Abstract
Postactivation depression of the Hoffmann (H) reflex is associated with a transient period of suppression following activation of the reflex pathway. In soleus, the depression lasts for 100–200 ms during voluntary contraction and up to 10 s at rest. A reflex root evoked potential (REP), elicited after a single pulse of transcutaneous stimulation to the thoracolumbar spine, has been shown to exhibit similar suppression. The present study systematically characterized the effect of transcranial magnetic stimulation (TMS) on postactivation depression using double-pulse H reflexes and REPs. A TMS pulse reduced the period of depression to 10–15 ms for both reflexes. TMS could even produce postactivation facilitation of the H reflex, as the second reflex response was increased to 243 ± 51% of control values at the 75-ms interval. The time course was qualitatively similar for the REP, yet the overall increase was less. While recovery of the H reflex was slower in the relaxed muscle, the profile exhibited a distinct bimodal shape characterized by an early peak at the 25-ms interval, reaching 72 ± 23% of control values, followed by a trough at 50 ms, and then a gradual recovery at intervals > 50 ms. The rapid recovery of two successively depressed H reflexes, ∼25 ms apart, was also possible with double-pulse TMS. The effect of the TMS-induced corticospinal excitation on postactivation depression may be explained by a combination of pre- and postsynaptic mechanisms, although further investigation is required to distinguish between them.
Keywords: postactivation depression, H reflex, root evoked potential, soleus, transcranial magnetic stimulation
in the period following the activation of a sensory-motor synapse, a monosynaptic reflex induced along the same pathway may be inhibited for 100–200 ms during contraction (Andrews et al. 2015; Stein et al. 2007) and up to 10 s at rest (Crone and Nielsen 1989; Pierrot-Deseilligny and Burke 2005). This phenomenon is known as either postactivation depression or homosynaptic depression and has been characterized in humans (Crone and Nielsen 1989; Magladery et al. 1952; Paillard 1955; Rothwell et al. 1986) and animals (Eccles et al. 1961; Frank and Fuortes 1957) after peripheral nerve stimulation. Postactivation depression of the Hoffmann (H) reflex is thought to be presynaptic to the α-motoneuron and has been associated with an increase in presynaptic inhibition (Crone and Nielsen 1989; Schieppati 1987) and changes in the presynaptic terminal leading to a temporary reduction of neurotransmitter release (Armitage and Siegelbaum 1998; Castellucci and Kandel 1974; Elliot et al. 1994; Lev-Tov and Pinco 1992; reviewed in Hultborn et al. 1996). A similar suppression has been observed with transcutaneous stimulation over the thoracolumbar spine whereby the excitability of a reflex root evoked potential (REP) will change for up to 10 s after an earlier stimulus (Andrews et al. 2015; Courtine et al. 2007; Minassian et al. 2007, 2009). After a first response, a soleus refex REP evoked 150 ms later will recover to 68% of the control response during a contraction and 20% at rest. This corresponds to ∼66% of the recovery observed using two H reflexes (Andrews et al. 2015). The difference may relate to the fact that the transcutaneous spinal stimulus activates multiple, bilateral lumbosacral roots (Courtine et al. 2007; Maertens de Noordhout et al. 1988; Minassian et al. 2007) in addition to the fibers located within the tibial nerve (TN).
Corticospinal input can interact with spinal interneurons, as transcranial magnetic stimulation (TMS) decreases postactivation depression of REPs during double-pulse (50 ms) stimulation (Roy et al. 2014) and, similarly, reduces presynaptic inhibition of the H reflex (Iles 1996; Meunier 1999; Valls-Solé et al. 1994). The effect of TMS on postactivation depression is transient, lasting ∼25 ms, and is maximal when descending inputs arrive at the motoneuron pool 8–13 ms before the afferent inputs produced by the transcutaneous spinal stimulus (Roy et al. 2014). Such a delay shares similarities to the period of facilitation measured with a single soleus REP (Knikou 2014; Roy et al. 2014) or H reflex (Nielsen and Petersen 1995; Serranova et al. 2008; Valls-Solé et al. 1994) conditioned by TMS. Postactivation depression of the soleus H reflex is reduced in Parkinson's disease, although the strength of the pathway can be enhanced by using subthalamic nucleus stimulation, potentially, through a reticulospinal pathway (Raoul et al. 2012). Thus descending projections from both pyramidal and extrapyramidal systems likely modulate postactivation depression of monosynaptic reflexes.
We previously reported that TMS can reduce postactivation depression of an REP in the hamstrings using transcutaneous spinal stimulation, and a similar effect was observed in the soleus muscle of most subjects (Roy et al. 2014). The present study builds on previous work by 1) directly comparing the recovery time course of the H reflex with the REP from postactivation depression in the presence of TMS and 2) characterizing the role of TMS intensity on the recovery. We employed double-pulse stimulation to elicit two soleus H reflexes (or REPs) with the TMS pulse time-locked to the second reflex response. To provide maximal recovery, the study was initially conducted during an isometric plantarflexion, given that voluntary drive increases motoneuron excitability (Pierrot-Deseilligny and Burke 2005) and reduces postactivation depression (Andrews et al. 2015; Burke et al. 1989; Hultborn and Nielsen 1998; Rothwell et al. 1986). The interaction on the H reflex was further characterized in a relaxed motor state, as the time course of recovery differs substantially between resting and active states.
METHODS
Participants.
Ten able-bodied volunteers (5 women, 5 men; age 23–59 yr) were recruited to participate in the study. Volunteers were screened for potential contraindications to the stimulation including a history of seizures, implanted devices, chronic back pain, or prior spinal surgery. Participants provided written consent to the experimental protocol approved by the Health Research Ethics Board at the University of Alberta.
Recording and stimulation.
