4 years; SD = 1.9 years) and were compensated for their time. One participant was excluded from analysis due to insufficient artifact-free EEG data. No participants reported a history of neurological or psychiatric illness. The study was approved by the University of Chicago Institutional Review Board. All subjects provided written, informed consent.
Dispositional Measure
All participants completed an online form at least 24 h prior to the EEG session on basic demographic information, including age, sex, ethnicity, and education, as well as three dispositional measures and a handedness inventory. Trait empathy was assessed with the Interpersonal Reactivity Index (IRI; Davis 1983), a well-validated multidimensional metric of empathy, which indexes specific facets of empathy, such as personal distress and empathic concern. Psychopathy was evaluated with the Levenson Self-Report Psychopathy Scale (LSRP; Levenson et al. 1995), designed for non-institutionalized populations. Sensitivity to justice was quantified using the Justice Sensitivity Inventory (Schmitt et al. 2010). Handedness was assessed via the Edinburgh Handedness Inventory (Oldfield 1971). Of the 38 participants, 33 were right-handed and 5 were left-handed.
Stimuli, Task, and Design
To examine affective sharing and empathic concern, participants viewed pictures of hands and feet in painful or neutral situations and were instructed to focus on the intensity of the pain experienced by the individual or the amount of concern they felt for him/her. Importantly, in the present study, before witnessing another person in distress, participants viewed the face of the individual that would be experiencing the pain to further contextualize the situation.
During the EEG session, participants viewed a standard set of 50 pictures of hands or feet in painful situations and 50 matched neutral pictures, used in several previous EEG and fMRI studies (Cheng et al. 2008; Decety et al. 2013b; Jackson et al. 2006; Jackson et al. 2005). The faces were selected from the NimStim set of facial expressions (Tottenham et al. 2009), and included nine male and nine female neutral expressions, presented randomly across trials.
For each trial, participants were presented with a fixation cross that lasted between 800 and 1,600 ms, then a face for 1,500 ms, followed by another fixation cross of 1,000 ms, then a painful or neutral stimulus (1,500 ms), and a 3,000 ms window where they responded with a visual analog scale. In that response phase, participants were asked to either evaluate the intensity of the pain or their empathic concern for the person depicted in each trial (VAS, with no pain/extreme pain and not sorry/very sorry as the anchors).
There were ten task blocks, five pain sharing and five empathic concern blocks, with each block consisting of 20 trials, for a total of 200 trials. Between blocks, a manually advanced instruction reminder was given to the participants. All 100 pictures were used twice, once in each task. This resulted in 50 trials per condition. Conditions were counterbalanced across subjects and stimuli were randomized across trials.
EEG Data Acquisition and Analysis
EEG data were collected from a 64-channel (EasyCap, 10–20 electrode locations) active electrode system (acti-Champ, Brain Products, Germany) at 2,000 Hz, referenced to Cz, with all electrode impedances kept below 10 kΩ. Electrophysiological data preprocessing was performed using Brain Vision Analyzer 2 Software (Brain Vision). Statistical analyses were performed using SPSS Statistics (IBM). All comparisons were based on specific a priori hypotheses; thus an alpha level of 0.05 was accepted.
ERP Data Analysis
Data were offline referenced to the average of linked mastoids and bandpass-filtered using an IIR filter of 0.1–30 Hz with a notch filter of 60 Hz. Ocular artifacts were removed using independent component analysis (ICA; Jung et al. 1998; Makeig et al. 1996). Artifacts were rejected in epoched data using a −200 μV to 200 μV threshold as well as manual inspection by trained research assistants. Epochs were created with a 200 ms baseline and 1,500 ms stimulus presentation following the onset of the painful or neutral stimuli. Epochs were baseline corrected and averaged by trial type.
ERP averages per individual were combined for each trial type to create grand averages for each of the four conditions. Data from all participants in the final sample (N = 38) had at least 25 artifact-free trials, with an average of 45.2 clean trials per condition, and an average of 5 trials rejected per condition. From the grand average and previous ERP studies of pain empathy, mean amplitudes within five time windows of interest were extracted: an early one (175–275 ms), a full late positive potential (LPP) (400-1,000 ms), an early LPP (400–600 ms), a middle LPP (600–800 ms), and a late LPP (800-1,000 ms). In addition, the inspection of the scalp topography and the grand averaged waveform was used to determine the time windows and electrode sites to be analyzed. Preliminary analyses of LPP effects across the three time windows suggested little differentiation; thus all results of LPP were based on the entire window (400-1,000 ms).
Statistical analyses of the early ERP component were carried out using a 2 (condition: affective sharing, empathic concern) by 2 (pain: painful, neutral) by 2 (sensor: O1, O2) repeated-measures ANOVA. For the LPP component, a 2 (condition: affective sharing, empathic concern) by 2 (pain: painful, neutral) by 4 (sensor: Cz, CPz, Pz, POz) repeated-measures ANOVA was performed. Dispositional measures of empathy, psychopathy, and justice sensitivity were correlated with the painful vs. neutral difference waves for empathic concern and affective sharing.
Spectral Data Analysis
As with the ERPs, the data were rereferenced to the average of the left and right mastoids, but a broader bandpass filter of 0.1–50 Hz was applied to allow for the examination of neuronal oscillations in the gamma range. Ocular artifact correction ICA, initial segmentation, artifact rejection, and baseline correction were performed using the same parameters as the ERP analysis. The preprocessed epochs were then divided by trial type and further segmented into 250 ms segments occurring from 0–1000 ms, and subsequently a fast Fourier transform (FFT) analysis was used. The resulting FFT data were averaged by trial type, and the alpha power density (8–13 Hz) was extracted and analyzed. Global alpha density was calculated by averaging across all sensors. The mu rhythm was analyzed using the mean alpha density of the central sensors (Cz, C1, C2, C3, C4, C5, C6). Pearson correlations were performed between the mu differences for painful vs. neutral stimuli and the dispositional measures of empathy and psychopathy.
For the gamma coherence analysis, the initial preprocessing steps were kept the same. FFT was performed on trial type epochs, and the coherence analysis was performed using a correlation across all combinations of electrodes. The resulting coherence values were exported into Matlab (Mathworks), where the gamma range (25–40 Hz) was extracted for all sensor combinations of interest for each participant. Local gamma coherence was calculated by averaging coherence between three combinations of sensors for left frontal (F3, F7, FC5), right frontal (F4, F8, FC6), left temporal (C3, T7, CP5), right temporal (C4, T8, CP6), left parietal (P3, P7, PO7), and right parietal (P4, P8, PO8) regions. Region by condition by pain ANOVAs were performed on these data. Dispositional measures were correlated with the average local coherence of all regions combined for painful vs. neutral stimuli within affective sharing and empathic concern conditions. Distal gamma coherence was calculated by averaging the coherence values between left frontal and right parietal (F3, F7, FC5 with P4, P8, PO8), left frontal and right temporal (F3, F7, FC5 with C4, T8, CP6), left temporal and right parietal (C4, T8, CP6 with P4, P8, PO8), as well as right frontal and left parietal (F4, F8, FC6 with P3, P7, PO7), right frontal and left temporal (F4, F8, FC6 with C3, T7, CP5), and right temporal to left parietal sites (C4, T8, CP6 with P3, P7, PO7). Distal coherence was analyzed as a regional connection by condition by pain repeated-measures ANOVA. Because the frontal to temporal and temporal to parietal connections were closer spatially than the frontal to parietal connections, the same ANOVA was also conducted separately on the closer and further connections. Pearson correlations were performed between dispositional measures and distal coherence values for each regional connection for painful vs. neutral stimuli in each condition.
