Abstract
Normative theories posit that value-based decision-making is context independent. However, decisions between two high-value options can be suboptimally biased by the introduction of a third low-value option. This context-dependent modulation is consistent with the divisive normalization of the value of each stimulus by the total value of all stimuli. In addition, an independent line of research demonstrates that pairing a stimulus with a high-value outcome can lead to attentional capture that can mediate the efficiency of visual information processing. Here we tested the hypothesis that value-based attentional capture interacts with value-based normalization to influence the optimality of decision-making. We used a binary-choice paradigm in which observers selected between two targets and the color of each target indicated the magnitude of their reward potential. Observers also had to simultaneously ignore a task-irrelevant distractor rendered in a color that was previously associated with a specific reward magnitude. When the color of the task-irrelevant distractor was previously associated with a high reward, observers responded more slowly and less optimally. Moreover, as the learned value of the distractor increased, electrophysiological data revealed an attenuation of the lateralized N1 and N2Pc responses evoked by the relevant choice stimuli and an attenuation of the late positive deflection (LPD). Collectively, these behavioral and electrophysiological data suggest that value-based attentional capture and value-based normalization jointly mediate the influence of context on free-choice decision-making.
Keywords: attention, decision-making, EEG, reward, value normalization
normative models suggest that decision-making requires representing the value of all available options and then selecting the option with the highest absolute value (Luce 1959; Samuelson 1947; Stephens and Krebs 1986). However, deviations from this ideal are often reported, and the relative value of competing alternatives has been shown to strongly modulate choice behavior (Bateson et al. 2003; Huber et al. 1982; Hunt et al. 2014; Louie et al. 2013; Shafir et al. 2002; Simonson 1989). For example, the preference for option A vs. option B can be influenced by the introduction of a tertiary option C, despite the fact that the differential value between A and B remains unchanged (Hunt et al. 2014; Louie et al. 2013).
A separate line of research has also demonstrated that value-based learning can enhance the effective salience of a stimulus, leading to a bias in the distribution of attentional priority across the visual field (Anderson et al. 2011a, 2011b; Anderson and Yantis 2012, 2013; Awh et al. 2012; Chelazzi et al. 2014; Hickey et al. 2010; Krebs et al. 2011; Lee and Shomstein 2014; MacLean and Giesbrecht 2015a, 2015b; Qi et al. 2013; Schiffer et al. 2014). For example, when a high-reward distractor appears in the visual field, attention is automatically captured and discrimination speed is slowed (Anderson et al. 2011a, 2011b; Anderson and Yantis 2012, 2013; Hickey et al. 2010). Thus value-driven attentional capture could be one of the key factors contributing to the context dependence of value-based decision-making. This possibility is also consistent with the observation that clinical populations who suffer deficits in value-based decision-making, such as drug addicts, are easily distracted by stimuli with a high learned value (Anderson et al. 2013; Field et al. 2004a, 2004b; Field and Cox 2008; Lubman et al. 2000; Stormark et al. 1997).
Here we designed a novel probabilistic binary-choice paradigm in which observers had to select between one of two colored choices associated with different amounts of monetary reward while simultaneously ignoring a distractor that could capture attention but that could never be selected (“response irrelevant”) and could never yield a reward (Fig. 1). Importantly, colors assigned to the relevant choices and to the distractor were shuffled across trials such that the color of the distractor on a given trial was previously associated with a reward on preceding trials (see red and blue arrows in Fig. 1). Across two experiments, human observers make slower and less optimal decisions as the value of the distractor increases. In addition, electroencephalography (EEG) data reveal that increasing distractor value reduces the lateralized difference between the N1 and the N2pc event-related potentials (ERPs) evoked by the two relevant choices. These early modulations of choice-related ERPs may then have a cascading influence on the speed and the outcome of downstream decision processes, consistent with the observation that the amplitude of the late positive deflection (LPD or P300) is reduced with increasing distractor value.
Fig. 1.
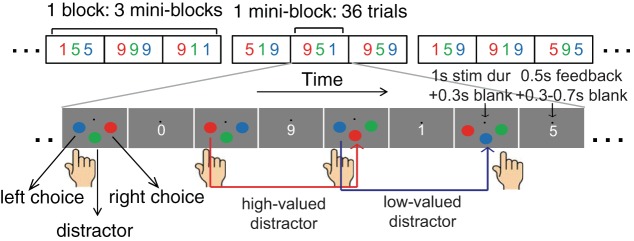
Behavioral binary-choice task. On each trial, subjects selected 1 of 2 choice stimuli that were on either side of fixation. A task-irrelevant distractor simultaneously appeared below the fixation point. Different reward values (1, 5, or 9) were assigned to different stimulus colors (red, green, and blue) every 36 trials. On each trial, one of the choice stimuli yielded a reward if it was selected (termed a baited stimulus) and the other did not (termed a decoy). If subjects selected the baited target, they received 1, 5, or 9 point(s). If they selected the decoy, they received 0 points. The left stimulus was baited on 50% of the trials, and on the other 50% of trials the right stimulus was baited. The distractor could never be selected and thus could never yield a reward (termed the response-irrelevant distractor). The colors assigned to the left and right choice stimuli and to the distractor were pseudorandomized across trials so that the color of either of the 2 choice stimuli in the current trial could become the color of the distractor in the following trials (red and blue arrows).
MATERIALS AND METHODS
Subjects.
Twenty-eight (15 women, 13 men; 3 left-handed; age 18–32 yr) and twenty-seven (16 women, 11 men; 2 left-handed; age 18–45 yr) human subjects were recruited to participate in a behavioral study (Exp1) and an EEG study (Exp2), respectively. All participants were neurologically intact and had normal or corrected-to-normal color vision, and all signed an informed consent form for the study, which was approved by the Institutional Review Board at the University of California, San Diego. Subjects were compensated $10 and $15 per hour and received up to $10 and $20 of additional compensation contingent on behavioral performance during Exp1 and Exp2, respectively. In Exp2, 1 subject did not complete the experiment, leaving 26 subjects in the final analysis. Exp1 lasted ∼1.5 h. Exp2 lasted ∼3 h, including EEG preparation and recording.
Stimuli and tasks.
We presented stimuli on a PC running Windows XP using MATLAB (MathWorks, Natick, MA) and the Psychophysics Toolbox (version 3.0.8; Brainard 1997; Pelli 1985). Participants sat 60 cm from the CRT monitor with a gray background of 42.68 ± 2.20 cd/m2 (60 Hz refresh rate) in a sound-attenuated and electromagnetically shielded room (ETS Lindgren, Cedar Park, TX).