Participants were seated with their left leg in a metal brace maintaining a 100° angle of both knee and ankle joints. EMG responses were recorded from the soleus and tibialis anterior muscles with a pair of silver-silver chloride surface electrodes (3.5 × 2.2 cm; Vermont Medical, Bellows Falls, VT) placed 3 cm below the distal border of the gastrocnemius and along the belly of the tibialis anterior, two-thirds of the distance from the lateral malleolus to the knee. The EMG was band-pass filtered between 10 and 1,000 Hz, amplified 1,000× (Octopus; Bortec Technologies, Calgary, AB, Canada), and digitized at 5 kHz with Axoscope Hardware (Digidata 1200 series; Axon Instruments, Union City, CA). The EMG was also full-wave rectified and low-pass filtered at 3 Hz so that the participants could monitor their level of EMG on an oscilloscope. At the start of the experiment, each participant's maximum voluntary contraction (MVC) was determined during an isometric plantarflexion.
The TN was stimulated with 1-ms pulses (SD9 Stimulator; Grass Instruments, West Warwick, RI) with the cathode on the popliteal fossa and the anode 4 cm more proximal (measured from the center of each electrode). The optimal position was determined with a handheld monopolar probe (1-cm tip) as the site that produced the largest soleus H reflex with minimal encroachment from the antagonistic tibialis anterior muscle. Threshold was determined as the minimum intensity capable of producing a visible EMG response.
Transcutaneous spinal (S) stimulation was performed with a constant-current stimulator (Digitimer DS7A; Digitimer, Welwyn Garden City, UK) with the pulse width set to 1 ms. Vertebral levels were identified and marked after palpation by a physiotherapist. The anode (7.5 × 13 cm; Axelgaard Manufacturing, Fallbrook, CA) was placed above the ipsilateral anterior superior iliac spine. A silver-silver chloride cathode (5 × 5 cm; WalkAide premium electrode; Innovative Neurotronics, Austin, TX) was initially placed on the back, midline over the 3rd and 4th lumbar intervertebral space. The electrode was then moved progressively more rostral in 2.5-cm increments until the position that produced the largest soleus REP was identified.
TMS was delivered over the motor cortex to elicit motor evoked potentials (MEPs) in soleus. Monophasic pulses were delivered with MagStim 2002 stimulators (Magstim, Whitland, UK). Paired pulses were delivered with a BiStim2 module (Magstim). The double-cone coil (P/N 9902-00: external wing diameter 110 mm) was orientated to produce posterior-to-anterior currents in the brain. The coil was initially positioned over the right motor cortex (1 cm lateral and 1 cm posterior of the vertex) and then moved in 1-cm increments until the optimal position for producing an MEP in the left soleus muscle was determined (range: 0–2 cm posterior and 1–2 cm lateral).
Recruitment curves were collected for the H reflex by gradually increasing the stimulus intensity from threshold and onward until reaching Hmax amplitude. For the M wave, the stimulus intensity was further increased beyond the level required to elicit Hmax to the intensity that produced Mmax. Separate recruitment curves were also collected for the MEP and REP. Double pulses of spinal stimulation were administered 50 ms apart to distinguish the reflex REP from the direct coactivation of motor axons in the ventral roots. The presence of a first response and the reduction of a second because of postactivation depression was indicative of a reflex REP (Courtine et al. 2007; Minassian et al. 2007; Roy et al. 2012). The stimulation intensity was increased from threshold up to REPmax or the intensity at which the first REP increased at the same rate as the second REP, which was consistent with the direct activation of motor axons.
Optimizing the TMS-H reflex and TMS-REP delay.
The optimal delay of the TMS pulse relative to a second TN stimulus (TMS-TN) was optimized prior to testing the effect of TMS on postactivation depression. TMS was set to 10–15% of the maximal stimulator output (MSO) above threshold. The stimulus intensity for the H reflex was adjusted to produce half of its maximal soleus EMG response, as both excitatory and inhibitory influences can be observed at this level of response activation (Zehr and Stein 1999). Postactivation depression was induced with two TN stimuli 100 ms apart, as this interval typically permitted a small amount of recovery of the second H reflex. In some subjects, the interpulse interval (IPI) was increased (150–275 ms) in order to produce a visible second reflex response. TMS was then delivered 5, 7, 10, 13, 15, 17, 20, and 25 ms before the second TN stimulus. The optimal delay was defined as the interval that produced maximum facilitation of the second reflex and was fixed for the duration of testing. As the latency of the REP was ∼12 ms shorter than the H reflex, the TMS-S delay was 12 ms longer than the TMS-TN delay to ensure that the timing of the inputs reaching the motoneuron pool was equivalent.
TMS on spinal reflexes during contraction.
The effect of TMS on the recovery from postactivation depression produced by double-pulse H reflexes was studied with a half-maximum reflex response amplitude during an isometric plantarflexion at 15–20% of MVC. Eight subjects were tested. To study the interaction across time, the IPI between the pairs of TN stimuli was systematically increased from 5 to 100 ms (i.e., 5, 10, 15, 25, 50, 75, and 100 ms). TMS was timed in relation to the second TN (or S) stimulus using the optimal delay described above, which remained fixed throughout the experiment. TMS was administered at five intensities (threshold, 5%, 10%, 15%, and 20% MSO above threshold) to test the effect of stimulus intensity on the recovery curves. Conditions without TMS were collected for comparison. Each condition was repeated four times. Testing was done at 0.2 Hz, as the effect of postactivation depression is sufficiently small after 5 s (Crone and Nielsen 1989; Pierrot-Deseilligny and Burke 2005). The above protocol was repeated with REPs to compare the effect of activating additional afferents on the recovery curve.
TMS on the H reflex at rest.
