Abstract
Obesity is a worldwide health problem that has reached epidemic proportions. To ameliorate this problem, one approach is the use of appetite suppressants. These compounds are frequently amphetamine congeners such as diethylpropion (DEP), phentermine (PHEN), and bupropion (BUP), whose effects are mediated through serotonin, norepinephrine, and dopaminergic pathways. The nucleus accumbens (NAc) shell receives dopaminergic inputs and is involved in feeding and motor activity. However, little is known about how appetite suppressants modulate its activity. Therefore, we characterized behavioral and neuronal NAc shell responses to short-term treatments of DEP, PHEN, and BUP. These compounds caused a transient decrease in weight and food intake while increasing locomotion, stereotypy, and insomnia. They evoked a large inhibitory imbalance in NAc shell spiking activity that correlated with the onset of locomotion and stereotypy. Analysis of the local field potentials (LFPs) showed that all three drugs modulated beta, theta, and delta oscillations. These oscillations do not reflect an aversive-malaise brain state, as ascertained from taste aversion experiments, but tracked both the initial decrease in weight and food intake and the subsequent tolerance to these drugs. Importantly, the appetite suppressant-induced weight loss and locomotion were markedly reduced by intragastric (and intra-NAc shell) infusions of dopamine antagonists SCH-23390 (D1 receptor) or raclopride (D2 receptor). Furthermore, both antagonists attenuated appetite suppressant-induced LFP oscillations and partially restored the imbalance in NAc shell activity. These data reveal that appetite suppressant-induced behavioral and neuronal activity recorded in the NAc shell depend, to various extents, on dopaminergic activation and thus point to an important role for D1/D2-like receptors (in the NAc shell) in the mechanism of action for these anorexic compounds.
Keywords: nucleus accumbens, appetite, amphetamine, anorexigenic, dopamine
obesity is a worldwide epidemic that predisposes individuals to a myriad of adverse health consequences (McPherson 2014). Although diet and exercise are the primary treatments for obesity, these activities are often supplemented by the use of appetite suppressants (Wilbert et al. 2011). Mild amphetamine analogs such as diethylpropion (DEP) and phentermine (PHEN) are among the most commonly used appetite suppressants (Hampp et al. 2013). Bupropion (BUP), an analog of DEP, also produces weight loss (Gadde and Xiong 2007). The mechanisms by which these pro-drugs produce their anorexic effect are complex, because they modulate the concentrations of serotonin, norepinephrine, and dopamine (Baumann et al. 2000) and evoke responses in various cortical and subcortical areas involved in feeding (Safta et al. 1976).
In humans the above-named amphetamine congeners produce the sensation of fullness (serotonin) and increase agitation, insomnia, and energy expenditure (norepinephrine), as well as affecting motivation and reward pathways (dopamine) (Moyers 2005; Offermeier and Potgieter 1972). To shed some light on their action, we measured in rats how repeated treatments of these three anorexigenic compounds alter feeding and motor behaviors and also how these behaviors are related to suppressant-evoked responses from the nucleus accumbens (NAc) shell, an area that receives dopaminergic input and is involved in reward, feeding, and locomotion (Carlezon and Thomas 2009; McGinty et al. 2013; Mogenson et al. 1980; Tellez et al. 2012).
Previous studies in rats found that intraperitoneal application of appetite suppressants increase dopamine levels in the NAc shell (Opacka-Juffry et al. 2014), increase locomotion, and, in the case of DEP, produce stereotypy (Reimer et al. 1995). The changes in locomotive behaviors have been attributed to the activation of dopamine D1-like and D2-like receptors (Janhunen et al. 2013). With regard to feeding, dopamine (DA) has been shown to play an important role (Costa et al. 2007; Rossi and Yin 2015). For example, alteration of dopamine levels (outside of their physiological range), such as a systemic increases or decreases, impede feeding behavior (Szczypka et al. 1999). Furthermore, after ingestion of food, obese persons exhibit a decrease of DA release in their NAc shell compared with that released by lean subjects (Wang et al. 2014). Moreover, compared with their lean littermates, obese rats exhibit a decreased number of D2 receptors (D2Rs) in their ventral striatum (Johnson and Kenny 2010). Likewise, morbidly obese persons have fewer D2Rs in their ventral striatum than persons with a normal body mass index (BMI) (Wang et al. 2009). Although these data point to an important role of DA in feeding, it is unknown to what extent the above-mentioned anorectic drugs produce their behavioral and neuronal effects via activation of DA receptors in the NAc shell.
In this study, we found that treatment with DEP, PHEN, or BUP transiently decreased food intake and increased weight loss, locomotion, and stereotypy. Single-unit NAc shell recordings showed that these appetite suppressants produced a net inhibitory imbalance in the various neuron types that tracked onset of locomotion and, stereotypy. Also, changes in the local field potential (LFP) evoked by the appetite suppressants tracked changes in weight loss and food intake (tolerance). A most relevant finding was that inhibition of D1R or D2Rs both intragastrically and infused in the NAc shell reversed to various extents both the DEP-induced behavioral and electrophysiological responses. These data demonstrate that D1-like and D2-like receptors are critically important factors in understanding how these anorectic compounds induce weight loss.
MATERIALS AND METHODS
Animals
A total of 147 male Sprague-Dawley rats ∼250–300 g were used for all experiments. Animals were housed individually and had ad libitum access to food and water except when they were removed from their home cages for testing in an operant box for multichannel recordings (see below) or in an open-field arena for locomotion and stereotypy studies. Room temperature was maintained at (21 ± 1°C) on a 12:12-h light-dark cycle (0600-1800). All procedures were approved by the CINVESTAV institutional animal care and use committee.
Drugs and Chemicals
The appetite suppressants diethylpropion hydrochloride (DEP) and phentermine hydrochloride (PHEN) were kindly provided by Productos Medix (Mexico). Bupropion hydrochloride (BUP), R(+)-SCH-23390 hydrochloride (SCH) and S(−)-raclopride (+)-tartrate salt (RAC), cholescystokinin-8 (CCK-8), and lithium chloride (LiCl) were obtained from Sigma-Aldrich. Unless otherwise mentioned, these compounds were dissolved in physiological saline (0.9% NaCl) and administered intraperitoneally for the dose-response effect of DEP on feeding, locomotion, gastric emptiness, and conditioned taste aversion experiments (Figs. 1, 2, and 6A). For all other experiments (Figs. 3–8) they were delivered intragastrically using a catheter (see below) in a volume of 1 ml/kg. Sodium saccharin was obtained from Sigma-Aldrich and was dissolved in water.