We employed a probabilistic binary-choice paradigm in which participants had to select between one of two choice stimuli that were associated with different amounts of monetary reward. At the same time, they had to ignore an additional distractor that yielded no reward and could never be selected (the response-irrelevant distractor; see Fig. 1). Each trial started with an array of three circular colored stimuli that were presented for 1 s (red, green, and blue, presented near physical isoluminance: 9.85 ± 0.44, 9.84 ± 0.45, and 9.75 ± 0.53 cd/m2, respectively; radius = 4.3° and 2.6° for Exp1 and Exp2, respectively). The relevant choice stimuli were located on the left and right sides of the fixation point, and the distractor stimulus was located directly below the fixation point. All stimuli were equidistant from the fixation point (7.6° and 17.5° for Exp1 and Exp2, respectively). Note that we increased the stimulus eccentricity and decreased stimulus size in Exp2 so that we could better isolate lateralized differences in the ERPs. On each trial, a reward was assigned to one of the two choice stimuli such that one of them would always yield a reward of 1, 5, or 9 point(s) if it was selected. The stimulus that would yield a reward if selected was termed the baited stimulus. The other choice stimulus was a decoy and yielded 0 points when selected. Thus there was a 50% probability of obtaining a reward on each trial, but the magnitude of the reward (when received) depended on the color of the selected stimulus. Importantly, the distractor stimulus could capture attention by virtue of the value that was previously associated with its color (Anderson et al. 2011b; Hickey et al. 2010), but it could never be selected and thus could never yield a reward (i.e., it was response irrelevant). Every 36 trials (a miniblock), different reward magnitudes were assigned to each color.
There were a total of 54 miniblocks needed to represent all of the 27 possible color-reward combinations (3 colors with any of the 3 potential reward levels). In each miniblock, there were six color-stimulus combinations with six trials each (i.e., red/green/blue, green/red/blue, green/blue/red, blue/green/red, blue/red/green, and red/blue/green for left choice stimulus/middle distractor/right choice stimulus). Within each miniblock, the order of trial presentation was pseudorandomized so that the trial sequence was unpredictable and the color of each choice stimulus on the current trial was equally likely to become the distractor color on the following trial (see blue and red arrows in Fig. 1 for examples). Note that it was also equally likely that the distractor color stayed the same on consecutive trials. The sequence of miniblocks was pseudorandomized so that subjects could not predict the color-reward pairings in advance.
Each observer participated in a total of 18 experimental blocks (1,944 trials), where each block lasted ∼4 min and contained 3 miniblocks. Subjects were instructed to maintain fixation while deciding between the left and right choice stimuli, and they made their selection by pressing one of two buttons on a keypad with either their right index finger or their right middle finger; 0.3 s after the offset of the stimulus array a feedback display indicating the number of points that subjects earned on that trial appeared for 0.5 s, followed by a 0.3- to 0.7-s intertrial interval (ITI). If subjects did not respond before the stimulus array disappeared, a “#” sign appeared in the center of the display and no points were awarded. Subjects were told that the reward magnitude associated with each color could change across successive trials, and they were encouraged to maximize cumulative points by actively learning the association between colors and reward magnitude. At the end of the study, the points that each subject earned were translated into bonus money (0.1 and 0.2 cents per 1 point for Exp1 and Exp2, respectively).
Behavioral analysis.
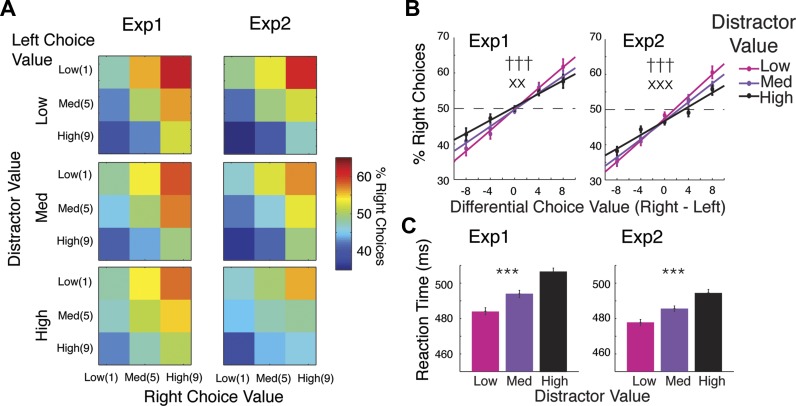
Trials in which subjects responded faster than 200 ms were discarded from the behavioral and EEG analyses (5.29% of trials ± 1.39% SE in Exp1 and 2.36% of trials ± 0.69% SE in Exp2). We first plotted the probability that subjects selected the right stimulus as a function of the potential reward associated with each choice stimulus as well as the learned value of the response-irrelevant distractor (low, medium, or high; Fig. 2A). Then, we plotted the data as a function of the differential value between the two choice stimuli (right minus left) and distractor value (Fig. 2B). We used a two-way repeated-measures ANOVA with within-subject factors for differential choice value (5 levels: −8, −4, 0, 4, and 8 points) and distractor value (3 levels: low, medium, and high) to the test for main effects and for an interaction between these two factors on the probability of selecting the right choice stimulus. We then bootstrapped the data for all subjects (resampled subject labels with replacement) to establish 95% confidence intervals (CIs). On each iteration of the bootstrapping procedure, we used MATLAB (fminsearch) to estimate the mean (μ) and the standard deviation (σ) of the cumulative Gaussian function that best fit the choice probability data derived from each distractor value condition. We repeated this resampling and refitting procedure 10,000 times and removed the mean value across distractor value conditions from each sampling literation to estimate within-subject 95% CIs for all fit parameters. Mean reaction times (RTs), collapsed across left and right choices and across distractor value conditions, were also plotted as a function of distractor value (Fig. 2C) and evaluated with a one-way repeated-measures ANOVA with a factor of distractor value.
Fig. 2.
Distractor value increases decision uncertainty and decision time. A: probability of right choices as a function of left and right choice values for when distractor value is low, medium, and high (top, middle, and bottom). B: same data as A plotted as a function of differential choice value (right minus left choice values) and distractor value (low, medium, and high). Overall, higher distractor value reduces the tendency of subjects to choose higher-valued over lower-valued choices. C: reaction times (RTs), collapsed across left/right choices and across differential choice value bins, plotted as a function of distractor value. Overall, RTs increase as distractor value increases. Error bars for choice probability and RT data represent within-subject SE. ***Significant main effect of distractor value (P < 0.001). †††Significant main effect of differential choice value (P < 0.001). xx,xxxSignificant interaction between distractor value and differential choice value (P < 0.01 and P < 0.001, respectively).
EEG recording, preprocessing, and analysis.