We investigated the interaction (i.e., TMS-TN) at rest to characterize the impact of motor state. Eight subjects were tested (2 new subjects; done ∼10–12 wk after the first experiment) with the same protocol described above for the H reflex. Given that the recovery curve at rest exhibited a bimodal shape, the time course was further reassessed with a finer resolution of IPIs (5, 8, 10, 12, 15, 20, 25, 30, 35, 40, 50, 75, 100, 150, and 200 ms). TMS was delivered at 15% MSO above threshold. Each IPI was tested four times, and eight stimuli were applied with TMS alone. The recovery of the H reflex from postactivation depression without TMS was also measured.
TMS on triple-pulse H reflexes at rest.
Postactivation depression during multipulse stimulation was evaluated with H reflexes produced by a short train of electrical stimuli (3 pulses, typically ∼40 Hz). We tested the same eight subjects involved in the previous study at rest. The chosen IPI was determined as the interval that produced the greatest recovery for IPIs < 50 ms (see above). The H reflex was adjusted to elicit a half-maximal amplitude. As preliminary experiments had shown that the excitation produced by one TMS pulse was too brief (see also Roy et al. 2014) to simultaneously facilitate both the second (H2) and third (H3) H reflexes, H2 and H3 were each conditioned by a TMS pulse. The interaction was tested at four TMS intensities (threshold, 5%, 10%, and 15% MSO above threshold), as well as without TMS. Each condition was repeated four times. On the basis that paired-pulse TMS will facilitate the MEP (Poon et al. 2008; Valls-Solé et al. 1992; Wassermann et al. 1996), the MEP doublet was also evaluated without TN stimulation.
Analysis.
The data were analyzed with MATLAB (MathWorks, Natick, MA). The amplitudes of the H reflex, REP, and MEP were measured peak to peak. H2 and REP2 were expressed as percentages of their unconditioned control responses (H1 and REP1), and full recovery was represented by 100%. At IPIs ≤ 15 ms, H1 (or REP1) could alter the shape of H2 (or REP2) since the peaks could overlap. This was particularly evident when TMS would also facilitate the first reflex because of spatiotemporal facilitation (Poon et al. 2008; Roy et al. 2014). To remove the contribution of H1 (or REP1) at IPIs ≤ 15 ms, the control reflex was 1) aligned with H1 (or REP1), 2) scaled with linear regression, and then 3) subtracted from the experimental data. Traces not having a visible H2 (or REP2) were given a value of zero. Latencies were determined by visual inspection of the waveforms. Statistical analysis was performed with SPSS analytics software (SPSS, Chicago, IL). H reflexes and REPs collected during a contraction were analyzed with a three-way repeated-measures analysis of variance (RM-ANOVA) treating “condition” (H reflex and REP), stimulus “intensity” (5 levels plus “without TMS” condition), and “interval” (7 levels) as within-subject factors. Two-way RM-ANOVAs were applied to the H reflex and REP conditions separately. A two-way RM-ANOVA was used across subjects for intervals > 25 ms to determine M-wave consistency. IPIs ≤ 25 ms were excluded, as waveforms tended to overlap at short intervals. A histogram was created with the rest data to illustrate the IPIs that most frequently showed an early effect by counting the number of times H2 recovered to within 50% of each subject's maximal early recovery. During multipulse stimulation, H reflexes were analyzed with a two-way RM ANOVA treating the reflex amplitude (H2 and H3) and TMS stimulus “intensity” (4 levels plus “without TMS” condition) as within-subject factors. Data are presented as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
TMS on spinal reflexes during contraction.
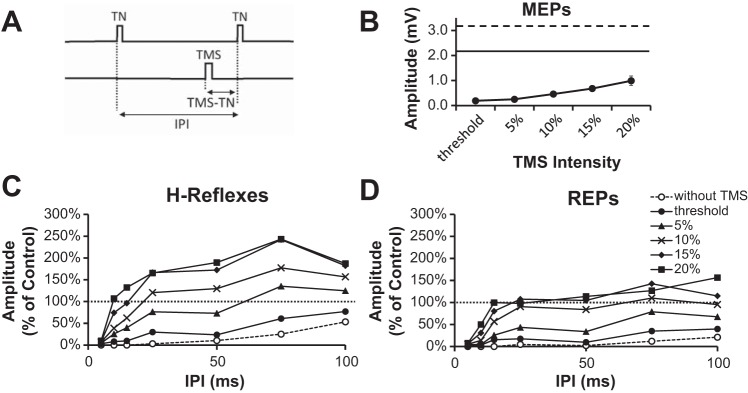
Figure 1A shows representative subject data where pairs of TN stimuli were applied 100 ms apart. The second H reflex (H2; Fig. 1A, top) was reduced to 71% of the first (H1) because of postactivation depression. Administering a TMS pulse 15 ms before the second TN stimulus (Fig. 1A, middle) resulted in the facilitation of H2 (233% of control; postactivation facilitation), which was also considerably greater than the size of the conditioning MEP (Fig. 1A, bottom). The interaction was modulated as a function of the TMS-TN delay (Fig. 1B).
Fig. 1.

Representative data showing the timing of the different stimuli. A: postactivation depression was produced with 2 tibial nerve (TN) stimuli delivered with an interpulse interval (IPI) of 100 ms. The second H reflex (H2) was reduced compared with the first (H1) (top). Transcranial magnetic stimulation (TMS) delivered before the second TN stimulus (TMS-TN delay) caused facilitation of H2 (middle). MEP, motor evoked potential. B: varying the TMS-TN delay resulted in changes in the size of H2. The curve was used to determine the optimal TMS-TN delay.