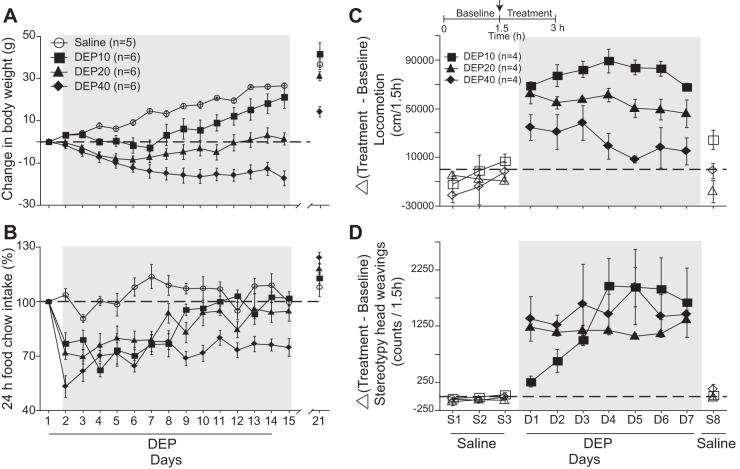
Fig. 1.
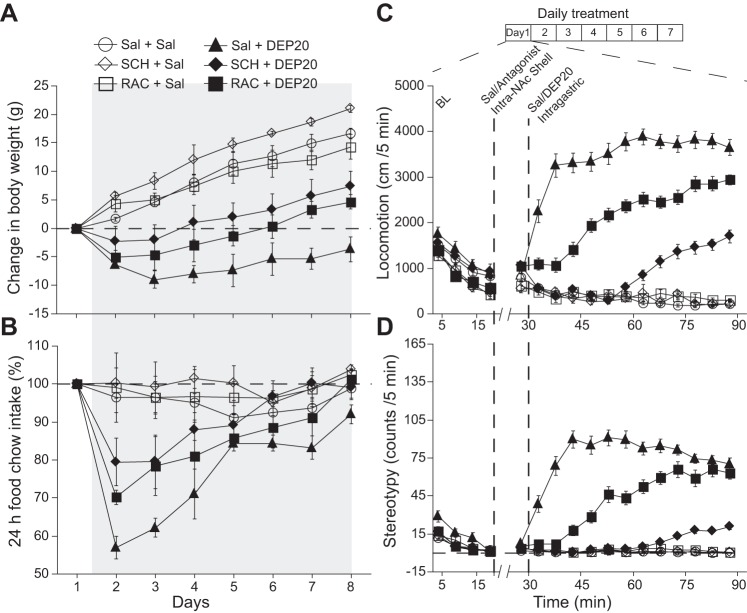
Effects of diethylpropion (DEP) on weight loss, feeding, locomotion, and stereotypic head movements. A: the change in body weight of control rats daily injected intraperitoneally with saline (○) from day 1 to day 14 compared with rats injected with DEP at 10 (■), 20 (▲), and 40 mg/kg (⧫) (hereafter DEP10, DEP20, and DEP40, respectively). Gray shading depicts the change in body weight measured 20 min before each DEP injection. The break in the axis indicates where the treatment was stopped. The horizontal dotted line represents no weight change. B: the DEP-induced change in food intake for the same subjects shown in A. C: the effects of DEP10, DEP20, and DEP40 (Δ, change relative to baseline) on the animal's locomotion (in cm/1.5 h). This protocol consisted of 3 days of saline treatment (S1–S3), 7 days of DEP treatment (D1–D7) and 1 final day of saline treatment (S8). Inset displays the daily protocol. D: the DEP-induced effect on stereotypic head movements (counts/1.5 h). Symbols represent means ± SE, and the shading indicates treatment times.
Fig. 2.
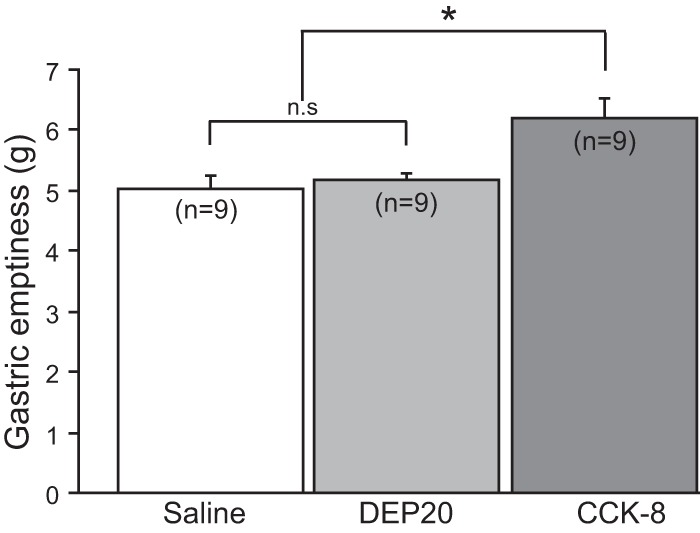
DEP20: a dose that suppresses food intake but does not delay gastric emptying. Graph shows the difference in weight between wet and dry stomach, in g, as a measure of gastric emptiness (see materials and methods for details). Note that DEP20 did not differ from saline, whereas cholescystokinin-8 (CCK-8) delayed gastric emptiness. Data are means ± SE. *P < 0.05; n.s., no significant difference.
Fig. 6.