RESULTS
Behavioral Ratings
Online subjective VAS ratings of the painful stimuli were analyzed in a 2 (condition) by 2 (sex) mixed-model ANOVA, which revealed a significant main effect of condition [F(1,36) = 7.189, P < 0.05, η2 = 0.166], wherein affective sharing ratings were greater than empathic concern. There was also a significant main effect of sex [F(1,36) = 12.753, P < 0.001, η2 = 0.262], characterized by higher ratings of both affective sharing and empathic concern for females compared with males. Finally, there was a significant interaction of condition and sex [F(1,36) = 9.511, P < 0.01, η2 = 0.209], wherein affective sharing ratings in male participants were greater than empathic concern ratings [t(18) = 3.164, P < 0.01]. However, there were no such differences in females [t(18) = 0.489, ns].
Additionally, individual differences in dispositional empathy, as indexed with the IRI, strongly predicted VAS ratings in affective sharing (IRI Total score: r = 0.472, P < 0.01; IRI Empathic Concern subscale: r = 0.658, P < 0.001) and in empathic concern (IRI Total score: r = 0.618, P < 0.001; IRI Empathic Concern subscale: r = 0.604, P < 0.001). Trait psychopathy (LSRP scores) negatively predicted ratings in both empathic concern (LSRP Total score: r = −0.394, P < 0.05; LSRP Primary Psychopathy subscale: r = −0.354, P < 0.05) and affective sharing (LSRP Total score: r = −0.410, P < 0.05; LSRP Primary Psychopathy subscale: r = −0.450, P < 0.01). None of the dispositional measures differed between sexes, with the exception of IRI Total score [t(36) = 2.429, P < 0.05] which was greater for females than males. As expected, several of the IRI and LSRP measures were negatively correlated (Table 1). Finally, dispositional measures of justice sensitivity were not related to either affective sharing or empathic concern.
Table 1.
Descriptive statistics of Interpersonal Reactivity Index (IRI) and Levenson Self Report Psychopathy (LSRP) assessments
| Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|
| IRI Total | 1.25 | 3.04 | 2.29 | 0.43 |
| IRI Perspective Taking | 0.57 | 3.57 | 2.48 | 0.74 |
| IRI Empathic Concern | 0.86 | 3.57 | 2.56 | 0.7 |
| IRI Personal Distress | 0 | 3 | 1.43 | 0.77 |
| LSRP Total | 1.31 | 3.04 | 2.04 | 0.35 |
| LSRP Primary | 1.06 | 2.94 | 1.98 | 0.43 |
| LSRP Secondary | 1.3 | 3.3 | 2.14 | 0.4 |
Shown are the minimum, maximum, mean and SD per item for each of the IRI and LSRP measures.
Electrophysiological Results
Early ERP component.
Results of a 2 (sensor) by 2 (condition) by 2 (pain) ANOVA for the early (175–275 ms window) component showed a main effect of pain [F(1,37) = 4.214, P < 0.05, η2 = 0.102], with painful stimuli eliciting a greater early positivity than neutral. There was also a main effect of condition [F(1,37) = 6.615, P < 0.05, η2 = 0.152], which was characterized by greater amplitudes for affective sharing than for empathic concern. Results from an ANOVA with the inclusion of sex as a between-subjects variable revealed no significant main or interaction effects of sex in the early ERP response (P > 0.1).
Late positive potential (LPP).
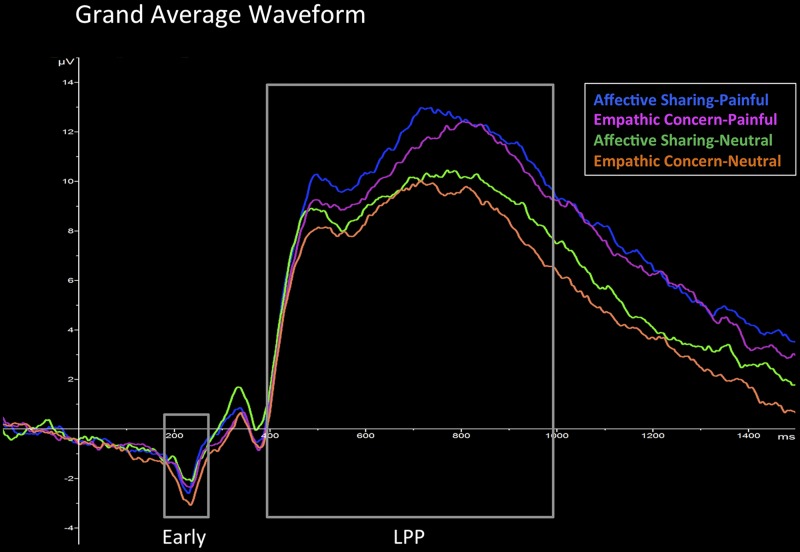
A 4 (sensor) by 2 (condition) by 2 (pain) ANOVA was performed for the LPP component (400–1,000 ms window). Results of this analysis showed a main effect of condition [F(1,37) = 4.989, P < 0.05, η2 = 0.119], with larger LPP responses for affective sharing than for empathic concern. There was also a main effect of pain [F(1,37) = 19.263, P < 0.001, η2 = 0.342], in which painful stimuli elicited greater LPP amplitudes. Results from an ANOVA with the inclusion of sex as a between-subjects variable revealed no significant main or interaction effects of sex in the late LPP response (P > 0.19) (see Fig. 1 for the grand average waveform).
Fig. 1.
Early and late (LPP) responses differ between painful and neutral stimuli and between conditions. Illustrated are the grand averaged ERP waveforms at the Cz/CPz/Pz/POz cluster for painful stimuli in affective sharing (blue) and empathic concern (pink), as well as neutral stimuli in sharing (green) and concern (orange) conditions.
Gamma coherence.
The 2 (condition) by 2 (pain) ANOVAs for local and distal gamma coherence failed to show main effects of pain or condition (P > 0.19).
Mu/alpha suppression.
No significant effects of global alpha power density or local mu suppression were found for painful vs. neutral stimuli, nor between the affective sharing and empathic concern conditions (P > 0.39).