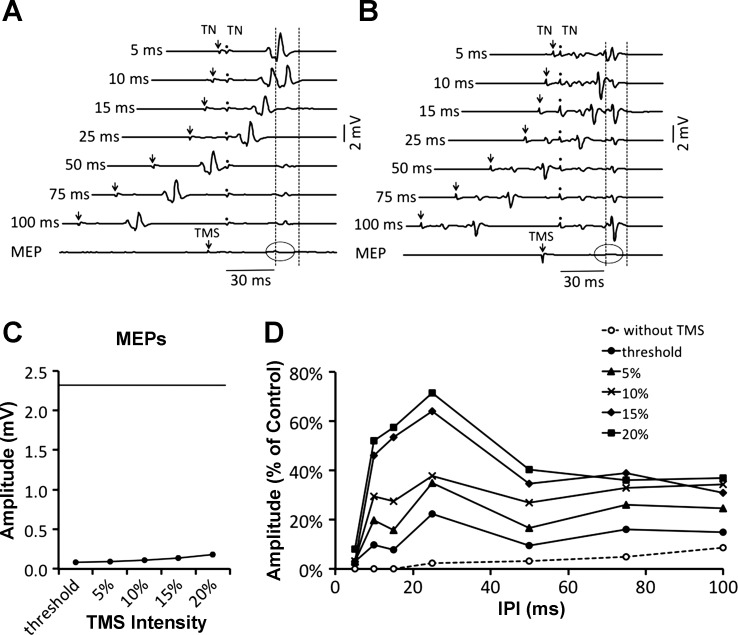
Figure 2 shows the recovery of the H reflex and the REP from postactivation depression in an example subject. Both the H reflex and REP were almost entirely suppressed at IPIs ≤ 50 ms (Fig. 2, A and B). When the depressed reflexes were conditioned with TMS, both reflexes showed marked recovery at all IPIs ≥ 10 ms (Fig. 2, C and D).
Fig. 2.
Single-subject traces showing the recovery of the H reflex (A and C) and root evoked potential (REP) (B and D) from postactivation depression with and without TMS. Consecutive traces show the recovery of the reflexes at IPIs ranging from 5 (top) to 100 ms (bottom). Effect of TMS on the recovery is shown in C and D. TMS was 15% maximal stimulator output (MSO) above threshold. Traces represent the average of 4 sweeps, and ovals highlight the MEP. Arrows and dots represent the times of 1st and 2nd TN and spinal (S) stimuli, respectively. Time of the TMS pulse is indicated with an arrow in MEP trace. H2 and REP2 were measured within the time window between the vertical lines.
Within the group, Hmax, REPmax, and MEPmax measured during a voluntary contraction were 3.6 ± 0.4 mV, 5.2 ± 0.7 mV, and 1.2 ± 0.2 mV (46%, 67%, and 15% of Mmax), respectively. Whenever present, M waves were maintained at a low level of response activation and did not vary significantly throughout experimentation [2-way RM ANOVA: F(2,14) = 2.159; P = 0.152]. As REPmax was larger than Hmax (P < 0.001), the control REP (REP1; 3.2 ± 0.6 mV) was also larger than the control H reflex (H1; 2.2 ± 0.3 mV, P < 0.001). Reflex responses were well matched at 61–62% of their maximum amplitude and were elicited at 43.7 ± 3.8 V (H reflex) and 45.0 ± 3.8 mA (REP). As the latencies of the MEP (32.2 ± 1.3 ms) and the H reflex (31.9 ± 1.1 ms) were similar, a TMS pulse administered 14.4 ± 0.9 ms before the TN stimulus caused the MEP waveform to overlap during the first part of the conditioned reflex. The overlap was comparable for the REP, as TMS was delivered ∼26 ms before the spinal stimulus because the latency of the REP (20.5 ± 0.6 ms) was ∼12 ms shorter than the H reflex.
Grouping the experimental data into three factors (“condition,” “interval,” and “intensity”), a three-way RM ANOVA showed a significant “condition” effect [F(1,7) = 7.27; P = 0.031], indicating that the facilitatory effect of the corticospinal input was greater on the H reflex compared with the REP. Separate two-way RM ANOVAs applied to the H reflex and REP data resulted in significant “interval” [H reflex: F(6,42) = 12.11, P < 0.001; REP: F(6,42) = 11.19, P < 0.001] and “intensity” [H reflex: F(5,35) = 16.77, P < 0.001; REP: F(5,35) = 36.51, P < 0.001] effects, suggesting that the recovery was affected by both interval and intensity. With TMS set to 20% MSO above threshold (i.e., 40.6 + 20% MSO; Fig. 3B), both H reflexes and REPs reached control values at IPIs as short as 10 and 15 ms, respectively (Fig. 3, C and D). Beyond the 50-ms IPI, reflex responses exceeded control values. In particular, postactivation depression was replaced by postactivation facilitation, as the recovered H reflex was 243 ± 51% of its control value at the 75-ms IPI (P < 0.05). This was also 667 ± 79% of the conditioning MEP amplitude (Fig. 3B). In the absence of TMS, postactivation depression of the H reflex was consistently less than the REP [2-way RM ANOVA: F(1,7) = 10.44, P = 0.014; compare open circles in Fig. 3].
Fig. 3.
A: schematic of the stimulation protocol. TMS was delivered prior to the 2nd TN stimulus (TMS-TN delay). The TMS-S delay was 12 ms longer when testing the interaction with the REP. B: average MEP amplitude plotted as a function of intensity. Control H reflex (solid line) and REP (dashed line) amplitudes are shown in B for reference. C and D: effect of corticospinal input on the recovery of the H reflex (C) or REP (D) from postactivation depression during a voluntary contraction. TMS was delivered at 5 different intensities (filled symbols). Data are from 8 subjects. Conditioned reflexes are represented as % of their control (H1 or REP1). Error bars in C and D have been omitted for clarity.
TMS on the H reflex at rest.