In Exp2, we examined neural modulations with EEG as a second means of testing the impact of distractor value on choice behavior. We first tested the impact of distractors on the amplitude of early lateralized ERPs in posterior-occipital electrodes contralateral and ipsilateral to the selected stimulus (the P1, N1, and N2pc components). Previous studies have interpreted modulations of the P1 and N1 components as an index of attentional gain in early visual cortex (e.g., Busse et al. 2005; Hillyard et al. 1998; Hillyard and Anllo-Vento 1998; Itthipuripat et al. 2014a; Johannes et al. 1995; Luck et al. 1990; Luck and Hillyard 1994; Mangun and Buck 1998; Mangun and Hillyard 1987, 1988, 1990, 1991; Noesselt et al. 2002; Störmer et al. 2009; Van Voorhis and Hillyard 1977; Woldorff et al. 1997; Zimmer et al. 2010). Recent studies have further linked these components to the value-based modulation of sensory and perceptual processing (Baines et al. 2011; Hickey et al. 2010; MacLean and Giesbrecht 2015b). The N2pc component has been used to index the focus of visuospatial attention as determined by explicit attention cues, stimulus salience, or learned stimulus value (Hickey et al. 2006, 2008, 2009, 2010; Kiss et al. 2008; Luck et al. 1997; Qi et al. 2013; San Martín et al. 2014; Woodman et al. 2009; Woodman and Luck 1999). If the efficiency of choice behavior is impaired via value-based attentional capture, then we should observe a reduction in the differential amplitude of these lateralized ERP components as a function of distractor value. Finally, we evaluated the influence of distractor value on the amplitude of the centroparietal late positive-going deflection across a 300–500 ms poststimulus window (the LPD or P300). This component has been previously linked to postsensory processing during decision-making, as it tracks decision difficulty and decision speed (Kelly and O'Connell 2013; O'Connell et al. 2012; Squires et al. 1973, 1975a, 1975b).
EEG data were recorded with a 64+8 channel BioSemi ActiveTwo system (Amsterdam, The Netherlands) at a sampling rate of 512 Hz. Two reference electrodes were placed at the mastoids. We monitored vertical eye movements and blinks via four extra electrodes placed below and above the eyes. Horizontal eye movements were assessed via another pair of electrodes placed near the outer canthi of the eyes. The EEG data were referenced online to the BioSemi CMS-DRL reference, and all offsets from the reference were maintained below 20 μV. The data were preprocessed with a combination of EEGlab11.0.3.1b (Delorme and Makeig 2004) and custom MATLAB scripts.
After data collection, we rereferenced the continuous EEG data to the mean of the two mastoid electrodes and applied 0.25-Hz high-pass and 55-Hz low-pass Butterworth filters (3rd order). An additional 22-Hz low-pass filter was applied to plot the data, but all reported statistics were performed on the 55-Hz low-pass filtered data (Luck 2005; also see similar methods in Hickey et al. 2010). The data were then segmented into epochs extending from 500 ms before to 2,000 ms after the trial onset and baseline-corrected based on the mean response from 0–200 ms before stimulus onset. Prominent eyeblink artifacts were first rejected by independent component analysis (Makeig et al. 1995). We then discarded epochs contaminated by residual eyeblinks and vertical eye movements (more than ±80–150 μV deviation from zero, with thresholds chosen for each individual subject), horizontal eye movements (more than ±75–100 μV deviation from zero), excessive muscle activity, or drifts, using threshold rejection and visual inspection (10.21% of trials ± 1.29% SE). The artifact-corrected epochs were then sorted on the basis of the hemifield of the selected choice (left or right), the absolute differential value of the selected and unselected choices (low, medium, or high), and then on the value of the response-irrelevant distractor (low, medium, or high). ERPs were then calculated in each of the resulting 18 condition bins with standard signal averaging procedures. Note that in Exp2 we collapsed across positive and negative differential choice values because we obtained an EEG measurement related to both stimuli on every trial. Thus there were only three levels of differential choice value in Exp2 as opposed to five levels in Exp1 (i.e., in Exp1 we had levels of −8, −4, 0, 4, and 8 points).
We then examined the impact of distractor value on the lateralized ERPs recorded from posterior-occipital sites (PO3, P3, and P5, for the left hemisphere and PO4, P4, and P6 for the right hemisphere), where the distractor value effects were maximal. We compared the mean differential amplitude between ERPs contralateral and ipsilateral to the selected choice stimulus as a function of the differential value between the two choice stimuli and the value of the distractor. This comparison was carried out across three temporal windows: from 100 to 125 ms (P1), from 160 to 185 ms (N1), and from 215 to 300 ms (N2Pc). For each of these ERP components, we used a two-way repeated-measures ANOVA with factors for the differential value between the two choice stimuli (3 levels: low, medium, and high) and distractor value (3 levels: low, medium, and high) to evaluate the influence of these two factors on the amplitude of the lateralized difference components. In addition, we defined the amplitude of the LPD component as the average response from 300 to 500 ms after stimulus in centroparietal electrodes (Cp1, CpZ, Cp2). A two-way repeated-measures ANOVA with factors for differential choice value and distractor value was used to evaluate modulations of LPD amplitude.
RESULTS
The value of a task-irrelevant distractor interferes with choice behavior and increases reaction times.
In both experiments, subjects exhibit a significant bias to select higher-valued choices over lower-valued choices (Fig. 2, A and B). This bias gives rise to a significant main effect of differential choice value (right minus left choice values) on the probability of choosing the right choice (% right choices) in both Exp1 [F(4,108) = 27.99, P < 0.0001] and Exp2 [F(4,100) = 68.31, P < 0.0001]. Importantly, this bias toward the higher-valued choice decreases as the value associated with the response-irrelevant distractor increases, leading to a significant interaction between differential choice value and distractor value in both Exp1 [F(8,216) = 3.14, P = 0.0022] and Exp2 [F(8,200) = 3.47, P = 0.00091]. We then fit a cumulative Gaussian function to the behavioral data for each distractor value condition to estimate the standard deviation (σ) and the mean (μ) of the underlying function (where the σ parameter determines the slope of the function and the μ parameter determines horizontal position; see Table 1 for statistics and CIs). Across the two experiments, σ increases as a function of increasing distractor value (i.e., the slope of the best-fitting cumulative Gaussian decreases). In Exp1, μ does not differ across distractor value conditions, indicating that there is no overall preference for the left or right stimulus. However, in Exp2 there is a slight bias to respond to the left choice. Similar response biases have been observed in a previous study (Louie et al. 2013); however, as in the present study, these biases seem to vary idiosyncratically across subjects/groups.
Table 1.
Parameters from fitting choice probability data from Fig. 2B with a cumulative Gaussian function
| σ[within-subject 95% CIs] |
μ[within-subject 95% CIs] |
|||||
|---|---|---|---|---|---|---|
| Exp | Low distractor value | Med distractor value | High distractor value | Low distractor value | Med distractor value | High distractor value |
| 1 | 27.5 | 37.2 | 45.9 | −0.04 | 0.3 | −0.6 |
| [18.5 32.3] | [32.9 45.4] | [40.5 54.3] | [−1.0 1.2] | [−0.4 1.0] | [−2.1 0.6] | |
| 2 | 25.6 | 30.6 | 40.8 | 1.6 | 2.4 | 3.3 |
| [19.8 29.9] | [26.6 34.3] | [35.9 48.2] | [1.0 2.2] | [1.9 2.9] | [2.6 4.3] | |
All confidence intervals (CIs) are based on the bootstrapping procedure described in materials and methods. σ, Standard deviation; μ, mean.