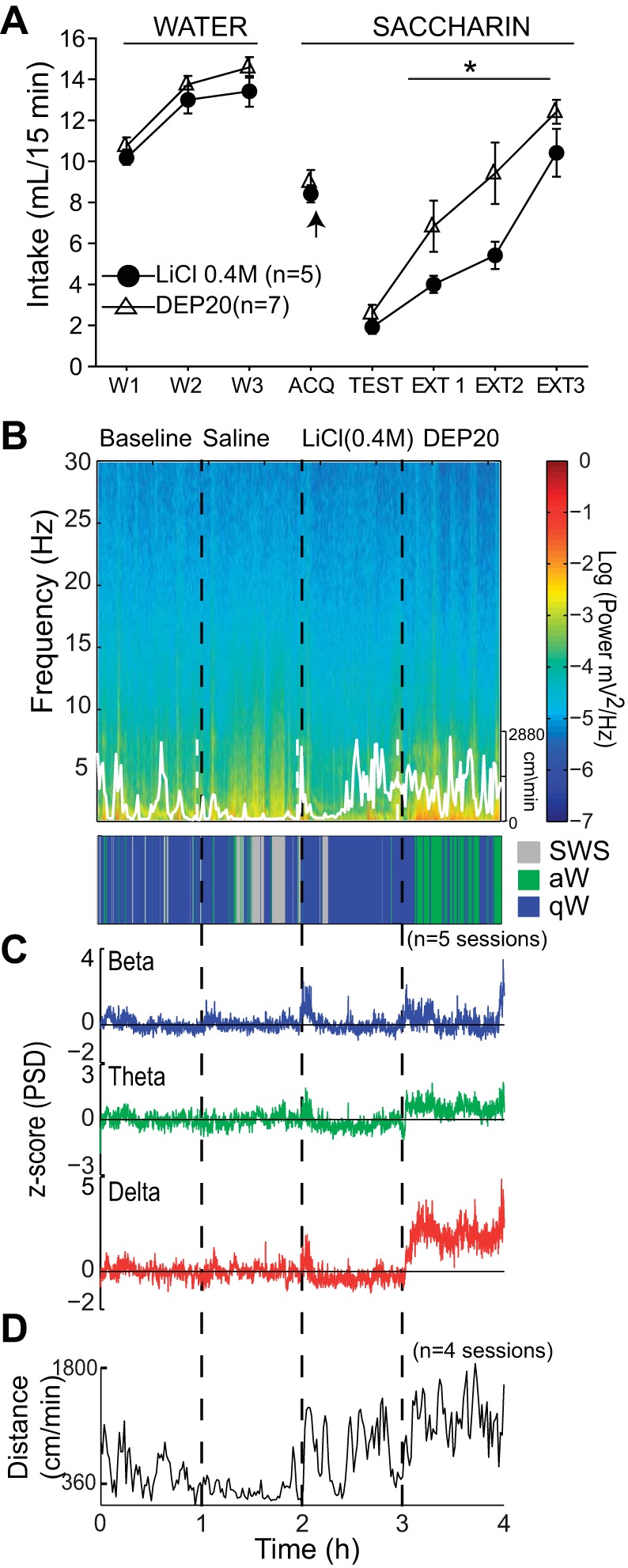
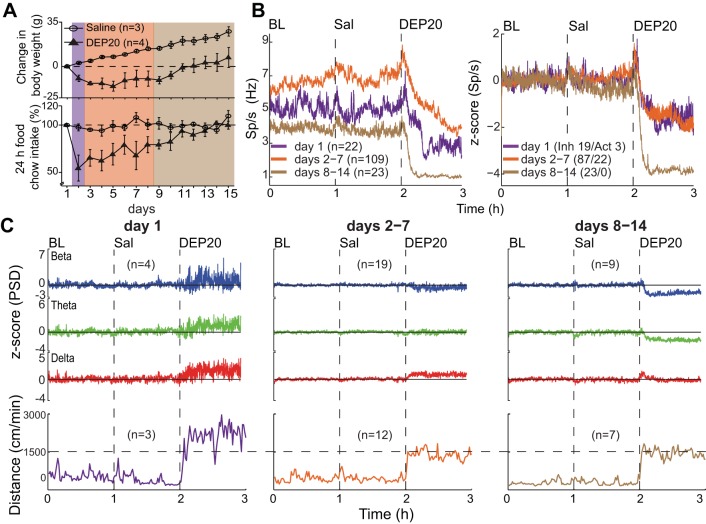
DEP20 induces taste aversion to a novel 0.1% saccharin solution, but DEP20-induced LFP oscillations are unrelated to an aversive malaise brain state. A: the daily 15-min intake (in ml) of water (W1–W3) and sodium saccharin (ACQ, Test, and EXT1–EXT3). The arrow (acquisition day, ACQ) indicates the time of injection of LiCl or DEP20, 15 min after consumption of the novel saccharin. The Test session occurred 72 h after ACQ; note that both groups rejected saccharin at similar levels, but during the 3 extinction days (EXT1–EXT3), DEP20 extinguishes the taste aversion faster than LiCl (*P <0.05, RM ANOVA). B: spectrogram (top) of the NAc shell LFP for the BL, Sal, LiCl (0.4 M), and DEP20 epochs, with corresponding hypnogram (bottom). The white trace shows the animal's locomotor activity (scale on right). C: normalized PSD at the beta (15–30 Hz), theta (6–9 Hz), and delta (1–4 Hz) frequency bands across the experiment. D: average locomotor activity (in cm/min) obtained during each epoch shows that LiCl upsets the animal, resulting in distress movements but not stereotypy (not shown) and exploratory locomotion.
Fig. 3.