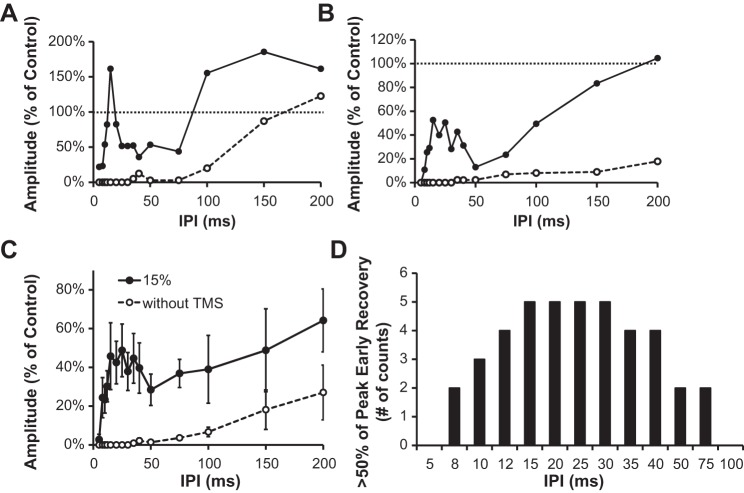
Figure 4 shows the effect of TMS on postactivation depression of the H reflex at rest in two example subjects. The profile of the first subject demonstrated a narrow peak at the 10-ms IPI, which was superimposed on a gradual later recovery starting at 50 ms (Fig. 4A). The second subject exhibited a peak at 15 ms, which was followed by a period of depression from 25 to 75 ms and then an increase to 176% at 100 ms (Fig. 4B). In both examples the MEP was <5% of the control H reflex, so its direct contribution was negligible. Within the group, all subjects showed evidence of bimodal recovery. The values of Hmax and MEPmax within the group were 3.6 ± 0.4 and 0.4 ± 0.2 mV (51% and 6% of Mmax), respectively, while the control H reflex was 2.3 ± 0.3 mV (64% of Hmax). The MEP (Fig. 4C) threshold at rest was 48.1 ± 3.9% MSO. A two-way RM ANOVA revealed a significant main effect for “intensity” [F(5,35) = 15.13, P < 0.001] and an “intensity” × “interval” interaction effect [F(30,210) = 1.84, P = 0.007]. Averaged across subjects, the H reflex recovered to 72 ± 23% of control values at the 25-ms IPI and decreased to 40 ± 10% at the 50-ms interval (Fig. 4D). In the six subjects who participated in both parts of the study, a three-way RM ANOVA showed a significant difference between the voluntary and rest conditions [F(1,5) = 24.31, P = 0.002; data not shown], which is indicative of state-dependent differences.
Fig. 4.
Example traces in 2 subjects showing the effect of corticospinal input on postactivation depression of the H reflex at rest. A and B are structured in the same manner as Fig. 2, C and D. C: average MEP amplitudes plotted as a function of intensity. Control H reflex (solid line) is shown for reference. D: group data from 8 subjects showing the effect of TMS intensity (filled symbols) on the size of the depressed H reflex (open circles). Error bars in D have been omitted for clarity.
The recovery profile of the resting H reflex was further examined with a finer range of IPIs. Two example subjects are shown in Fig. 5, A and B, to highlight some of the differences in the time course. The first subject had a narrow peak from 15 to 25 ms (Fig. 5A), while the second subject had a broader peak from 10 to 35 ms superimposed on a gradual later recovery (Fig. 5B). Within the group, there was a significant “interval” effect [F(14,98) = 1.93, P = 0.032; Fig. 5C] and the IPIs that most frequently produced the short-latency increase were from 15 and 30 ms (Fig. 5D).
Fig. 5.
A and B: single-subject data showing the effect of corticospinal input on postactivation depression of the resting H reflex. Recovery of H2 is shown with (filled circles) and without (open circles) TMS. C: group average. D: no. of times the 2nd H reflex recovered to within 50% of the peak early recovery. Data are from 8 subjects.
TMS on triple-pulse H reflexes at rest.
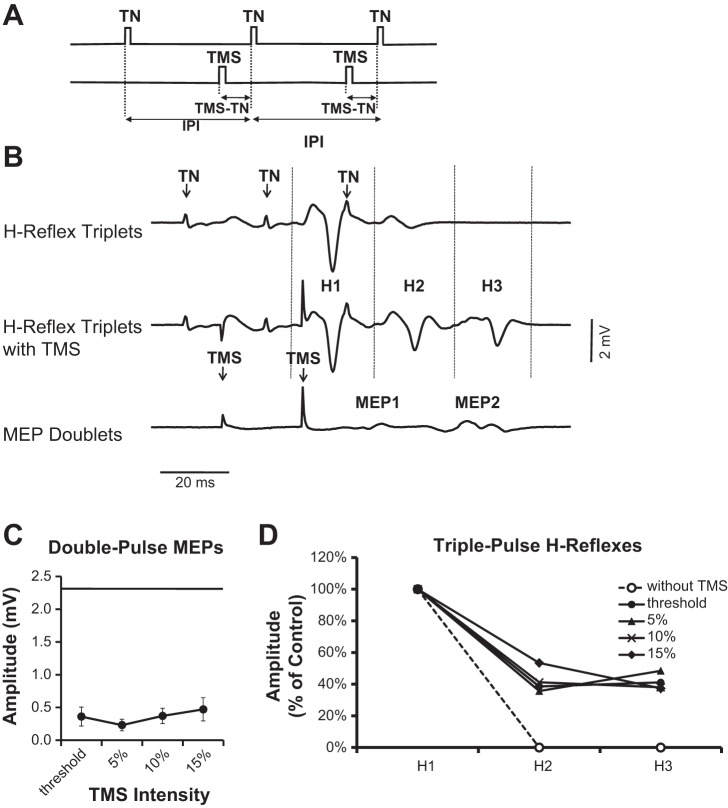
Figure 6B shows the recovery of two depressed H reflexes with double-pulse TMS in an example subject. TN triplets were delivered with a 25-ms IPI, and two TMS pulses were used to condition the second and third H reflexes (Fig. 6A). While both H2 and H3 were completely suppressed (see Fig. 6B, top), corticospinal input caused the partial recovery of both depressed reflexes (see Fig. 6B, middle). Figure 6D illustrates the group recovery of H2 and H3 at the different TMS intensities. The H reflexes were significantly facilitated by TMS [F(1,15) = 12.71, P = 0.003], and the increases to H2 and H3 were not different [F(1,7) = 0.18, P = 0.9]. There were also no differences between the facilitatory effects of the four TMS intensities [F(3,21) = 28, P = 0.84], presumably since the amplitudes of the corresponding MEP doublets were similar (Fig. 6C). Averaged across all TMS intensities, H2 and H3 recovered to 42 ± 17% of control values. This was three times larger than the MEP doublet, which on its own was only 14 ± 11% of the control H reflex.