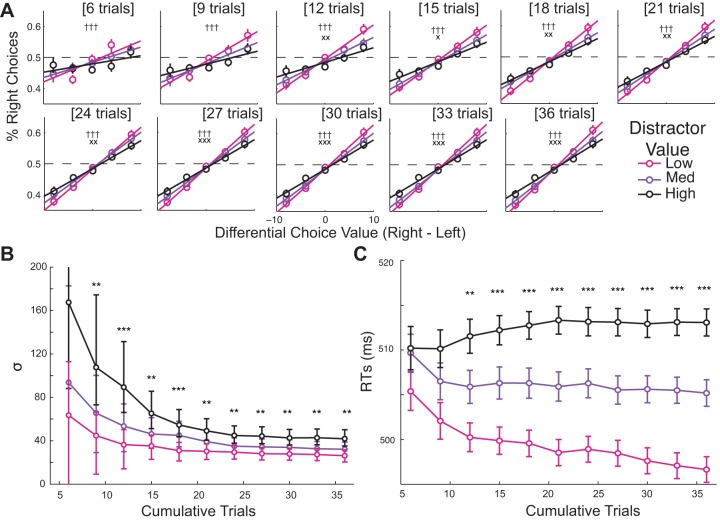
In addition, RTs significantly increase as a function of distractor value in both Exp1 [F(2,54) = 9.57, P = 0.00028] and Exp2 [F(2,50) = 12.44, P < 0.0001; see Fig. 2C]. Auxiliary analyses also demonstrate that subjects gradually learned the values associated with each color within the first six trials of a minisession, and the magnitude of these decision biases increases over the course of each miniblock (see Fig. 3 and Table 2 for results and statistics). The influence of distractor value on decision biases (i.e., the interaction between distractor value and differential choice value) and RTs emerges later (within the first 12 trials). Overall, the behavioral data from Exp1 and Exp2 suggest that even though the distractor is neither relevant nor available for selection, the learned value associated with the distractor color systematically captures attention and leads to less optimal and slower decisions.
Fig. 3.
Choice behavior and RT data across trials. A: probability of right choices as a function of differential choice value (right minus left choice values) and distractor value (low, medium, and high) computed across the first 6–36 cumulative trials within each miniblock of 36 trials. Data from Exp1 and Exp2 are combined to improve analysis power. Overall, subjects exhibit a bias toward higher-valued choices within the first 6 trials, and the modulation of this bias by distractor value emerges within the first 12 trials. Error bars represent within-subject SE. B: corresponding σ parameters from fitting the choice probability data with a cumulative Gaussian function. Error bars indicate within-subject 95% confidence intervals. C: RTs plotted as a function of distractor value across the first 6–36 cumulative trials. Similar to choice behavior, the modulation of RTs by distractor value emerges within the first 12 trials. Error bars represent within-subject SE. **,***Significant main effect of distractor value [P < 0.01 and P < 0.001 with false discovery rate (FDR) correction for multiple comparisons]. †††Significant main effect of differential choice value (P < 0.001, FDR-corrected). x,xx,xxxSignificant interactions between differential choice value and distractor value (P ≤ 0.012, P < 0.01, and P < 0.001, FDR-corrected).
Table 2.
Statistical results for cumulative trial analyses on choice behavior and RTs in Fig. 3
| Choice Behavior (Fig. 3A) |
||||
|---|---|---|---|---|
| No. of Cumulative Trials | Main effect of differential choice value F(4,212) [P value] | Main effect of distractor value F(2,106) [P value] | Interaction F(8,424) [P value] | RTs (Fig. 3C) Main Effect of Distractor Value F(2,106) [P value] |
| 6 | 8.77 | 0.41 | 1.76 | 0.95 |
| [<0.0001†††] | [0.66] | [0.083] | [0.39] | |
| 9 | 21.52 | 1.02 | 1.94 | 2.54 |
| [<0.0001†††] | [0.37] | [0.052] | [0.084] | |
| 12 | 37.35 | 1.15 | 3.16 | 6.64 |
| [<0.0001†††] | [0.32] | [0.0017xx] | [0.0019**] | |
| 15 | 47.42 | 0.48 | 2.48 | 9.28 |
| [<0.0001†††] | [0.62] | [0.012x] | [0.0002***] | |
| 18 | 54.95 | 0.22 | 3.25 | 11.68 |
| [<0.0001†††] | [0.81] | [0.0013xx] | [<0.0001***] | |
| 21 | 65.00 | 0.13 | 3.01 | 15.14 |
| [<0.0001†††] | [0.87] | [0.0027xx] | [<0.0001***] | |
| 24 | 75.89 | 0.34 | 3.06 | 14.12 |
| [<0.0001†††] | [0.71] | [0.0023xx] | [<0.0001***] | |
| 27 | 81.69 | 0.00 | 4.41 | 15.44 |
| [<0.0001†††] | [1.00] | [<0.0001xxx] | [<0.00001***] | |
| 30 | 82.52 | 0.22 | 4.47 | 17.42 |
| [<0.0001†††] | [0.80] | [<0.0001xxx] | [<0.001***] | |
| 33 | 84.06 | 0.30 | 5.41 | 20.05 |
| [<0.0001†††] | [0.74] | [<0.0001xxx] | [<0.0001***] | |
| 36 | 80.05 | 0.48 | 6.50 | 20.63 |
| [<0.0001†††] | [0.62] | [<0.0001xxx] | [<0.0001***] | |
RT, reaction time.
Significant main effects of distractor value [P < 0.01 and P < 0.001, false discovery rate (FDR)-corrected].
Significant main effect of differential choice value (P < 0.001, FDR-corrected).
Significant interactions between distractor value and differential choice value (P ≤ 0.012, P < 0.01, and P < 0.001, FDR-corrected).
Distractor value reduces the amplitude of lateralized ERP differences evoked by choice stimuli.