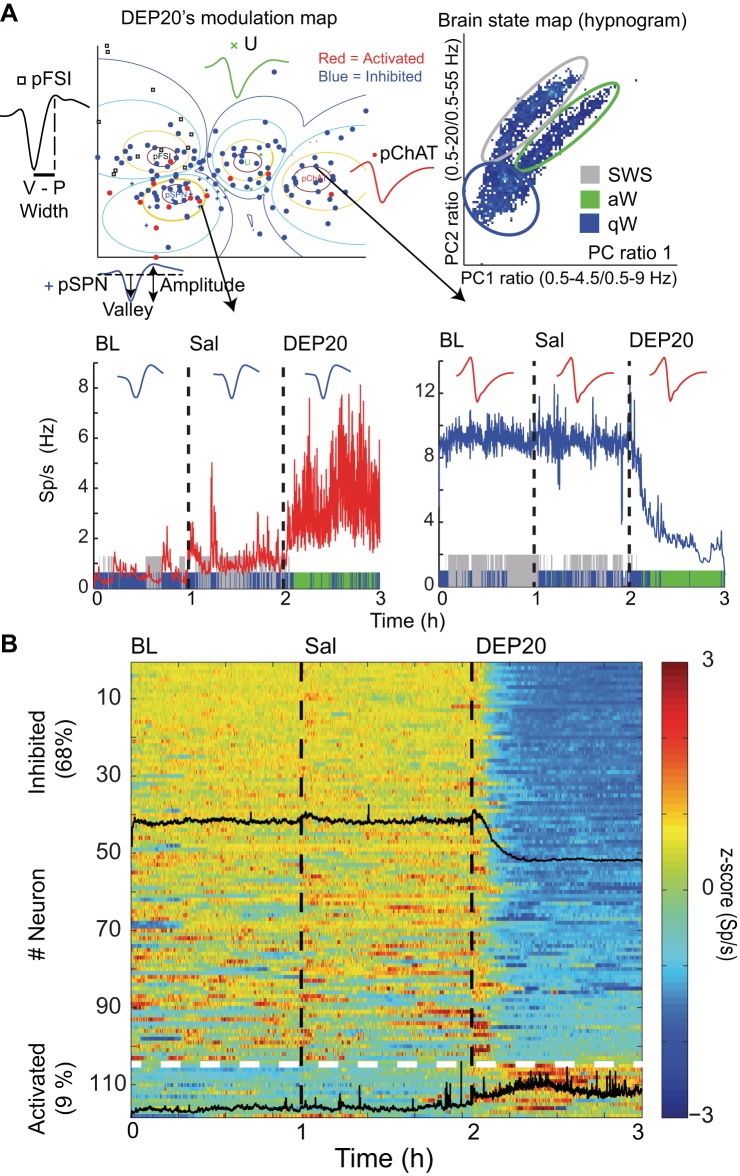
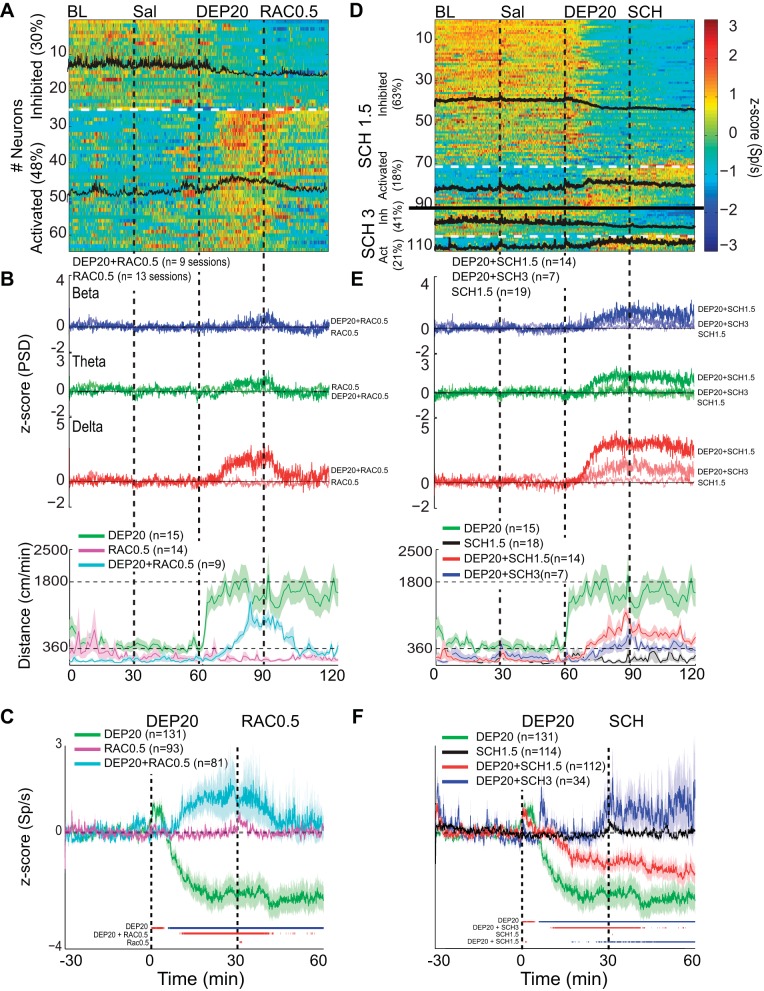
Intragastric infusions of DEP20 modulate nucleus accumbens (NAc) shell spiking activity. A, top left: a modulation map for DEP20 responses was computed with 154 neurons by using a fuzzy cluster algorithm to classify them into 4 putative cell types: pSPN, medium spiny projection neuron (+); pFSI, fast-spiking interneuron (□), pChAT, choline acetyltransferase interneurons (●); and U, unidentified (×) (see Table 1). For each cell type, a representative waveform is given as well as significantly inhibited (blue dots) and activated responses (red dots). V-P is the valley-to-peak width and a waveform illustrating the amplitude. Top right: a brain state map (hypnogram) was computed from the local field potentials (LFPs) in the NAc shell (see Fig. 4A) illustrating the 3 major behavioral states of an animal during the trial: active awake (aW), quiet awake (qW), and slow-wave sleep (SWS) (see Table 3). Each dot represents the principal component (PC) ratio between 2 power ranges for each second of LFP activity. Each dot falling into the gray ellipsoid corresponds to periods when the animals were in SWS, and the blue and green ellipsoids represent periods when the animals were in the qW and aW states, respectively. Bottom left: excitatory changes from a representative pSPN during baseline (BL), saline (Sal), and DEP20 infusion. During the transitions between SWS and qW states, the firing rate transiently increased but dramatically increased after infusion of DEP20. The colors on the abscissa represent blue, gray, and green for the qW, SWS, and aW states, respectively. Bottom right: inhibitory response of a representative pChAT neuron for the BL, Sal, and DEP20 epochs. B: population responses of 118 (of 154) neurons recorded during the BL, Sal, and DEP20 epochs. The activity of each neuron is normalized to its z-score value and is plotted in a color-coded population peristimulus time histogram (PSTH) showing that 68% (n = 104; black overlap line indicates average population response) were inhibited by DEP20 and 9% (n = 14) were activated. The other neurons (23%) were unaffected. The horizontal dotted white line indicates the division between inhibited and activated responses.
Fig. 8.