Fig. 6.
H-reflex triplets paired with double-pulse TMS. A: schematic of the stimulation protocol. Two time-locked TMS pulses were paired with H2 and H3. B: traces show the effect of stimulating the TN 3 times (top). Only H1 is evident while the M waves are present. H2 and H3 are evident when conditioned by TMS (middle). The 3 H reflexes fall within the windows separated by vertical dashed lines. Double-pulse TMS produced two MEPs (i.e., MEP1 and MEP2; bottom). C: amplitude of double-pulse MEP is presented in the same manner as in Fig. 4C. D: group data from 8 subjects showing the effect of the different TMS intensities on H2 and H3.
DISCUSSION
This series of experiments demonstrated that TMS can reduce and even reverse postactivation depression of the H reflex and REP in soleus. In the presence of a suprathreshold TMS pulse, full recovery of H2 and REP2 was possible in as little as 10–15 ms during voluntary contraction. To characterize the effect of motor state, we further explored the strength of this interaction using the H reflex at rest. While the recovery was weaker at rest, the profile exhibited a more distinct bimodal recovery pattern, having an early peak of excitation (∼25 ms) superimposed on a later, more gradual recovery. The early excitation was also present with triple-pulse stimulation at ∼25-ms IPIs, given that two consecutively depressed H reflexes could be facilitated by two time-locked MEPs.
Transient TMS-induced corticospinal excitation on spinal neurons.
TMS caused a reduction in postactivation depression of the H reflex and REP at all IPIs ≥ 10 ms. The strength of the interaction progressively increased from the threshold TMS intensity (see Fig. 3C) and was typically maximal when TMS was 15% MSO above threshold. While the effect of TMS-induced corticospinal excitation on postactivation depression of the REP can last for ∼25 ms (see Roy et al. 2014), the strength of the interaction was maximal when the first corticospinal volley reached the motoneuron pool ∼14 ms before the segmental input. This timing agrees with previous reports using TMS and single H reflexes (Nielsen and Petersen 1995; Serranova et al. 2008; Valls-Solé et al. 1994). A similar profile has also been observed after subthalamic nucleus stimulation, presumably through the activation of corticospinal tract fibers within the internal capsule (Costa et al. 2011). Several mechanisms have been proposed to explain the facilitatory time course in soleus, including a decrease in the amount of presynaptic inhibition acting on Ia afferents (see Costa et al. 2011) along with the temporal summation of excitatory postsynaptic potentials from slow corticospinal fibers and/or indirectly from polysynaptic pathways (Nielsen and Petersen 1995).
Comparing motor state and reflex type.
With TMS, recovery of the H reflex was greater during a contraction relative to rest, which is similar to the trend observed for the soleus REP (Roy et al. 2014). The effect of motor state is likely attributed to the well-known reduction in postactivation depression that occurs during voluntary contraction (Burke et al. 1989; Hultborn and Nielsen 1998; Rothwell et al. 1986). In general, the recovery time courses of the H reflex and REP with TMS were comparable, suggesting that the pathways underwent similar modulation. However, the recovery was less for the REP, even though both reflexes were matched as a percentage of their maximum amplitude. Weaker recovery of the REP by TMS can likely be explained by findings that REPs (compared to H reflexes) are more susceptible to suppression during double-pulse spinal stimulation (Andrews et al. 2015).
Potential mechanism of recovery.
Without TMS, postactivation depression was remarkably robust at IPIs < 50 ms and was partly caused by a combination of presynaptic (Crone and Nielsen 1989) and postsynaptic (Poon et al. 2008; Roy et al. 2014) inhibition. Motoneurons are inhibited after an H reflex (Poon et al. 2008) or an REP (Knikou et al. 2014; Roy et al. 2014), likely because of the afterhyperpolarization of motoneurons (Matthews 1996) and recurrent inhibition (Windhorst 1996). With TMS, the second reflexes (REP2 and H2) could overcome the afterhyperpolarization at IPIs ≥ 10 ms even though the period of afterhyperpolarization in soleus motoneurons can last for 100 ms (Matthews 1996).
Postactivation depression is thought to be associated with a temporary reduction of neurotransmitter release (Armitage and Siegelbaum 1998; Castellucci and Kandel 1974; Elliot et al. 1994; Lev-Tov and Pinco 1992; reviewed in Hultborn et al. 1996) and/or changes in the presynaptic terminal leading to increased presynaptic inhibition (Crone and Nielsen 1989; Schieppati 1987). Studies in animals have suggested an interaction between presynaptic inhibition and postactivation depression. For example, Davies et al. (1985) demonstrated that the administration of benzodiazepines will produce a prolongation of presynaptic inhibition that in turn will alter postactivation depression. In addition, repetitive activation of peripheral afferents has been shown to decrease the efficacy of presynaptic inhibition (Enriquez-Denton et al. 2002). As descending drive can decrease the amount of presynaptic inhibition of Ia fibers acting on soleus motoneurons with TMS (Iles 1996; Valls-Solé et al. 1994), the marked recovery of the H reflex and the REP may, in part, be attributed to the modulation of neurotransmitter release from Ia fibers to soleus motoneurons. This mechanism is consistent with the finding that the control and recovered (second) reflexes had similar morphologies.