Relation between EEG/ERPs and Dispositional Measures
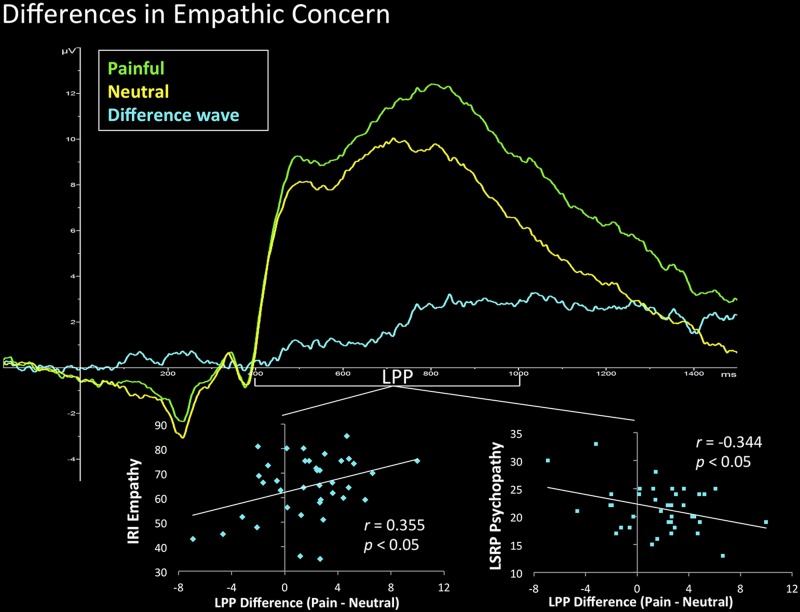
Dispositional empathy (total IRI score) positively predicted modulations in LPP response over central and parietal midline locations for painful vs. neutral stimuli in empathic concern (Cz/CPz/Pz/POz cluster, r = 0.355, P < 0.05; see Fig. 2), but not affective sharing (P > 0.23). Importantly, dispositional psychopathy was negatively related to differences in LPP in empathic concern and, again, not in affective sharing (P > 0.35). Psychopathy, as indexed by total LSRP score and primary psychopathy subscale, was negatively associated with LPP differences in the empathic concern condition in POz (Total score: r = −0.388, P < 0.05; LSRP primary psychopathy subscale: r = −0.340, P < 0.05). Additionally, scores on the LSRP secondary psychopathy subscale negatively predicted this LPP effect (Cz/CPz/Pz/POz cluster, r = −0.344, P < 0.05). Individual differences in early ERP responses to painful vs. neutral stimuli in either the affective sharing or empathic concern condition were not related to dispositional measures of empathy, psychopathy, or justice sensitivity (all P > 0.19). Finally, a comparison of beta-weights for the IRI and LSRP measures and painful vs. neutral LPP differences in empathic concern and affective sharing conditions revealed no significant effects (Table 2).
Fig. 2.
Individual differences in dispositional empathy and psychopathy predict LPP during empathic concern condition. Grand averaged ERP waveforms for painful (green) vs. neutral (yellow) stimuli in the empathic concern condition and the corresponding difference wave (blue) at the centro-parietal midline cluster are illustrated. The difference wave during the LPP window was positively predictive of trait empathy (left) and inversely related to trait psychopathy (right). IRI, Interpersonal Reactivity Index; LSRP, Levenson Self Report Psychopathy.
Table 2.
Pearson correlations between and within self-report measures and all painful vs. neutral difference waves for early and late ERP components
| Correlation Coefficients |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IRI |
LSRP |
Early ERP |
LPP |
||||||||
| Total | PT | EC | PD | Total | 1° | 2° | Share | EC | Share | EC | |
| LPP EC | 0.39* | 0.24 | 0.14 | 0.33* | −0.39* | −0.34* | −0.32 | −0.25 | 0.20 | 0.07 | |
| LPP Share | 0.11 | −0.14 | 0.09 | 0.25 | −0.12 | −0.11 | −0.09 | 0.52* | 0.41* | ||
| Early EC | −0.16 | 0.06 | −0.05 | −0.22 | 0.13 | 0.16 | 0.02 | −0.36* | |||
| Early Share | −0.04 | −0.2 | −0.04 | −0.01 | 0.11 | 0.11 | 0.06 | ||||
| LSRP 2° | −0.15 | −0.25 | −0.14 | 0.27 | 0.74* | 0.42* | |||||
| LSRP 1° | −0.59* | −0.35* | −0.53* | −0.23 | 0.92* | ||||||
| LSRP Total | −0.5* | −0.37* | −0.44* | −0.05 | |||||||
| IRI PD | 0.42* | −0.07 | −0.05 | ||||||||
| IRI EC | 0.78* | 0.62* | |||||||||
| IRI PT | 0.67* | ||||||||||
| IRI Total | |||||||||||
ERP, event-related potential; LPP, late positive potential; EC, Empathic Concern; Share, Affective Sharing; LSRP 1°, LSRP Primary Psychopathy; LSRP 2°, LSRP Secondary Psychopathy; IRI PD, IRI Personal Distress; IRI EC, IRI Empathic Concern; IRI PT, IRI Perspective Taking.
Significant correlations (P < 0.05) are in bold.
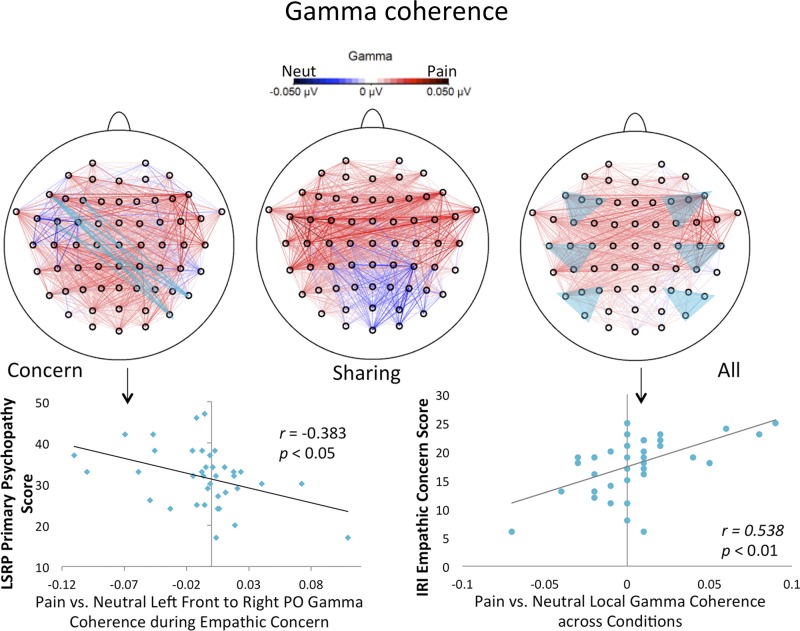
Local and distal gamma coherence for painful vs. neutral stimuli were also predicted by individual differences in dispositional empathy and psychopathy (Fig. 3). Scores on the IRI empathic concern subscale positively predicted local gamma coherence in affective sharing (r = 0.342, P < 0.05), as well as across both conditions (r = 0.538, P < 0.01). Dispositional psychopathy negatively predicted distal gamma coherence, but only in the empathic concern condition. LSRP primary psychopathy subscale scores negatively predicted left frontal to right parietal coherence (r = −0.383, P < 0.05) and left frontal to right temporal coherence (r = −0.370, P < 0.05). LSRP total score also predicted coherence between left frontal and right temporal regions (r = −0.333, P < 0.05).