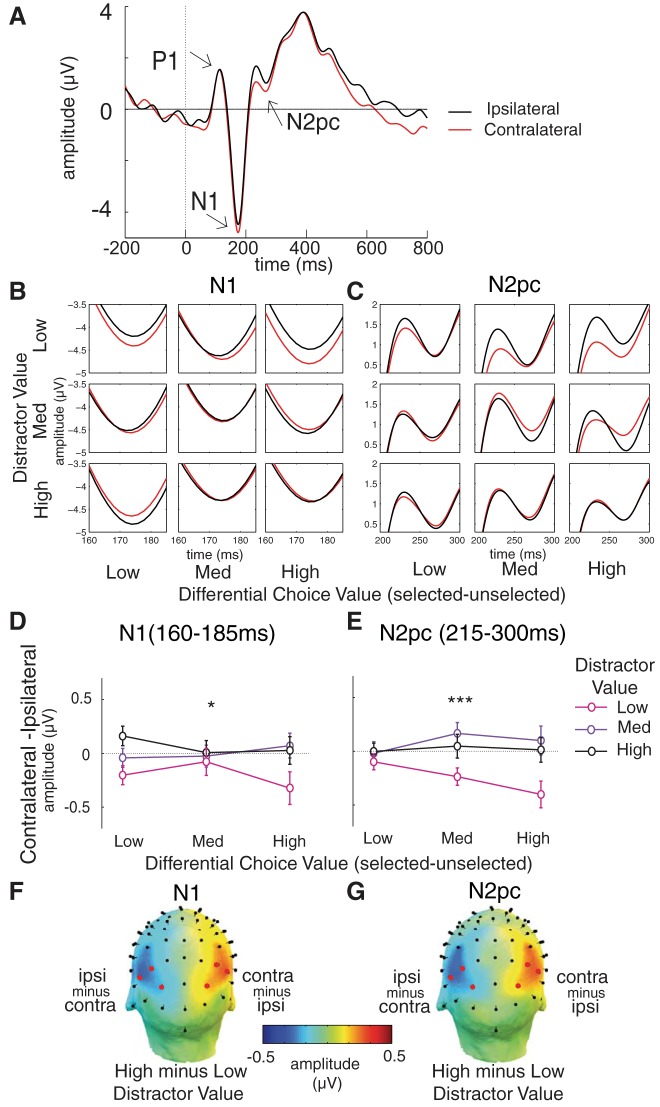
Figure 4A illustrates averaged stimulus-locked ERPs measured from electrodes that are contralateral and ipsilateral to the selected stimulus (red and black traces, respectively) in the posterior-occipital electrodes. For illustrative purposes, data are from trials with a high differential choice value and a low distractor value, as this condition yields the largest difference between the lateralized ERPs. Overall, the magnitude of the lateralized N1 difference (contralateral minus ipsilateral) decreases as distractor value increases [Fig. 4, B and D; F(2,50) = 4.83, P = 0.012]. There is a similar modulation for the lateralized N2pc difference [Fig. 4, C and E; F(2,50) = 8.16, P = 0.00086]. There is no main effect of distractor value across the P1 window [F(2,50) = 1.97, P = 0.15]. Together, significant modulations of the lateralized N1 and N2pc components suggest that distractor value interferes with the early processing of choice stimuli (indexed by the modulation of the N1) and draws spatial attention away from the relevant choices (indexed by the modulation of the N2pc).
Fig. 4.
Distractor value reduces the amplitudes of lateralized event-related potentials (ERPs). A: averaged stimulus-locked posterior-occipital ERPs contralateral to selected (red) and unselected (black) choice stimuli in the condition with high differential choice value but low distractor value. B and C: zoomed-in view of lateralized N1 and N2pc components plotted as a function of differential choice value and distractor value. D and E: mean amplitude difference of the lateralized ERPs across the N1 and N2pc windows, plotted as a function of differential choice value and distractor value. Overall, the amplitude difference of the early N1 and attention-shift-related N2pc components decreases (became less negative) as distractor value increases. F and G: topographical maps of the lateralized N1 contralateral-minus-ipsilateral difference and the N2pc contralateral-minus-ipsilateral difference between the high and low distractor value conditions (shown in the right hemisphere of the head model; the left hemisphere is the mirror image of the right hemisphere with the opposite sign). The effect of distractor value on these 2 components is maximal at the lateral posterior-occipital and posterior electrodes (red circles). Error bars represent within-subject SE. *,***Significant main effects of distractor value (P < 0.05 and P < 0.001, respectively).
Distractor value modulates the amplitude of the late positive-going deflection.
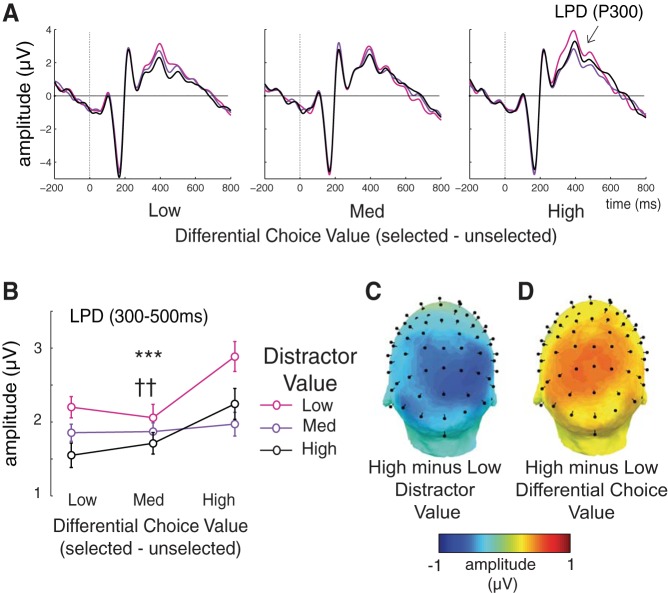
LPD amplitude decreases significantly with increasing distractor value [Fig. 5; F(2,50) = 10.25, P = 0.00018]. This modulation suggests slower postsensory processing (O'Connell et al. 2012) and is consistent with the observed increase in RTs as a function of distractor value (Fig. 2C). In addition, LPD amplitude decreases as differential choice value decreases [F(2,50) = 7.77, P = 0.0012], potentially reflecting increased decision uncertainty or decision conflict when the value of each choice is more similar (cf. Cavanagh et al. 2011; Hillyard et al. 1971; Itthipuripat et al. 2014a; O'Connell et al. 2012; Shenhav et al. 2014; Wiener and Thompson 2015).
Fig. 5.
Distractor value reduces the amplitude of the late positive-going deflection (LPD or P300). A: stimulus-locked centroparietal ERPs across differential choice values (low to high from left to right) and distractor values (low, medium, and high). B: mean amplitude of the LPD component, plotted as a function of differential choice value and distractor value. LPD amplitude decreases as distractor value increases (see topography in C). In contrast, LPD amplitude increases as differential choice value increases (see topography in D). Error bars represent within-subject SE. ††,***Significant main effect of differential choice value (P < 0.01) and distractor value (P < 0.001), respectively.
DISCUSSION
Attentional capture induced by the learned value of an irrelevant visual stimulus has been shown to impair performance during visual search (Anderson et al. 2011a, 2011b; Anderson and Yantis 2012, 2013; Hickey et al. 2010; MacLean and Giesbrecht 2015a; Qi et al. 2013). Here we tested the hypothesis that this value-driven attention capture contributes to context-dependent modulations during value-based decision-making (e.g., Hunt et al. 2014; Louie et al. 2013). Across two experiments, human subjects respond more slowly and are less likely to choose the higher-value choice as distractor value increases. Simultaneously recorded EEG data show that the amplitudes of the lateralized N1 and N2pc difference waves, as well as the amplitude of decision-related LPD, all decrease as distractor value increases. These results suggest that distractor value interferes with early sensory and attention-related processing of relevant choices, and we speculate that these early modulations then propagate to influence the speed and the outcome of decision-making. Taken together, our data strongly speak against normative theories of decision-making (Luce 1959; Samuelson 1947; Stephens and Krebs 1986) that posit context independence during decision-making. Moreover, our data suggest that value-based attentional capture by a response-irrelevant stimulus is one important factor that contributes to this context dependence.