Short-latency recovery of the H reflex.
The recovery profile of the H reflex at rest (see Fig. 4C) exhibited a bimodal pattern that consisted of a short-latency period of recovery at ∼25 ms superimposed on a gradual later recovery. The two distinct phases were less apparent during a contraction, likely because volitional drive both reduced postactivation depression and increased the size of the soleus MEP, which may accelerate the second phase of recovery. While a reduction in presynaptic inhibition by TMS may have induced the recovery of the H reflex and REP over all intervals ≥ 10 ms, the existence of a short-latency contribution, which tended to subside by 50 ms, suggests involvement of another mechanism.
After an initial activation, motoneurons are relatively refractory for 3–4 ms, after which time the threshold for a second response will be decreased for a period of ∼20 ms (Burke et al. 2001). This can be explained by the existence of supernormal excitability that occurs after direct depolarization of motor axons (Burke et al. 2001). This period of increased excitability may allow more axons to reach threshold, thereby producing the early peak in excitability observed in the present study. The profile of supernormality is dynamic and shifts to longer IPIs after a particularly strong initial depolarization (Burke et al. 1998) and may explain why the optimal IPI, which was typically clustered from 15 to 30 ms, varied between subjects. In line with the time course of supernormality, motor units are known to fire twice in close succession (2–20 ms) (Stein and Parmiggiani 1979) because of excitatory properties within the soma (Jones et al. 1995). Motor unit doublets occur at the onset of movement (Piotrkiewicz et al. 2013) and are responsible for maximizing twitch force during rapid volitional contraction. The presence of double (or even triple) reflexes in this experimental paradigm may therefore relate to the physiological adaptation for improving musculoskeletal contractions.
As many spinal inhibitory circuits are also under supraspinal influence (Baldissera et al.1981), changes in the strength of spinal inhibitory pathways may also contribute to the early excitation. For instance, corticospinal excitation can cause Renshaw cells to be temporarily inhibited for ∼25 ms (Mazzocchio et al. 1994), leading to an increase in motoneuron excitability. Collateral activation of Renshaw cells during the first reflex will produce recurrent inhibition on the motoneuron. This extra inhibition normally lasts for ∼40 ms (Pierrot-Deseilligny and Burke 2005) and leads to a strong reduction in excitability of motoneurons. Removal of recurrent inhibition by TMS may therefore cause motoneurons to remain depolarized for longer. As Renshaw cells are only active for ∼40 ms, the effect will naturally diminish at longer intervals.
The purpose of this study was to characterize the effect of TMS-induced corticospinal excitation on postactivation depression of the H reflex and REP. The recovery profile exhibited a distinct bimodal curve characterized by an early peak superimposed on a later, more gradual recovery. It is likely that the two phases of recovery are mediated by distinct mechanisms and may be explained by a combination of pre- and postsynaptic mechanisms. Still, further investigation is required to distinguish between them.
GRANTS
This study was funded in part by the Canadian Institutes for Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.C.A., R.B.S., and F.D.R. conception and design of research; J.C.A., R.B.S., and F.D.R. performed experiments; J.C.A. and F.D.R. analyzed data; J.C.A., R.B.S., and F.D.R. interpreted results of experiments; J.C.A., R.B.S., and F.D.R. edited and revised manuscript; J.C.A., R.B.S., and F.D.R. approved final version of manuscript; J.C.A. prepared figures; J.C.A. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Monica A. Gorassini for the loan of some equipment and Dr. David F. Collins for helpful feedback on the manuscript.
REFERENCES
- Andrews JC, Stein RB, Roy FD. Post-activation depression in the human soleus muscle using peripheral nerve and transcutaneous spinal stimulation. Neurosci Lett 589: 144–149, 2015. [DOI] [PubMed] [Google Scholar]
- Armitage BA, Siegelbaum SA. Presynaptic induction and expression of homosynaptic depression at Aplysia sensorimotor neuron synapses. J Neurosci 18: 8770–8779, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc, 1981, sect. 1, vol. II, p. 509–595. [Google Scholar]
- Burke D, Adams RW, Skuse NF. The effects of voluntary contraction on the H reflex of human limb muscles. Brain 112: 417–433, 1989. [DOI] [PubMed] [Google Scholar]
- Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophsyiol 112: 1575–1585, 2001. [DOI] [PubMed] [Google Scholar]
- Burke D, Mogyoros I, Vagg R, Kiernan MC. Quantitative description of the voltage dependence of axonal excitability in human cutaneous afferents. Brain 12: 1975–1983, 1998. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci USA 71: 5004–5008, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J, Guzman J, Valldeoriola F, Rumia J, Tolosa E, Casanova-Molla J, Valls-Sole J. Modulation of the soleus H-reflex by electrical subcortical stimuli in humans. Exp Brain Res 212: 439–448, 2011. [DOI] [PubMed] [Google Scholar]
- Courtine G, Harkema SJ, Dy CJ, Gerasimenko YP, Dyhre-Poulsen P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol 582: 1125–1139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post-activation depression of the soleus H-reflex in man. Exp Brain Res 78: 28–32, 1989. [DOI] [PubMed] [Google Scholar]
- Davies MF, Esplin B, Capek R. Effects of benzodiazepines on spinal homosynaptic depression. Neuropharmacology 24: 301–307, 1985. [DOI] [PubMed] [Google Scholar]