Fig. 3.
Dispositional empathy and psychopathy are associated with differential gamma coherence. Gamma coherence (top) is presented for painful vs. neutral stimuli in the empathic concern (left) and affective sharing (middle) conditions separately and combined (right). The bottom left plot depicts the negative relation between trait psychopathy and gamma coherence from left frontal to right parieto-occipital sites (highlighted in light blue) in the empathic concern condition for painful vs. neutral stimuli. The bottom right plot shows the positive association between trait empathic concern and local gamma coherence across all six groups of nearby sites (highlighted in light blue) for painful vs. neutral stimuli.
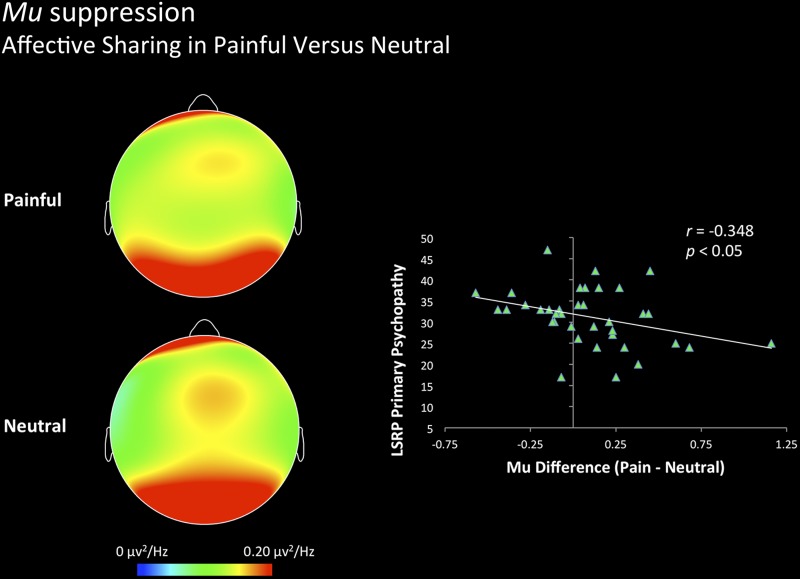
Dispositional psychopathy was positively related to the degree of mu suppression when perceiving painful stimuli vs. neutral stimuli in the affective sharing condition, with lower mu predicted by LSRP total score (r = −0.472, P < 0.01), primary psychopathy score (r = −0.441, P < 0.01), and secondary psychopathy score (r = −0.336, P < 0.05). Global alpha was not affected by any of the dispositional measures (Fig. 4).
Fig. 4.
Relation between trait psychopathy and mu suppression in the affective sharing condition. Topographic maps of alpha power density for painful (top) vs. neutral (bottom) stimuli in the affective sharing condition are depicted on the left. Individual differences in trait psychopathy predicted greater mu suppression as illustrated on the right.
DISCUSSION
The ability to be moved by the distress of others and feel concern for their well-being is critical for the development of moral behavior and smooth interpersonal relations. A wealth of studies in social psychology and developmental science has reliably documented that empathic concern plays a primary role in eliciting prosocial behavior (Batson 2012), particularly when other-oriented concern develops in concert with the understanding of others' internal states (Davidov et al. 2013; Patil and Silani 2014; Williams et al. 2014). While this is true for most people, there are individuals for whom this is either less or not the case. In particular, individuals with psychopathy are characterized by shallow affect, and a lack of concern for the feelings and welfare of others (Hare 1999; Kiehl 2014; Remmel and Glenn 2015). Examining the extent to which psychopathic traits, in a community sample, modulate the specific neural processing of the distress cues of others is informative in understanding the nature of the empathy-psychopathy relation. This study utilized a novel extension of a commonly used method for investigating neural responses to the pain of others, by contextualizing it with actual conspecifics, allowing for a more ecologically valid experimental paradigm that can scaffold the engagement of affective sharing and empathic concern. In addition, examining the spatiotemporal neural dynamics in response to others in distress permits the disentangling of the mechanisms that modulate pain perception, namely emotion and selective attention (Godinho et al. 2006).
In the current study, participants provided subjective ratings of affective sharing and empathic concern in response to the viewing of individuals suffering from physical bodily harm while electrophysiological data were collected. Behavioral results indicate that all participants' ratings of affective sharing were significantly higher than ratings of empathic concern, and this differentiation was qualified by sex. Male participants displayed greater sensitivity when focusing on the pain of others (affective sharing) than when asked about how concerned they were for the target in pain. Female participants engaged in both affective sharing and empathic concern equally. Consistent with a wealth of literature, dispositional empathy (as assessed by IRI total score) was higher in females and, across both sexes, positively predicted subjective reports of both sharing and concern (Baron-Cohen and Wheelwright 2004; Michalska et al. 2013). Moreover, as hypothesized and keeping with previous studies (Marcoux et al. 2013; Seara-Cardoso et al. 2012; but see Lishner et al. 2012), trait psychopathy showed the opposite pattern of relation.
In line with EEG/ERP investigations of pain empathy, all participants showed an automatic, early differentiation between painful and neutral stimuli, as indexed by a greater early positivity (175–275 ms after stimulus onset) at posterior sites or a greater negativity at anterior sites (e.g., Cheng et al. 2014; Fan and Han 2008; Han et al. 2008). As has been extensively documented, the polarity (negativity/positivity) of EEG signals is highly dependent upon a myriad of nonneurophysiological factors, including the electrode reference schematic, rendering an interpretation of polarity functionally insignificant (Otten and Rugg 2005). This response is generally interpreted as an automatic attention allocation to salient visual stimuli (Olofsson et al. 2008). In most previous EEG investigations, this early component was not modulated by task demands (e.g., Li and Han 2010). One notable exception comes from a study that compared physicians and controls when viewing medical procedures and found that medical expertise attenuated this very early component difference (Decety et al. 2010).
Interestingly, in the present study, this component was affected by condition instructions (focusing on either the pain of the other or being concerned for her/his well-being), demonstrating an automatic differentiation in neural processes based on task demands toward the same cues of distress from others. Task-related differences (difference wave) in the mean amplitudes of this early component were not correlated with any individual dispositional measures of empathy or justice sensitivity. Importantly, psychopathic traits were also not related to differences in this early component. This result is contrary to what has been previously reported in one study with juvenile incarcerated psychopaths (Cheng et al. 2012). In that study, high callous-unemotional offenders failed to show such an early differentiation when viewing painful vs. neutral stimuli, suggesting a fundamental distinction between forensic psychopathic populations and dimensional psychopathy in community samples.