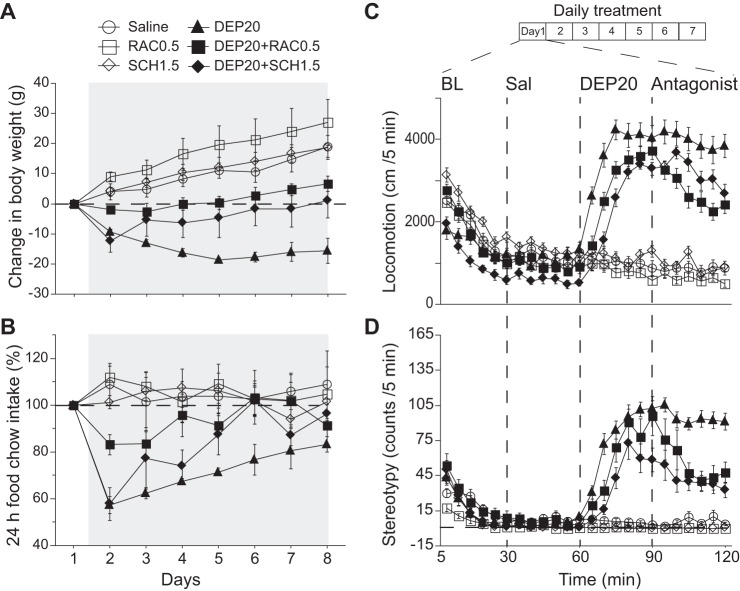
Intragastric D1 dopamine receptor (D1R) antagonist SCH-23390 (SCH1.5) and D2R antagonist raclopride (RAC0.5) attenuate DEP20’s effect on weight loss, food intake, locomotion, and stereotypic head movements. A: the change in body weight during 7 days of treatment with daily intragastric infusions of Sal (○), RAC0.5 (□), SCH1.5 (◇), DEP20 (▲), DEP20+RAC0.5 (■), and DEP20+SCH1.5 (⧫) starting on day 1 and terminating on day 7 (gray shading). The weight loss produced by DEP20 was reduced in the presence of either RAC0.5 or SCH 1.5. Note that neither D2 (□) nor D1 (◇) antagonists alone induced weight loss. B: graph showing the change in 24-h food intake under the same conditions as in A. Infusion of DEP20+RAC0.5 or DEP20+SCH1.5 increased food intake compared with DEP20 alone. C: effect of these DA antagonists on locomotion in the same subjects and grouping as in A measured in an open-field arena. All groups displayed a gradual decay in exploratory activity within 20 min from introduction to the open field (BL). Intragastric infusions were then made at 30-min intervals with saline, at 60 min with DEP20, and at 90 min with and/or without RAC0.5 or SCH1.5. Compared with DEP20 alone, repeated administrations of DEP20+RAC0.5 or DEP20+SCH1.5 decreased the magnitude and delayed the onset of locomotion. D: graph showing the reduction in the stereotypy induced by DEP20+RAC0.5 or DEP20+SCH1.5.
Locomotion and Stereotypy
Measurements of locomotion and stereotypy in an open field.
Locomotion was measured using an RV2 video processor (TDT Systems, Alachua, FL; digital videotaping and real-time tracking). The RV2 video analyzer was placed outside an open-field arena (40-cm length × 40-cm width × 30-cm height) containing a charge-coupled device camera (top view) with specialized software (RVMap software, VGAC machine vision color camera; TDT Systems) to track the animal's position in x and y coordinates. For stereotypy measurements, we focused on head weavings because they were the most readily measurable and quantifiable. We note that other stereotypic behaviors, e.g., licking the walls and wood gnawing behavior, were also observed (mainly in group DEP40; see Supplementary Video 1). (Supplemental material is available online at the Journal of Neurophysiology website.) We did not quantify the other types of stereotypy, because for the dose primarily used in most experiments (DEP20), head weavings were the most prominent stereotypy. For head weavings, three color points (red, green, and blue) were glued to the animal and selected as targets for the nose, head, and body, respectively, and the same apparatus described above was used. In both cases, homemade MATLAB scripts were used to calculate the locomotion (in cm) and stereotypy (e.g., Figs. 1, C and D, 7B, 8C, and 9, C and D). Stereotypy is expressed as the occurrence of an angle more than 45° between the nose and head color mark targets (see Supplementary Video 1).
Fig. 7.
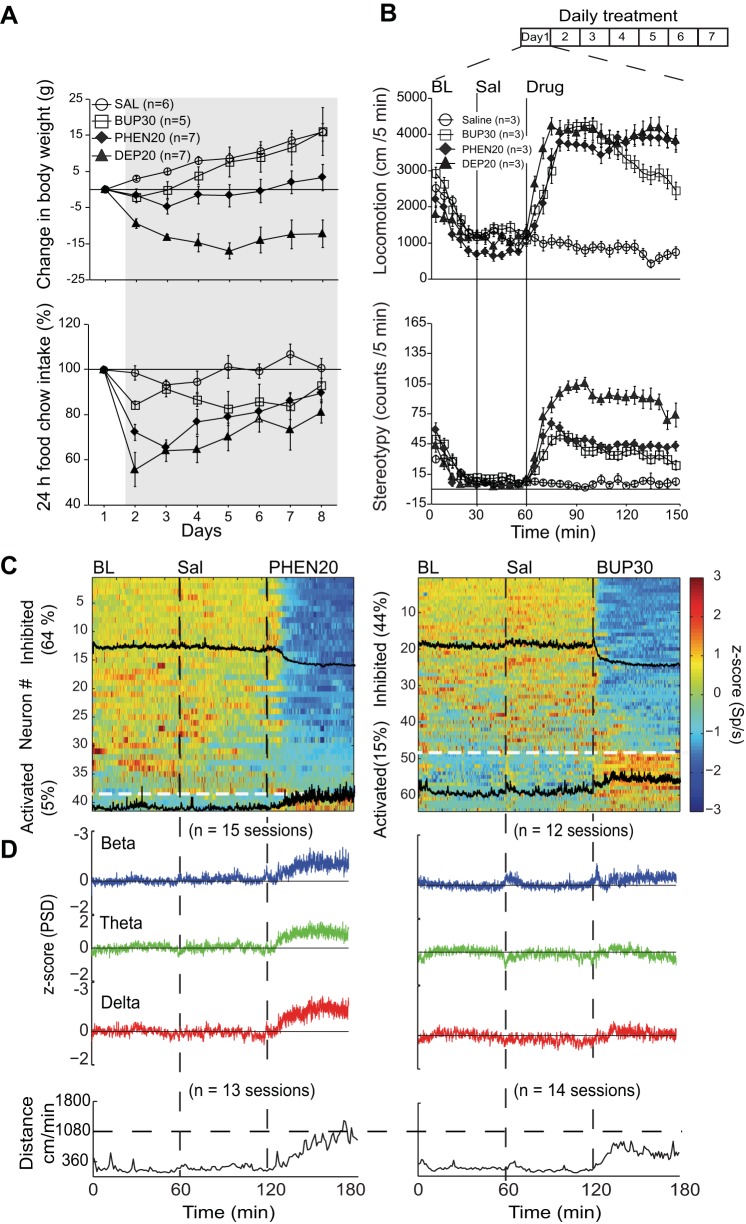
Appetite suppressants phentermine (PHEN) and bupropion (BUP) also modulate appetite, locomotion, and NAc shell activity. A, top: graph showing the change in weight over a 7-day intragastric treatment (gray shading) with saline, PHEN20, BUP30, and DEP20. Relative to saline, the 3 treatments produced a significant weight loss with DEP20 exhibiting the greatest loss. The weight loss with BUP30 was significant for only 2 days. In contrast to DEP20, during treatment, PHEN20- and BUP30-treated animals began to recover their weight. Bottom: for the same animals, the change in 24-h food intake was unmodified with saline infusions but transiently decreased with PHEN20 and DEP20. BUP30 gradually and slightly decreased food intake during treatment. B, top: effect on locomotion of repeated infusions of PHEN20, BUP30, and DEP20 measured in an open-field arena. All groups displayed a gradual decrease in exploratory activity within 20 min from introduction into the open field (BL). Intragastric