- Delwaide PJ, Cordonnier M, Charlier M. Functional relationships between myotatic reflex arcs of the lower limb in man: investigation by excitability curves. J Neurol Neurosurg Psychiatry 39: 545–554, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol 159: 147–166, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot LS, Kandel ER, Hawkins RD. Modulation of spontaneous transmitter release during depression and post-tetanic potentiation of Aplysia sensory-motor neuron synapse isolated in culture. J Neurosci 14: 3280–3292, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Denton M, Morita H, Christensen LO, Petersen N, Sinkjaer T, Nielsen JB. Interaction between peripheral afferent activity and presynaptic inhibition of Ia afferents in the cat. J Neurophysiol 88: 1664–1674, 2002. [DOI] [PubMed] [Google Scholar]
- Frank K, Fuortes MG. Presynaptic and postsynaptic inhibition of monosynaptic reflexes. Fed Proc 16: 39–40, 1957. [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108: 450–462, 1996. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Nielsen J. Modulation of transmitter release from Ia afferents by their preceding activity—a “post-activation depression.” In: Presynaptic Inhibition and Neural Control. New York: Oxford Univ. Press, 1998, p. 178–191. [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol 491: 197–207, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Calancie B, Hall A, Bawa P. Time course of excitability changes of human α-motoneurons following single spikes and doublets. In: Alpha and Gamma Motor Systems, edited by Taylor A, Gladden MH, Durbaba R. New York: Plenum, 1995, p. 103–105. [Google Scholar]
- Knikou M. Transpinal and transcortical stimulation alter corticospinal excitability and increase spinal output. PLoS One 7: e102313, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Tov A, Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J Physiol 447: 149–169, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Rothwell JC, Thompson PD, Day BL, Marsden DC. Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psychiatry 51: 174–181, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magladery JW, Teasdale RD, Park AM, Languth HW. Electrophysiological studies of reflex activity in patients with lesions of the nervous system. I. A comparison of spinal motoneurone excitability following afferent nerve volleys in normal persons and patients with upper motor neurone lesions. Bull Johns Hopkins Hosp 91: 219–244, 1952. [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol 492: 597–628, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocchio R, Rossi A, Rothwell JC. Depression of Renshaw recurrent inhibition by activation of corticospinal fibers in human upper and lower limb. J Physiol 481: 487–498, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S. Modulation by corticospinal volleys of presynaptic inhibition to Ia afferents in man. J Physiol (Paris) 93: 387–394, 1999. [DOI] [PubMed] [Google Scholar]
- Minassian K, Hofstoetter US, Rattay F, Mayr W, Dimitrijevic MR. Posterior root-muscle reflexes and the H-reflex in humans: electrophysiological comparison (Abstract). Neuroscience Meeting Planner 2009: program no. 658.12.2009, 2009. [Google Scholar]
- Minassian K, Persy I, Rattay F, Dimitrijevic MR, Hofer C, Kern H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 35: 327–336, 2007. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Evidence favouring different descending pathways to soleus motoneurons activated by magnetic brain stimulation in man. J Physiol 486: 779–788, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard J. Reflexes et Regulations D'Origine Proprioceptive chez l'Homme. Paris: Arnette, 1955. [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge, UK: Cambridge Univ. Press, 2005, p. 7, 14, 152, 188. [Google Scholar]
- Piotrkiewicz M, Sebik O, Binboga E, Mlozniak D, Kuraszkiewicz B, Turker KS. Double discharges in human soleus muscle. Front Hum Neurosci 7: 843, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon DE, Roy FD, Gorassini MA, Stein RB. Interaction of paired cortical and peripheral nerve stimulation on human motor neurons. Exp Brain Res 188: 13–21, 2008. [DOI] [PubMed] [Google Scholar]
- Raoul S, Roualdes V, Deligny C, Leduc D, Lamy JC, Lackmy-Vallee A, N'guyen JP, Damier P, Katz R. Subthalamic nucleus stimulation reverses spinal motoneuron activity in parkinsonian patients. Brain 135: 139–147, 2012. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Berardelli A, Marsden CD. Habituation and conditioning of the human long latency stretch reflex. Exp Brain Res 63: 197–204, 1986. [DOI] [PubMed] [Google Scholar]
- Roy FD, Bosgra D, Stein RB. Interaction of percutaneous spinal stimulation and transcranial magnetic stimulation in human leg muscles. Exp Brain Res 232: 1717–1728, 2014. [DOI] [PubMed] [Google Scholar]
- Roy FR, Gibson G, Stein RB. Effect of percutaneous stimulation at different spinal levels on the activation of sensory and motor roots. Exp Brain Res 223: 281–289, 2012. [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 28: 345–376, 1987. [DOI] [PubMed] [Google Scholar]
- Serranova T, Valls-Sole J, Munoz E, Genis D, Jech R, Seeman P. Abnormal corticospinal tract modulation of the soleus H reflex in patients with pure spastic paraparesis. Neurosci Lett 437: 15–19, 2008. [DOI] [PubMed] [Google Scholar]
- Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res 182: 309–319, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Everaert DG, Roy FD, Chong S, Soleimani M. Facilitation of corticospinal connections in able-bodied people and people with central nervous system disorders using eight interventions. J Clin Neurophysiol 30: 66–78, 2013. [DOI] [PubMed] [Google Scholar]
- Stein RB, Parmiggiani F. Optimal motor patterns for activating mammalian muscle. Brain Res 175: 372–376, 1979. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Alvarez R, Tolosa ES. Vibration-induced presynaptic inhibition of the soleus H reflex is temporarily reduced by cortical magnetic stimulation in human subjects. Neurosci Lett 170: 149–152, 1994. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85: 355–364, 1992. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res 109: 158–163, 1996. [DOI] [PubMed] [Google Scholar]
- Windhorst U. On the role of recurrent inhibitory feedback in motor control. Prog Neurobiol 49: 517–587, 1996. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. Interaction of the Jendrássik maneuver with segmental presynaptic inhibition. Exp Brain Res 124: 474–480, 1999. [DOI] [PubMed] [Google Scholar]