The perception of painful vs. neutral stimuli robustly elicited a late ERP response as reflected in a differentiated LPP component, in keeping with previous work (Chen et al. 2012; Cheng et al. 2014; Decety et al. 2010; Fan and Han 2008; Li and Han 2010). In addition to this prototypical response, participants in the present study also showed a difference in LPP between the affective sharing and empathic concern conditions. Overall, the LPP was more positive-going when participants were engaged in the affective sharing condition. Dispositional empathy was positively associated with LPP difference waves for painful vs. neutral stimuli in the empathic concern condition but not in the affective sharing condition. Trait psychopathy was inversely related to this difference in LPP during the empathic concern condition. Modulations in the LPP are argued to index controlled reappraisal and more elaborative processing of emotional stimuli (Cheng et al. 2014; Hacjak et al. 2010; Ibanez et al. 2012; and Decety 2014; Zhang and Zhou 2014), necessities for eliciting a motivation to care when attending to people in distress. Results from the present experiment suggest that dimensional psychopathy is unrelated to differences in automatic (early ERP) and rudimentary reactions to the pain of others. Yet, trait psychopathy drives differences in the more controlled components (LPP) that underlie empathic concern. This novel finding neatly supports both clinical diagnostic criterion for psychopathy in forensic and community samples, as well as theories of psychopathy that emphasize the lack of concern and callous disregard for others' feelings as a hallmark of the disorder (Cleckley 1941; Hare 1999; Kiehl 2014).
Another novel finding of the study is the association between dispositional measures of empathy and trait psychopathy and gamma coherence. Synchronization of neural oscillations in the gamma range has been interpreted as an integration of bottom-up and top-down influences on perception and cognition (Fell et al. 2003; Herrmann et al. 2004). It is therefore illuminating that increased gamma coherence was associated with dispositional empathic concern when participants viewed people in distress. Conversely, trait psychopathy was negatively related to gamma coherence only during the empathic concern condition. This spectral finding and the ERP data converge to highlight the lack of binding between bottom-up (perceiving others in pain) and top-down (being concerned) processes in psychopathy.
Finally, no significant differences were detected for either global alpha or mu suppression (8–12 Hz over the central electrodes) when participants perceived others in pain, regardless of the two experimental conditions. The attenuation of the alpha component, known as the mu rhythm, is considered to reflect event-related desynchronization of the EEG-induced signal by an enhancement of neural activity in somatosensory and premotor cortex leading to asynchronous neural firing. It should be noted, however, that in the same frequency range, the modulation of mu rhythm differs from that of the alpha in several ways. In contrast to alpha, mu rhythm is not considered to be modulated primarily by visual stimulation or attention, but its power is attenuated during motor activity and during observation of human actions (Perry and Bentin 2010). However, there are recent studies showing that modification of mu rhythm over the somatosensory cortex and inferior frontal gyrus can, in fact, be driven by attention to tactile stimuli (Sacchet et al. 2015).
Global alpha density is considered a marker of attentional allocation (Sauseng et al. 2005). Our findings indicate that attention was equally distributed across stimuli and tasks, for all participants, regardless of their personality traits. Some previous EEG studies using similar stimuli have reported a suppression of the mu rhythm over the somatosensory cortex, often interpreted as reflecting sensorimotor resonance (Cheng et al. 2008; Perry et al. 2010; Yang-Teng et al. 2010). However, recent findings have revealed that differential mu suppression is associated with the affective valence of an outcome rather than an immediate negative reaction (Chen et al. 2012). The role of sensorimotor resonance (as reflected by mu suppression) in the facilitation of empathy remains highly controversial (Decety 2011a,c).
Interestingly, trait psychopathy in the current study was associated with greater mu suppression during the affective sharing condition for painful vs. neutral stimuli. This supports and extends neurophysiological findings wherein psychopathy was not coupled with a deficit in somatosensory resonance, as measured by mu suppression (Cheng et al. 2012), corticospinal inhibition (Fecteau et al. 2008), and somatosensory gating (Marcoux et al. 2013), but in fact just the opposite, i.e., psychopathic traits led to increased somatosensory resonance. Indeed, in our study, individuals with high psychopathy scores exhibited increased mu suppression when focusing on the pain of others. Taken together, these findings cast doubt on the idea that sensorimotor or somatosensory resonance is the mechanism underlying emotional sharing (emotional empathy), since psychopaths are emotionally shallow and less sensitive to signs of distress, and yet exhibit greater mu suppression when perceiving others' pain (Fig. 4). Motor resonance should thus not be equated with emotional contagion (Decety 2011c; Decety and Cowell In press; Hickok 2014; Lamm and Majdandzic 2015) as is often the case.
Conclusion
In forensic neuroscience, psychopathy is often considered as a discrete category rather than a dimensional variable present to some extent in the general population. It is thus particularly interesting that we could disentangle specific spatiotemporal markers associated with much milder forms of the condition in a noninstitutionalized sample in response to the perception of distress cues. Trait psychopathy fundamentally altered the later controlled response to the distress of others, particularly in relation to empathic concern, but unexpectedly, not the early orienting/attentional responses. As these early responses (<200 ms) to the perception of the pain of others are argued to index selective attention and are unmodified by emotions (Godinho et al. 2006), then it may come as no surprise that trait psychopathy attenuated later electrophysiological responses but not early ones. Taken together, these findings demonstrate that even when processing the same visual stimuli depicting the distress of others, distinct facets of empathy, elicited by specific instructions, operate on different time courses, and probably neural computations. Moreover, these electrophysiological components are modulated by individual differences in dispositional empathy and trait psychopathy. Electrophysiology, including ERPs and gamma coherence, thus serves as a valuable method for examining the complex relations between various facets of empathy and trait psychopathy in normal populations.
GRANTS
The study was supported by grants from the John Templeton Foundation (Wisdom Research at the Univ. of Chicago) and from the National Institutes of Health (R01-MH-087525; R01-MH-084934) to J. Decety.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D. and J.M.C. conception and design of research; J.D., K.L.L., and J.M.C. performed experiments; J.D., K.L.L., and J.M.C. analyzed data; J.D., K.L.L., and J.M.C. interpreted results of experiments; J.D., K.L.L., and J.M.C. prepared figures; J.D., K.L.L., and J.M.C. drafted manuscript; J.D., K.L.L., and J.M.C. edited and revised manuscript; J.D., K.L.L., and J.M.C. approved final version of manuscript.