infusions were then made at 30-min intervals with saline, and at 60 min with the corresponding appetite suppressant. Bottom: quantification of head weavings (stereotypy) caused by these 3 appetite suppressants. Relative to saline, DEP20 induced the largest effect and PHEN20 and BUP30 the smallest. C: color-coded PSTH showing the number of NAc shell neurons that were inhibited or activated by PHEN20 (left) and BUP30 (right). Each neuron was normalized to z-score values (see materials and methods). The black tracings are the mean PSTH of the inhibited and activated responses. The horizontal line separates these populations. D, top: normalized PSDs of mean LFPs at beta, theta, and delta frequencies during BL, Sal, and PHEN20 (left) and BUP30 epochs (right). Both compounds increased the LFP oscillations for the 3 frequency bands. Bottom: PHEN20 and BUP30 both increased locomotion, albeit with a delay.
Fig. 9.
Intra-NAc shell infusion of either D1R (SCH) or D2R (RAC) antagonists attenuated the effect of intragastric infusion of DEP20-induced effects on weight loss, food intake, locomotion, and stereotypic head movements. A: the change in body weight across 7 days of treatment. Note that all groups received 2 injections: one directly in the NAc shell (Sal, SCH, or RAC) and a second one intragastrically (Sal or DEP20). Thus the group Sal+Sal received saline (intra-NAc) + saline (intragastric), whereas the group Sal+DEP20 received saline (Intra-NAc) + DEP20 (intragastric), and so on for all other groups. B: graph showing the change over 7 days in chow food intake per 24 h with the same protocol as in A. C: effect of SCH and RAC antagonists on locomotion measured in the open-field arena, in the same subjects shown in A. At 20 min, animals were briefly removed from the open field and received an intra-NAc infusion; note the interruption in the x-axis at 20–25 min. At 30 min, all animals received the corresponding intragastric infusion. D: graph plotting head-weaving stereotypy under the same conditions as in C.
Measurement of locomotion during multichannel recordings from the NAc shell.
During multichannel recordings (see below), the position of the rat's center of mass was tracked using Ethovision XT10 (Noldus Information Technology, Wageningen, The Netherlands) and reported in centimeters per minute. Because this method only tracks one body point (the center of mass), stereotypy could not be measured during these recordings. In addition, only webcam videos in which locomotion could be clearly decoded were included in the analysis (e.g., Figs. 4D, 5C, 6D, 7D, and 10B). Onset of locomotion was computed by using a cumsum statistic (Gutierrez et al. 2006) to obtain the first bin after drug infusion where locomotion significantly increased above locomotion in the saline epoch.
Fig. 4.
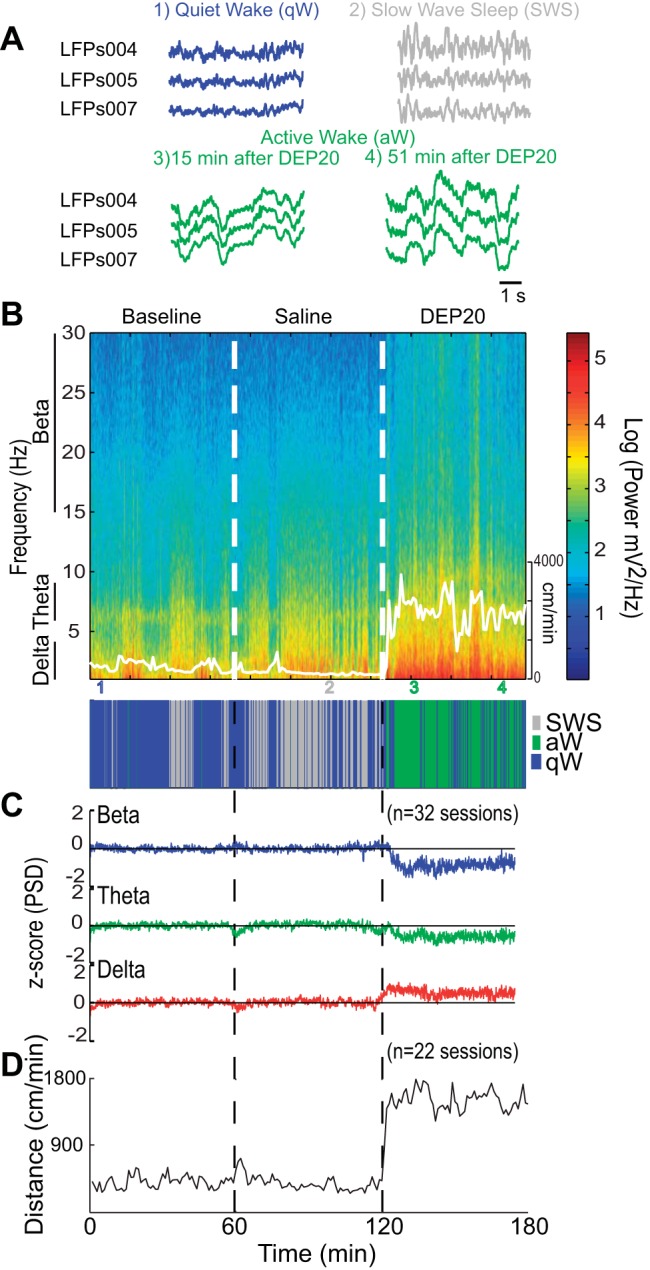
DEP20 increases oscillations in the NAc shell. A: raw traces of LFPs recorded while an animal was in the qW (blue), SWS (gray), and aW states (green), with the latter shown 15 and 51 min after intragastric infusion of DEP20. B, top: spectrogram of the LFPs taken from 1 to 30 Hz for the BL, Sal, and DEP20 epochs (recorded on day 1). The animal's locomotor activity is shown as a thin white trace (scale on right). Note that after DEP20, rats are primarily in the aW state (green; see Table 3). The delta power (1–4 Hz) increases during SWS (bottom) and exhibits larger amplitude after DEP20. The colored numbers indicate the times at which the raw LFP traces were obtained (plotted in A). C: normalized and smoothed power spectral densities (PSDs) at the beta (15–30 Hz), theta (6–9 Hz), and delta (1–4 Hz) frequency bands obtained for BL, Sal, and DEP20 epochs across the entire 14-day treatment period. During the DEP20 epoch, delta oscillations increased whereas beta and theta oscillations decreased. D: locomotor activity measurements (cm/min) obtained during BL, Sal, and DEP20 epochs. These data show that DEP20 clearly increases locomotion (see also Table 6).