Recent studies using ternary-choice tasks have shown that preference for the highest-value option over the second-highest-value option can be impacted by the value of a third low-value option (Hunt et al. 2014; Louie et al. 2013). These observations can be explained by divisive normalization (Carandini and Heeger 2012), a computational principle that has been used to account for gain control in sensory systems (Heeger 1992; Rabinowitz et al. 2011; Reynaud et al. 2012; Tsai et al. 2012; Zoccolan 2005) and attentional modulation of sensory signals (Herrmann et al. 2010; Itthipuripat et al. 2014b; Reynolds et al. 1999; Reynolds and Heeger 2009). Specifically, value-based normalization models postulate that the neural representation of each option is divided by the summed activity that represents the value of all available options (Louie et al. 2011, 2013; Rangel and Clithero 2012). Thus an increase in the value of the third stimulus will normalize the differential value of the other two stimuli and decision-making will be less consistent.
The present observation that increased distractor value leads to less efficient decision-making and attenuated ERP responses is consistent with divisive normalization. However, there are several differences between the present task and tasks used in past studies that support value-based normalization models (e.g., Hunt et al. 2014; Louie et al. 2011, 2013). First, we used a binary-choice task instead of a ternary-choice task in which all three stimuli could be selected by the observer. This design change allowed us to selectively examine the impact of attentional capture by a response-irrelevant distractor. Second, the present task had a shorter response window and less predictable reward rates (i.e., a 50% probability of reward combined with a change in the association between reward and color every 36 trials). This speed pressure and increase in uncertainty may have led subjects to adopt a fundamentally different strategy than in previous experiments (e.g., Hunt et al. 2014; Louie et al. 2011, 2013). We view this as unlikely, however, as subjects learned the value that was associated with each color within the first six trials following a change (Fig. 3). Thus, even though our experimental design differed somewhat compared with previous studies, our behavioral data suggest that distractor value has an impact on decision-making in a manner that is consistent with value-based normalization (Louie et al. 2011, 2013; Rangel and Clithero 2012). In addition, across all trial types and value manipulations, the probability that a left or right choice stimulus would be rewarded was equated. Therefore, the effect of distractor value on behavioral choice cannot be attributed to a difference in reward uncertainty across conditions. Finally, the dynamic modulation of EEG markers is consistent with previous observations that cortical areas along the dorsal visual pathway encode value in a relative manner (Anderson et al. 2014; Buschschulte et al. 2014; Hickey and Peelen 2015; Louie et al. 2011; Persichetti et al. 2015; Schiffer et al. 2014; Serences 2008; Serences and Saproo 2010; Stănişor et al. 2013) and that divisive normalization operates to regulate responses in these regions (Carandini and Heeger 2012; Louie et al. 2011, 2013; Reynolds and Heeger 2009). Taken together, the data suggest that value-driven attentional capture interacts with divisive normalization to mediate context effects during decision-making.
Reward learning in this task may bias decision-making by mediating attentional priority via interactions between reward-related mesolimbic, attentional control, and sensory systems. Previous work suggests that attentional selection depends on a neuronal network centered on the basal ganglia that receives converging inputs from substantia nigra, ventral tegmental area, thalamus, amygdala, and cerebral cortex (Anderson et al. 2014; Bromberg-Martin et al. 2010; Gottlieb et al. 2014; Hickey and Peelen 2015; Hikosaka et al. 2013; Krauzlis et al. 2014; Nakano et al. 1990; Peck et al. 2013; Selemon and Goldman-Rakic 1985; Zorrilla and Koob 2013). Consistent with this idea, past studies have demonstrated that reward-based learning can enhance the saliency of a stimulus and can flexibly and selectively alter attentional priority maps (Anderson et al. 2011a, 2011b; Anderson and Yantis; 2012, 2013; Awh et al. 2012; Chelazzi et al. 2014; Hickey et al. 2010; Krebs et al. 2011; Lee and Shomstein 2014; Schiffer et al. 2014) thought to be encoded by population-level activity throughout occipital and parietal cortex (Itti and Koch 2001; Serences and Yantis 2007; Sprague and Serences 2013). Moreover, patients with dysfunctions in the mesolimbic dopaminergic pathway, such as drug addicts and patients with Parkinson's disease, exhibit deficits in visual attention (Botha and Carr 2012; Fénelon 2008; Field et al. 2004a, 2004b; Field and Cox 2008; Hepp et al. 2013; Horowitz et al. 2006; Lubman et al. 2000; Maddox et al. 1996; Stormark et al. 1997). Thus the interplay between the reward-related mesolimbic pathway and attentional priority maps may allow the brain to control the balance between bottom-up and top-down inputs that are necessary for representing the value of individual alternatives during decision-making. Future research could extend these results by using different measurement techniques combined with modeling methods (Hunt et al. 2014; Louie et al. 2013; Sprague and Serences 2013) to examine how interactions between reward-related and attentional systems jointly influence value-based decision-making.
GRANTS
This work was supported by a Howard Hughes Medical Institute international student fellowship to S. Itthipuripat and by National Institute of Mental Health Grant R01-MH-092345 and a James S. McDonnell Foundation grant to J. T. Serences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.I. and J.T.S. conception and design of research; S.I., K.C., and N.R. performed experiments; S.I., K.C., and N.R. analyzed data; S.I. and J.T.S. interpreted results of experiments; S.I. prepared figures; S.I. drafted manuscript; S.I. and J.T.S. edited and revised manuscript; S.I., K.C., N.R., and J.T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Siyi Chen for help with data collection and Thomas Sprague, Vy Vo, Edward Ester, Stephanie Nelli, Scott Freeman, and Evan Carr for useful discussions.