REFERENCES
- Aharoni E, Sinnott-Armstrong W, Kiehl KA. Can psychopathic offenders discern moral wrongs? A new look at the moral/conventional distinction. J Abnorm Psychol 121: 484–497, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniskiewicz AS. Autonomic components of vicarious conditioning and psychopathy. J Clin Psychol 35: 60–67, 1979. [DOI] [PubMed] [Google Scholar]
- Balconi M, Pozzoli U. Arousal effect on emotional face comprehension: frequency band changes in different time intervals. Physiol Behav 97: 455–462, 2009. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord 34: 163–175, 2004. [DOI] [PubMed] [Google Scholar]
- Batson CD. The empathy-altruism hypothesis: Issues and implications. In: Empathy—From Bench to Bedside, edited by Decety J. Cambridge, MA: MIT Press, 2012, p. 41–54. [Google Scholar]
- Batson CD. These things called empathy: eight related but distinct phenomena. In: The Social Neuroscience of Empathy, edited by Decety J, Ickes W. Cambridge, MA: MIT Press, 2009, p. 3–15. [Google Scholar]
- Behrens TE, Hunt LT, Rushworth MF. The computation of social behavior. Science 324: 1160–1164, 2009. [DOI] [PubMed] [Google Scholar]
- Bell DC, Richard AJ. Caregiving: the forgotten element in attachment. Psychol Inq 11: 69–83, 2000. [Google Scholar]
- Betti V, Zappasodi F, Rossini PM, Aglioti SM, Tecchio F. Synchronous with your feelings: sensorimotor gamma band and empathy for pain. J Neurosci 29: 12384–12392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Responsiveness to distress cues in the child with psychopathic tendencies. Pers Individ Dif 27: 135–145, 1999. [Google Scholar]
- Book AS, Quinsey VL, Langford D. Psychopathy and the perception of affect and vulnerability. Crim Justice Behav 34: 531–544, 2007. [Google Scholar]
- Brook M, Kosson DS. Impaired cognitive empathy in criminal psychopathy: evidence from a laboratory measure of empathic accuracy. J Abnorm Psychol 122: 156–66, 2013. [DOI] [PubMed] [Google Scholar]
- Carretie L, Martin-Loeches M, Hinojosa JA, Mercado F. Emotion and attention interaction studied through event-related potentials. J Cogn Neurosci 13: 1109–1128, 2001. [DOI] [PubMed] [Google Scholar]
- Carretie L, Hinojosa JA, Martin-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: neural correlates. Hum Brain Mapp 22: 290–299, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Yang CY, Cheng Y. Sensorimotor resonance is an outcome but not a platform to anticipating harm to others. Soc Neurosci 7: 578–590, 2012. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chen C, Decety J. An EEG/ERP investigation of the development of empathy during early childhood. Dev Cogn Neurosci 10: 160–169, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Hung AY, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Dev Psychopathol 24: 623–636, 2012. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Yang CY, Lin CP, Lee PR, Decety J. The perception of pain in others suppresses somatosensory oscillations: a magnetoencephalography study. Neuroimage 40: 1833–1840, 2008. [DOI] [PubMed] [Google Scholar]
- Cleckley H. The Mask of Sanity: An attempt to Reinterpret the So-Called Psychopathic Personality. St. Louis, MO: Mosby, 1941. [Google Scholar]
- Cohen MX, David N, Vogeley K, Elger CE. Gamma-band activity in the human superior temporal sulcus during mentalizing from nonverbal social cues. Psychophysiology 46: 43–51, 2009. [DOI] [PubMed] [Google Scholar]
- Coplan A. Understanding empathy: its features and effects. In: Empathy—Philosophical Perspectives, edited by Coplan A, Goldie P. New York: Oxford Univ. Press, 2011, p. 3–44. [Google Scholar]
- Cowell JM, Decety J. The neuroscience of implicit moral evaluation and its relation to generosity in early childhood. Curr Biol 25: 1–5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff BMP, Brown SJ, Taylor L, Howat DJ. Empathy: a review of the concept. Emot Rev (December 1, 2014). EPub ahead of print. [Google Scholar]
- Dadds MR, Hawes DJ, Frost ADJ, Vassallo S, Bunn P, Hunter K, Merz S. Learning to “talk the talk”: the relationship between psychopathic traits to deficits in empathy across childhood. J Child Psychol Psychiatry 50: 599–606, 2009. [DOI] [PubMed] [Google Scholar]
- Davidov M, Zahn-Waxler C, Roth-Hanania R, Knafo A. Concern for others in the first year of life: theory, evidence, and avenues for research. Child Dev Perspect 7: 126–131, 2013. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol 44: 113–126, 1983. [Google Scholar]
- Decety J. The neural pathways, development and functions of empathy. Curr Opin Behav Sci 3: 1–6, 2015. [Google Scholar]
- Decety J. Dissecting the neural mechanisms mediating empathy. Emot Rev 3: 92–108, 2011a. [Google Scholar]
- Decety J. The neuroevolution of empathy. Ann NY Acad Sci 1231: 35–45, 2011b. [DOI] [PubMed] [Google Scholar]
- Decety J. Promises and challenges of the neurobiological approach to empathy. Emot Rev 3: 115–116, 2011c. [Google Scholar]
- Decety J, Cowell JM. The complex relation between morality and empathy. Trends Cogn Sci 18: 337–339, 2014a. [DOI] [PubMed] [Google Scholar]
- Decety J, Cowell JM. Friends or foes: is empathy necessary for moral behavior? Perspect Psychol Sci 9: 525–537, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Cowell JM. Empathy, justice and moral behavior. Am J Bioethics Neurosci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Cacioppo S. The speed of morality: a high-density electrical neuroimaging study. J Neurophysiol 108: 3068–3072, 2012. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev 3: 71–100, 2004. [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville J. Shared representations between self and others: a social cognitive neuroscience view. Trends Cogn Sci 7: 527–533, 2003. [DOI] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski CL, Kiehl KA. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Front Hum Neurosci 7: 489, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski CL, Kiehl KA. Socioemotional processing of morally-laden behavior and their consequences on others in forensic psychopaths. Hum Brain Mapp 36: 2015–2026, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey B. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol 80: 203–211, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Porges EC. Imagining being the agent of actions that carry different moral consequences: an fMRI study. Neuropsychologia 49: 2994–3001, 2011. [DOI] [PubMed] [Google Scholar]
- Decety J, Skelly LR, Kiehl KA. Brain response to empathy-eliciting scenarios in incarcerated individuals with psychopathy. JAMA Psychiatry 70: 638–645, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Yang CY, Cheng Y. Physicians down-regulate their pain empathy response: An event-related brain potential study. Neuroimage 50: 1676–1682, 2010. [DOI] [PubMed] [Google Scholar]
- Decety J, Skelly LR, Yoder KJ, Kiehl KA. Neural processing of dynamic facial expressions in psychopaths. Soc Neurosci 9: 36–49, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Wheatley T. The Moral Brain—A multidisciplinary perspective. Cambridge, MA: MIT Press, 2015. [Google Scholar]