Fig. 5.
Behavioral and neuronal changes of NAc shell activity across 14 days of DEP20 treatment. A: plots of weight loss and food intake over 14-day treatments of saline or DEP20. With intragastric saline the animal's weight monotonically increased across days, whereas for DEP20, after a decrease that lasted until day 9, the animals gained weight at the same rate as the saline-treated animals. The DEP20 group transiently reduced food intake and then increased it with increasing treatment days. Purple shading indicates day 1, orange days 2–7, and brown days 8–14. B, left: a plot of the neuronal population changes observed across repeated intragastric DEP20 infusions. The colors codes are the same as in A. Relative to treatment day 1, during days 2–7, DEP20 increased global firing rates during BL and Sal epochs, whereas after DEP20 for days 8–14, the activity decreased. For days 8–14 relative to baseline, the activity is lower in all 3 epochs. Right, normalized activity in the BL, Sal, and DEP20 epochs. The latter reveals a much larger inhibition during days 8–14. The ratios of inhibitory to activated responses are also presented. C, top: changes in the LFP PSDs at beta, theta, and delta frequencies across days. All DEP20-evoked oscillations were positive at day 1, but all decreased across days, with the beta and delta oscillations becoming negative at days 8–14. Bottom, locomotion evoked by DEP20 across days.
Fig. 10.
Intragastric dopamine D1R and D2R antagonists reverse the DEP20-induced inhibition/activation asymmetry in the rat's NAc shell. A: a plot of the activity of 64 (of 81) NAc shell neurons that were significantly inhibited (30%) or activated (48%) by DEP20 across 7 days of chronic treatment with RAC0.5. Each of the 4 epochs was 30 min long. Note that compared with DEP20 alone (only using neurons recorded over 7 days from Fig. 3B), the percentage of neurons inhibited during the DEP20 epoch in the presence of chronic treatments with RAC0.5 decreased and the percentage activated increased. The black traces are the mean activity of the 2 respective categories. B, top: the grand average of 9 experiments showing the normalized PSD at delta frequency from the same experiments shown in A over all days. Infusion of RAC0.5 greatly diminished the changes in all DEP20-induced oscillations. Note that infusion of RAC0.5 alone (thin traces) did not change LFP oscillations. Bottom, locomotion increases evoked by DEP20 were also decreased by RAC0.5 (cyan trace), whereas RAC0.5 alone did not increase locomotion (pink trace). C: a plot of the normalized global population responses of all neurons recorded (n = 131) with DEP20 alone (green; same data as in Fig. 3, aligned to DEP injection time = 0 min) vs. all 81 neurons recorded with DEP20 and chronic treatment with RAC0.5 (cyan trace over same treatment days, DEP20+RAC0.5). Note that after the infusion of RAC0.5, the population firing rate returned to near baseline levels. Finally, the infusion of RAC0.5 alone did not produce any inhibitory imbalance (pink trace). The colored lines at bottom indicate the bins (1-min resolution) with significant increases (red) or decreases (blue) of population activity relative to saline firing rates (time interval: −30 to 0 min). D: results showing repeated administration of two doses of D1R antagonist (SCH1.5 and SCH 3) infused after DEP20. The firing rate modulation of 91 out of 112 (and 21 out of 34) single neurons was recorded in the NAc shell during 30-min BL, Sal, DEP20+SCH1.5, and DEP20+SCH3 infusions, respectively. Under repeated administration SCH, DEP20 did not produce a robust spiking inhibition [SCH1.5 (63%) and SCH3 (41%) compared with DEP20 alone (63%, 82/131)]; rather, it increased its activation [SCH1.5 (18%) and SCH3 (21%) compared with DEP20 alone (11%, 14/131)]. E: a plot of the LFP PSD (top) and locomotor activity (bottom) induced by SCH1.5 (red trace), SCH3 (blue trace), and SCH1.5 alone (black trace). D1R antagonist attenuated in a dose-dependent manner the DEP20-induced delta oscillations and delayed the onset and diminished the magnitude of locomotion evoked by DEP20. Note all oscillations are positive. The infusion of SCH1.5 alone (thinnest lines) did not change LFP oscillations, with values around 0 z-score. F: the average firing rate of all neurons recorded under repeated treatment of DEP20 followed by SCH1.5 (red trace) and SCH3 (blue trace) and treatment of SCH1.5 alone (black trace). Note that SCH in a dose-dependent manner reversed the inhibition/activation imbalance induced by DEP20.