REFERENCES
- Anderson BA, Faulkner ML, Rilee JJ, Yantis S, Marvel CL. Attentional bias for non-drug reward is magnified in addiction. Exp Clin Psychopharmacol 21: 499–506, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Learned value magnifies salience-based attentional capture. PLoS One 6: e27926, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proc Natl Acad Sci USA 108: 10367–10371, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Res 1587: 88–96, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Yantis S. Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Atten Percept Psychophys 74: 1644–1653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Yantis S. Persistence of value-driven attentional capture. J Exp Psychol Hum Percept Perform 39: 6–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn Sci 16: 437–443, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines S, Ruz M, Rao A, Denison R, Nobre AC. Modulation of neural activity by motivational and spatial biases. Neuropsychologia 49: 2489–2497, 2011. [DOI] [PubMed] [Google Scholar]
- Bateson M, Healy SD, Hurly TA. Context-dependent foraging decisions in rufous hummingbirds. Proc Biol Sci 270: 1271–1276, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H, Carr J. Attention and visual dysfunction in Parkinson's disease. Parkinsonism Related Disord 18: 742–747, 2012. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68: 815–834, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschschulte A, Boehler C, Strumpf H, Stoppel C, Heinze HJ, Schoenfeld MA, Hopf JM. Reward- and attention-related biasing of sensory selection in visual cortex. J Cogn Neurosci 26: 1049–1065, 2014. [DOI] [PubMed] [Google Scholar]
- Busse L, Roberts KC, Crist RE, Weissman DH, Woldorff MG. The spread of attention across modalities and space in a multisensory object. Proc Natl Acad Sci USA 102: 18751–18756, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci 13: 51–62, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci 14: 1462–1467, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Estocinova J, Calletti R, Lo Gerfo E, Sani I, Della Libera C, Santandrea E. Altering spatial priority maps via reward-based learning. J Neurosci 34: 8594–8604, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Fénelon G. Psychosis in Parkinson's disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr 13: 18–25, 2008. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend 97: 1–20, 2008. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Eye movements to smoking-related cues: effects of nicotine deprivation. Psychopharmacology (Berl) 173: 116–123, 2004a. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology (Berl) 176: 88–93, 2004b. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Hayhoe M, Hikosaka O, Rangel A. Attention, reward, and information seeking. J Neurosci 34: 15497–15504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Vis Neurosci 9: 181–197, 1992. [DOI] [PubMed] [Google Scholar]
- Hepp DH, da Hora CC, Koene T, Uitdehaag BM, van den Heuvel OA, Klein M, van de Berg WD, Berendse HW, Foncke EM. Cognitive correlates of visual hallucinations in non-demented Parkinson's disease patients. Parkinsonism Relat Disord 19: 795–799, 2013. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Montaser-Kouhsari L, Carrasco M, Heeger DJ. When size matters: attention affects performance by contrast or response gain. Nat Neurosci 13: 1554–1559, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. J Neurosci 30: 11096–11103, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Di Lollo V, McDonald JJ. Target and distractor processing in visual search: decomposition of the N2pc. OPAM Proceedings-Visual Cognition 16: 90–143, 2008. [Google Scholar]
- Hickey C, Di Lollo V, McDonald JJ. Electrophysiological indices of target and distractor processing in visual search. J Cogn Neurosci 21: 760–775, 2009. [DOI] [PubMed] [Google Scholar]
- Hickey C, McDonald JJ, Theeuwes J. Electrophysiological evidence of the capture of visual attention. J Cogn Neurosci 18: 604–613, 2006. [DOI] [PubMed] [Google Scholar]
- Hickey C, Peelen MV. Neural mechanisms of incentive salience in naturalistic human vision. Neuron 85: 512–518, 2015. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Yamamoto S, Yasuda M, Kim HF. Why skill matters. Trends Cogn Sci 17: 434–441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA 95: 781–787, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Squires KC, Bauer JW, Lindsay PH. Evoked potential correlates of auditory signal detection. Science 172: 1357–1360, 1971. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci 353: 1257–1270, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz TS, Choi WY, Horvitz JC, Côté LJ, Mangels JA. Visual search deficits in Parkinson's disease are attenuated by bottom-up salience and top-down information. Neuropsychologia 44: 1962–1977, 2006. [DOI] [PubMed] [Google Scholar]
- Huber J, Payne JW, Puto C. Adding asymmetrically dominated alternatives: violations of regularity and the similarity hypothesis. J Consum Res 9: 90–98, 1982. [Google Scholar]
- Hunt LT, Dolan RJ, Behrens TE. Hierarchical competitions subserving multi-attribute choice. Nat Neurosci 17: 1613–1622, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itthipuripat S, Ester EF, Deering S, Serences JT. Sensory gain outperforms efficient readout mechanisms in predicting attention-related improvements in behavior. J Neurosci 34: 13384–13398, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itthipuripat S, Garcia JO, Rungratsameetaweemana N, Sprague TC, Serences JT. Changing the spatial scope of attention alters patterns of neural gain in human cortex. J Neurosci 34: 112–123, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci 2: 194–203, 2001. [DOI] [PubMed] [Google Scholar]
- Johannes S, Muente TF, Heinze HJ, Mangun GR. Luminance and spatial attention effects on early visual processing. Cogn Brain Res 2: 189–205, 1995. [DOI] [PubMed] [Google Scholar]
- Kelly SP, O'Connell RG. Internal and external influences on the rate of sensory evidence accumulation in the human brain. J Neurosci 33: 19434–19441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Van Velzen J, Eimer M. The N2pc component and its links to attention shifts and spatially selective visual processing. Psychophysiology 45: 240–249, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Bollimunta A, Arcizet F, Wang L. Attention as an effect not a cause. Trends Cogn Sci 18: 457–464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Egner T, Woldorff MG. The neural underpinnings of how reward associations can both guide and misguide attention. J Neurosci 31: 9752–9759, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Shomstein S. Reward-based transfer from bottom-up to top-down search tasks. Psychol Sci 25: 466–475, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Glimcher PW. Reward value-based gain control: divisive normalization in parietal cortex. J Neurosci 31: 10627–10639, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Khaw MW, Glimcher PW. Normalization is a general neural mechanism for context-dependent decision making. Proc Natl Acad Sci USA 110: 6139–6144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, Deakin JF. Attentional bias for drug cues in opiate dependence. Psychol Med 30: 169–175, 2000. [DOI] [PubMed] [Google Scholar]
- Luce RD. Individual Choice Behavior: A Theoretical Analysis. New York: Wiley, 1959. [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press, 2005. [Google Scholar]
- Luck SJ, Girelli M, McDermott M, Ford M. Bridging the gap between monkey neurophysiology and human perception: an ambiguity resolution theory of visual selective attention. Cogn Psychol 33: 64–87, 1997. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalogr Clin Neurophysiol 75: 528–542, 1990. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology 31: 291–308, 1994. [DOI] [PubMed] [Google Scholar]
- MacLean MH, Giesbrecht B. Irrelevant reward and selection histories have different influences on task-relevant attentional selection. Atten Percept Psychophys 22: 222–223, 2015a. [DOI] [PubMed] [Google Scholar]