- Decety J, Yoder KJ. Empathy and motivation for justice: Cognitive empathy and concern, but not emotional empathy, predict sensitivity to injustice for others. Soc Neurosci. First published April 2, 2015; doi: 10.1080/17470919.2015.1029593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Hollerbach P, Vohs K, Mokros A, Habermeyer E. Emotional empathy and psychopathy in offenders: an experimental study. J Pers Disord 27: 67–84, 2013. [DOI] [PubMed] [Google Scholar]
- Escobar MJ, Huepe D, Decety J, Sedeno L, Messow MK, Baez S, Rivera-Rei A, Canales-Johnson A, Morales JP, Gomez D, Schröeder J, Manes F, López V, Ibañez A. Brain signatures of moral sensitivity in adolescents with early social deprivation. Sci Rep 4: 5354, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Han S. Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia 46: 160–173, 2008. [DOI] [PubMed] [Google Scholar]
- FeldmanHall O, Dalgleish T, Evans D, Mobbs D. Empathic concern drives costly altruism. Neuroimage 105: 347–356, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Fernandez G, Klaver P, Elger CE, Fries P. Is synchronized neuronal gamma relevant for selective attention? Brain Res Rev 42: 265–272, 2003. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Theoret H. Psychopathy and the mirror neuron system: preliminary findings from a non-psychiatric sample. Psychiatry Res 160: 137–144, 2008. [DOI] [PubMed] [Google Scholar]
- Godinho F, Magnin M, Frot M, Perchet C, Garcia-Larrea L. Emotional modulation of pain: is it the sensation or what we recall? J Neurosci 26: 11454–11461, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsell JN, Inzlicht M. Empathy constrained: prejudice predicts reduced mental simulation of actions during observation of outgroups. J Exp Soc Psychol 46: 841–845, 2010. [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: time-course of the late positive potential. Clin Neurophysiol 120: 505–510, 2009. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: The Oxford Handbook of Event-Related Potential Components, edited by Kappenman ES, Luck SJ. New York: Oxford Univ. Press, 2011, p. 1–38. [Google Scholar]
- Hall JR, Benning SD, Patrick CJ. Criterion-related validity of the three-factor model of psychopathy: personality, behavior, and adaptive functioning. Assessment 11: 4–16, 2004. [DOI] [PubMed] [Google Scholar]
- Han S, Fan Y, Mao L. Gender difference in empathy for pain: an electrophysiological investigation. Brain Res 1196: 85–93, 2008. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare PCL-R: Some issues concerning its use and misuse. Legal Criminol Psych 3: 99–119, 1998. [Google Scholar]
- Hare RD. Without Conscience. New York: Guilford Press, 1999. [Google Scholar]
- Hare RD, Strachan C, Forth AE. Psychopathy and crime: an overview. In: Clinical Approaches to the Mentally Disordered Offender, edited by Hollin CR, Howells K. Chichester, UK: Wiley, 1993, p. 165–178. [Google Scholar]
- Hare RD, Neumann CS. Psychopathy as a clinical and empirical construct. Annu Rev Clin Psychol 4: 217–246, 2008. [DOI] [PubMed] [Google Scholar]
- Hermann CS, Munk MHJ, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci 8: 347–355, 2004. [DOI] [PubMed] [Google Scholar]
- Hickok G. The myth of mirror neurons: the real neuroscience of communication and cognition. New York: Norton, 2014. [Google Scholar]
- Ibáñez A, Melloni M, Huepe D, Helgiu E, Rivera-Rei A, Canales-Jojnson A, Baker P, Moya A. What event-related potentials (ERPs) bring to social neuroscience? Soc Neurosci 7: 632–649, 2012. [DOI] [PubMed] [Google Scholar]
- Ibáñez A, Hurtado E, Lobos A, Escobar J, Trujillo N, Baez S, Huepe D, Manes F, Decety J. Subliminal presentation of other faces (but not own face) primes behavioral and evoked cortical processing of empathy for pain. Brain Res 1398: 72–85, 2011. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy and the neural mechanisms involved in imagining how I feel versus how you would feel pain: an event-related fMRI study. Neuropsychologia 44: 752–761, 2006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others: a window into the neural processes involved in empathy. Neuroimage 24: 771–779, 2005. [DOI] [PubMed] [Google Scholar]
- Jung TP, Humphries C, Lee TW, Makeig S, McKeown MJ, Iragui V, Sejnowski TJ. Extended ICA removes artifacts from electroencephalographic recordings. Adv Neural Inf Process Syst 10: 890–900, 1998. [Google Scholar]
- Kanske P, Plitschka J, Kotz SA. Attentional orienting towards emotion: P2 and N400 ERP effects. Neuropsychologia 49: 3121–3129, 2011. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. The Psychopath Whisperer: Inside the Minds of Those without a Conscience. New York, NY: Crown, 2014. [Google Scholar]
- Kotov R, Ruggero CJ, Krueger RF, Watson D, Yuan Q, Zimmerman M. New dimensions in the quantitative classification of mental illness. Arch Gen Psychiatry 68: 1003–1011, 2011. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54: 2492–2502, 2011. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci 19: 42–58, 2007. [DOI] [PubMed] [Google Scholar]
- Lamm C, Majdandzic J. The role of shared neural activations, mirror neurons, and morality in empathy—a critical comment. Neurosci Res 90: 14–24, 2015. [DOI] [PubMed] [Google Scholar]
- Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? J Cogn Neurosci 22: 362–376, 2010. [DOI] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. J Pers Soc Psychol 68: 151–158, 1995. [DOI] [PubMed] [Google Scholar]
- Li W, Han S. Perspective taking modulates event-related potentials to perceived pain. Neurosci Lett 469: 328–332, 2010. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. J Pers Assess 66: 488–524, 1996. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Latzman RD, Watts AL, Smith SF, Dutton K. Correlates of psychopathic personality traits in everyday life: Results from a large community survey. Frontiers Psychol 5: 540, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishner D, Vitacco M, Hong P, Mosley J, Miska Stocks E K. Evaluating the relation between affective psychopathy and empathy: Two preliminary studies. Int J Offender Ther Comp Criminol 56: 11–61–1181, 2012. [DOI] [PubMed] [Google Scholar]
- Lockwood PL, Sebastian CL, McCrory EJ, Hyde ZH, Gu X, De Brito SA, Viding E. Association of callous traits with reduced neural response to others' pain in children with conduct disorder. Curr Biol 23: 901–905, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maibom HL. Moral unreason: the case of psychopathy. Mind Lang 20: 237–257, 2005. [Google Scholar]
- Maibom HL. Feeling for others: empathy, sympathy and morality. Inquiry 52: 483–499, 2009. [Google Scholar]
- Makeig S, Bell AJ, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. Adv Neural Inf Process Syst 8: 145–151, 1996. [Google Scholar]
- Malterer MB, Lilienfeld SO, Neumann CS, Newman JP. Concurrent validity of the psychopathic personality inventory with offender and community samples. Assessment 17: 3–15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux LA, Michon PE, Voisin JIA, Lemelin S, Vachon-Presseau E, Jackson PL. The modulation of somatosenory resonance by psychopathic traits and empathy. Front Hum Neurosci 7: e274, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz IT, Schechter JC, Pine DS, Decety J, Blair RJR. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J Child Psychol Psychiatry 54: 900–901, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella E, Ramsey R, Conson Hamilton A. Brain systems for visual perspective taking and action perception. Soc Neurosci 8: 248–267, 2013. [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Kinzler KD, Decety J. Age-related sex differences in explicit measures of empathy do not predict brain responses. Dev Cogn Neurosci 3: 22–32, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, De Oliveira-Souza R. Moral judgments, emotions and the utilitarian brain. Trends Cogn Sci 11: 319–321, 2007. [DOI] [PubMed] [Google Scholar]
- Moll J, Schulkin J. Social attachment and aversion in human moral cognition. Neurosci Biobehav Rev 33: 456–465, 2009. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Bosworth R, Nott Z, Louis WR, Smith JR, Vohs KD, Amiot C, Decety J. The influence of group membership and individual differences in psychopathy and perspective taking on neural responses when punishing and rewarding others. Hum Brain Mapp 35: 4989–4999, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother's responses to infant's attachment behaviors. Biol Psychiatry 63: 415–423, 2008. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biol Psychol 77: 247–265, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Interpreting event-related brain potentials. In: Event-Related Potentials: A Methods Handbook, edited by Handy TC. Cambridge, MA: MIT Press, 2005, p.4–16. [Google Scholar]
- Ozerdem A, Guntekin B, Atagun I, Turp B, Basar E. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J Affect Disord 132: 325–332, 2011. [DOI] [PubMed] [Google Scholar]
- Parsons CE, Stark EA, Young KS, Stein A, Kringelbach ML. Understanding the human parental brain: a critical role of the orbitofrontal cortex. Soc Neurosci 8: 525–543, 2013. [DOI] [PubMed] [Google Scholar]
- Patil I, Silani G. Reduced empathic concern leads to utilitarian moral judgment in trait alexithymia. Front Psychol 5: 501, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Bentin S. Does focusing on hand grasping intentions modulate EEG mu and alpha suppression? Neuroreport 21: 1050–1054, 2010. [DOI] [PubMed] [Google Scholar]
- Perry A, Bentin S, Bartal IBA, Lamm C, Decety J. Feeling the pain of those who are different from us: Modulation of EEG in the mu/alpha range. Cogn Affect Behav Neurosci 10: 493–504, 2010. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Singer W. In search of common foundations for cortical computation. Behav Brain Sci 20: 657–722, 1997. [DOI] [PubMed] [Google Scholar]
- Remmel RJ, Glenn AL. Immorality in the adult brain. In: The Moral Brain—A Multidisciplinary Perspective, edited by Decety J, Wheatley T. Cambridge, MA: MIT Press, 2015, p. 239–251. [Google Scholar]
- Roskies AL. A puzzle about empathy. Emot Rev 3: 278–280, 2011. [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective taking with social emotions. J Cogn Neurosci 16: 988–999, 2004. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe? A neuroimaging study of conceptual perspective taking. Eur J Neurosci 17: 2475–2480, 2003. [DOI] [PubMed] [Google Scholar]
- Sacchet MD, LaPlante RA, Wan Q, Pritchett DL, Hämäläinen M, Moore CL, Kerr CE, Jones SR. Attention drives synchronization of alpha and beta rhythms between right inferior frontal and primary sensory neocortex. J Neurosci 35: 2074-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci 22: 2917–2926, 2005. [DOI] [PubMed] [Google Scholar]
- Schmitt MJ, Baumert A, Gollwitzer M, Maes J. The Justice Sensitivity Inventory: factorial validity, location in the personality facet space, demographic pattern, and normative data. Soc Justice Res 23: 211–238, 2010. [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37: 257–261, 2000. [PubMed] [Google Scholar]
- Seara-Cardoso A, Neumann C, Roiser J, McCrory E, Viding E. Investigating associations between empathy, morality and psychopathic personality traits in the general population. Pers Diff 52: 67–71, 2012. [Google Scholar]
- Shamay-Tsoory SG, Harari H, Aharon-Peretz J, Levkovitz Y. The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex 46: 668–677, 2010. [DOI] [PubMed] [Google Scholar]
- Skeem JL, Cooke DJ. Is criminal behavior a central component of psychopathy? Conceptual directions for resolving the debate. Psychol Assess 22: 433–445, 2010. [DOI] [PubMed] [Google Scholar]
- Skeem JL, Poythress N, Edens JF, Lilienfeld SO, Cale EM. Psychopathic personality or personalities? Exploring potential variants of psychopathy and their implications for risk assessment. Aggress Violent Behav 8: 513–546, 2003. [Google Scholar]
- Skeem JL, Polaschek DL, Patrick CJ, Lilienfeld SO. Psychopathic personality bridging the gap between scientific evidence and public policy. Psychol Sci Public Interest 12: 95–162, 2011. [DOI] [PubMed] [Google Scholar]
- Sobhani M, Bechara A. A somatic marker perspective of immoral and corrupt behavior. Soc Neurosci 6: 640–652, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Konrath S, Brown SL, Finegood ED, Akce LB, Dayton Ho SS CJ. Parenting and beyond: Common neurocircuits underlying parental and altruistic caregiving. Parent Sci Practice 12: 1–9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber-Thomas BC, Asp EW, Koenigs M, Sutterer M, Anderson SW, Tranel D. Arrested development: early prefrontal lesions impair the maturation of moral judgment. Brain 137: 1254–1261, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res 168: 242–249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heiden L, Scherpiet S, Konicar L, Birbaumer N, Veit R. Inter-individual differences in successful perspective taking during pain perception mediates emotional responsiveness in self and others: An fMRI study. Neuroimage 65, 367–394, 2013. [DOI] [PubMed] [Google Scholar]
- Von Borries AKL, Volman I, de Bruijn ERA, Bulten H, Verkes RJ, Rielofs K. Psychopaths lack the automatic avoidance of social threat: relation to instrumental aggression. Psychiatry Res 200: 761–766, 2012. [DOI] [PubMed] [Google Scholar]
- Williams A, O'Driscoll K, Moore C. The influence of empathic concern on prosocial behavior in children. Front Psychol 5: 425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Teng F, Decety J, Chia-Yen Y, Lui JL, Cheng Y. Unbroken mirror neurons in autism spectrum disorders: an EEG study. J Child Psychol Psychiatry 51: 981–988, 2010. [DOI] [PubMed] [Google Scholar]
- Yoder KJ, Decety J. Spatiotemporal neural dynamics of moral judgments: a high-density EEG/ERP study. Neuropsychologia 60: 39–45, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Decety J. The good, the bad, and the just: Justice sensitivity predicts neural response during moral evaluation of actions performed by others. J Neurosci 34: 4161–4166, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Dungan J. Where in the brain is morality? Everywhere and maybe nowhere. Soc Neurosci 7: 1–10, 2012. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Radke-Yarrow M. The origins of empathic concern. Motiv Emot 24: 107–130, 1990. [Google Scholar]
- Zhang J, Zhou R. Individual differences in automatic emotion regulation affect the asymmetry of the LPP component. PLoS One 9: e88261, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]