- MacLean MH, Giesbrecht B. Neural evidence reveals the rapid effects of reward history on selective attention. Brain Res 1606: 86–94, 2015b. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Filoteo JV, Delis DC, Salmon DP. Visual selective attention deficits in patients with Parkinson's disease: a quantitative model-based approach. Neuropsychology 10: 197–218, 1996. [Google Scholar]
- Makeig S, Bell A, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. Adv Neural Inf Process Syst 8: 222–223, 1995. [Google Scholar]
- Mangun GR, Buck LA. Sustained visual-spatial attention produces costs and benefits in response time and evoked neural activity. Neuropsychologia 36: 189–200, 1998. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. The spatial allocation of visual attention as indexed by event-related brain potentials. Hum Factors 29: 195–211, 1987. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Spatial gradients of visual attention: behavioral and electrophysiological evidence. Electroencephalogr Clin Neurophysiol 70: 417–428, 1988. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Allocation of visual attention to spatial locations: tradeoff functions for event-related brain potentials and detection performance. Percept Psychophys 47: 532–550, 1990. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol Hum Percept Perform 17: 1057–1074, 1991. [DOI] [PubMed] [Google Scholar]
- Nakano K, Hasegawa Y, Tokushige A, Nakagawa S, Kayahara T, Mizuno N. Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the Japanese monkey, Macaca fuscata. Brain Res 537: 54–68, 1990. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jäncke L, Tempelmann C, Hinrichs H, Heinze HJ. Delayed striate cortical activation during spatial attention. Neuron 35: 575–587, 2002. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Kelly SP. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat Neurosci 15: 1729–1735, 2012. [DOI] [PubMed] [Google Scholar]
- Peck CJ, Lau B, Salzman CD. The primate amygdala combines information about space and value. Nat Neurosci 16: 340–348, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. Uncertainty explains many aspects of visual contrast detection and discrimination. J Opt Soc Am A 2: 1508–1532, 1985. [DOI] [PubMed] [Google Scholar]
- Persichetti AS, Aguirre GK, Thompson-Schill SL. Value is in the eye of the beholder: early visual cortex codes monetary value of objects during a diverted attention task. J Cogn Neurosci 27: 893–901, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Zeng Q, Ding C, Li H. Neural correlates of reward-driven attentional capture in visual search. Brain Res 1532: 32–43, 2013. [DOI] [PubMed] [Google Scholar]
- Rabinowitz NC, Willmore BD, Schnupp JW, King AJ. Contrast gain control in auditory cortex. Neuron 70: 1178–1191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Clithero JA. Value normalization in decision making: theory and evidence. Curr Opin Neurobiol 22: 970–981, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud A, Masson GS, Chavane F. Dynamics of local input normalization result from balanced short- and long-range intracortical interactions in area V1. J Neurosci 32: 12558–12569, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci 19: 1736–1753, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron 61: 168–185, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson PA. Foundations of Economic Analysis. Cambridge, MA: Harvard Univ. Press, 1947. [Google Scholar]
- San Martín R, Appelbaum LG, Huettel SA, Woldorff MG. Cortical brain activity reflecting attentional biasing toward reward-predicting cues covaries with economic decision-making performance. Cereb Cortex (August 19, 2014). doi: 10.1093/cercor/bhu160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer AM, Muller T, Yeung N, Waszak F. Reward activates stimulus-specific and task-dependent representations in visual association cortices. J Neurosci 34: 15610–15620, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 5: 776–794, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT. Value-based modulations in human visual cortex. Neuron 60: 1169–1181, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Saproo S. Population response profiles in early visual cortex are biased in favor of more valuable stimuli. J Neurophysiol 104: 76–87, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cereb Cortex 17: 284–293, 2007. [DOI] [PubMed] [Google Scholar]
- Shafir S, Waite TA, Smith BH. Context-dependent violations of rational choice in honeybees (Apis mellifera) and gray jays (Perisoreus canadensis). Behav Ecol Sociobiol 51: 180–187, 2002. [Google Scholar]
- Shenhav A, Straccia MA, Cohen JD, Botvinick MM. Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat Neurosci 17: 1249–1254, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson I. Choice based on reasons: the case of attraction and compromise effects. J Consum Res 16: 158–174, 1989. [Google Scholar]
- Sprague TC, Serences JT. Attention modulates spatial priority maps in the human occipital, parietal and frontal cortices. Nat Neurosci 16: 1879–1887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires KC, Hillyard SA, Lindsay PH. Cortical potentials evoked by confirming and disconfirming feedback following an auditory discrimination. Percept Psychophys 13: 25–31, 1973. [Google Scholar]
- Squires KC, Squires NK, Hillyard SA. Vertex evoked potentials in a rating-scale detection task relation to signal probability. Behav Biol 3: 21–34, 1975a. [DOI] [PubMed] [Google Scholar]
- Squires KC, Squires NK, Hillyard SA. Decision-related cortical potentials during an auditory signal detection task with cued observation intervals. J Exp Psychol Hum Percept Perform 1: 268–279, 1975b. [DOI] [PubMed] [Google Scholar]
- Stănişor L, van der Togt C, Pennartz CM, Roelfsema PR. A unified selection signal for attention and reward in primary visual cortex. Proc Natl Acad Sci USA 110: 9136–9141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DW, Krebs JR. Foraging Theory. Princeton, NJ: Princeton Univ. Press, 1986. [Google Scholar]
- Stormark KM, Field NP, Hugdahl K, Horowitz M. Selective processing of visual alcohol cues in abstinent alcoholics: an approach-avoidance conflict? Addict Behav 22: 509–519, 1997. [DOI] [PubMed] [Google Scholar]
- Störmer VS, McDonald JJ, Hillyard SA. Cross-modal cueing of attention alters appearance and early cortical processing of visual stimuli. Proc Natl Acad Sci USA 106: 22456–22461, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JJ, Wade AR, Norcia AM. Dynamics of normalization underlying masking in human visual cortex. J Neurosci 32: 2783–2789, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis S, Hillyard SA. Visual evoked potentials and selective attention to points in space. Percept Psychophys 22: 54–62, 1977. [Google Scholar]
- Wiener M, Thompson JC. Repetition enhancement and memory effects for duration. Neuroimage 113: 268–278, 2015. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Fox PT, Matzke M, Lancaster JL, Veeraswamy S, Zamarripa F, Seabolt M, Glass T, Gao JH, Martin CC, Jerabek P. Retinotopic organization of early visual spatial attention effects as revealed by PET and ERPs. Hum Brain Mapp 5: 280–286, 1997. [DOI] [PubMed] [Google Scholar]
- Woodman G, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature 400: 867–869, 1999. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Arita JT, Luck SJ. A cuing study of the N2pc component: an index of attentional deployment to objects rather than spatial locations. Brain Res 1297: 101–111, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer U, Itthipanyanan S, Grent-'t-Jong T, Woldorff MG. The electrophysiological time course of the interaction of stimulus conflict and the multisensory spread of attention. Eur J Neurosci 31: 1744–1754, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolan D, Cox DD, DiCarlo JJ. Multiple object response normalization in monkey inferotemporal cortex. J Neurosci 25: 8150–8164, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Amygdalostriatal projections in the neurocircuitry for motivation: a neuroanatomical thread through the career of Ann Kelley. Neurosci Biobehav Rev 37: 1932–1945, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]