Behavioral Procedures
Dose-response effect of DEP on weight-loss and food intake.
To determine whether DEP affects body weight and food intake in a dose-response manner, we assigned 23 lean male rats to 4 groups [saline 0.9% (n = 5), DEP10 (n = 6), DEP20 (n = 6), and DEP40 (n = 6), where numbers indicate doses of 10, 20, and 40 mg/kg, respectively] that were provided with 100 g per day of standard rat chow (Purina Mexico) and ad libitum water. After 7 days (to obtain a stable baseline; data not shown), rats received a daily intraperitoneal injection of one of the four treatments for 14 consecutive days (days 1–14), followed by 7 days (days 15–21) of recovery without treatment (withdrawal period). These behavioral experiments were carried out between 1700 and 1800 (i.e., commencement of their active dark phase). Their body weight and 24-h food intake were measured daily 20 min before an intraperitoneal injection of the corresponding treatment was administered (see Fig. 1, A and B).
Dose-response effect of DEP on locomotor activity and stereotypy in an open-field arena.
In a different group of rats, we evaluated in a dose-dependent manner whether intraperitoneal injections of DEP affect locomotion and stereotypy. For this experiment, 12 rats were assigned to 3 groups (DEP10, DEP20, and DEP40; n = 4 per group). All experiments were conducted during the inactive light period. Locomotion and stereotypy (head weavings indicated by head oscillations) were measured for 90 min before (baseline) and after injection by the RV2 video processor described above (see Fig. 1, C and D). These experiments lasted 11 days, of which 3 days (S1–S3; Fig. 1C) were for habituation with only injection of saline. For the next 7 days (D1–D7), rats received daily injections of DEP followed by 1 day (S8) of saline injection. The ambulation and the stereotypic head movements were measured across the days as a delta (Δ) by subtracting the values of treatment by the values observed at baseline (see Fig. 1C, inset).
Effect of DEP20 on gastric emptiness.
We assigned 27 naive rats into 3 equally sized groups. Briefly, animals were water and food deprived for 24 h, at which time they received a single intraperitoneal injection of either saline, DEP20, or 2 μg/kg CCK-8. Then, 15 min after one of the injections, animals received a 5-ml oral bolus of Ensure chocolate flavor via an oral gavage needle, following the protocol described by Conover et al. (1988). Finally, after 10 min, animals were killed and their stomachs were isolated with both sphincters occluded with silk threads. The wet stomach was weighed and inverted, rinsed with water, and placed in an oven at 45°C for 24 h. The dry stomach was weighed, and the difference in weight between wet and dry measurements was used as an indicator of gastric emptiness (see Fig. 2).
Behavioral effect of DEP20 as unconditioned aversive stimuli.
Twelve naive rats were randomly divided into two groups, LiCl (n = 5) and DEP20 (n = 7), following the protocol described by Gutierrez et al. (2003). Briefly, animals were water deprived for 23.75 h, and for 3 days they had a daily period of 15-min access to water (W1–W3; see Fig. 6A). On day 4 (ACQ, acquisition day), animals were allowed to drink a novel saccharin solution (0.1% wt/vol) for 15 min that served as a conditioned stimulus, and 15 min later rats received a single intraperitoneal injection of either 0.4 M LiCl (7.5 ml/kg) or DEP20 (1 ml/kg) that served as an unconditioned aversive stimulus. On days 5 and 6, animals were given only water to allow recovery from gastric malaise. On the Test day (day 7) rats were presented with 15 min (1730–1745) of 0.1% (wt/vol) saccharin followed by another 15 min of water (1747–1802). Following the Test session, rats received 3 more extinction days (EXT1–EXT3). Intake (ml) was measured at 0.5-ml resolution and displayed in Fig. 6A.
Locomotion and stereotypy induced by DEP20, PHEN20, and BUP30.
In the open-field arena described above for a new cohort of rats (n = 3 per group), locomotion and stereotypy (head weavings) were measured over 7 days of repeated intragastric infusions of saline, DEP20, PHEN20, and BUP30 (see Fig. 7B). For implantation of the intragastric catheter, we followed the protocol described by Lukas and Moreton (1979) and Ueno et al. (2012). Briefly, the hair on the mid-abdomen and dorsal neck areas was clipped and cleaned. To expose the stomach, a midline incision of 2–3 cm was made in the abdomen. With a 30-gauge needle we punctured the fundus and inserted 1 cm of the catheter (14-cm length and 0.76-mm diameter of Silastic laboratory tubing; Dow Corning, Midland, MI) with 1 sterile rubber band brace glued at 1 cm from the tip. We then joined the catheter's rubber band and the rat's stomach using a nonabsorbable silk suture (USP 6-0; Atramat, Mexico City, Mexico). We passed the opposite end of the catheter subcutaneously until it exited the dorsal neck incision. Finally, the peritoneal cavity was carefully stitched together using chromic catgut, whereas the abdominal and dorsal incision was stitched together using a silk suture. After the animals were permitted to recover, at least 1 wk from surgery before starting the treatment, they were habituated to the open field for 3 days. In each experiment, a tube (30-cm length) was connected to the catheter and to a syringe that was manually activated outside of the behavioral box. The catheter patency was always confirmed at the end of all experiments before perfusion of the animal.
Effect of intragastric D1 (SCH-23390) and D2 (raclopride) antagonists on DEP20’s effect on weight loss, food intake, locomotion, and stereotypy.
To understand the participation of D1Rs and D2Rs on DEP's behavioral effects, we tested and compared four groups, each comprising three animals: saline, DEP20, DEP20+RAC0.5, and DEP20+SCH1.5, where the numbers are doses in mg/kg. Briefly, rats were habituated to the open-field arena for 2 days (data not shown), and then each group received a daily intragastric infusion for 7 days during which their locomotion and stereotypy were measured. We initially used this method because intragastric infusions of appetite suppressants mimic how humans take these compounds. As shown below, we also infused these antagonists directly into the NAc shell. To determine whether DEP-induced neuronal modulation could be reversed by intragastric infusion of dopamine receptor antagonists, we first infused DEP20, followed 30 min later by one of the two antagonists (see Fig. 8). The antagonist concentrations were selected on the basis of previous studies showing that RAC at a systemic dose of 0.5 mg/kg antagonized the locomotion effects induced by amphetamine or methamphetamine (Broening et al. 2005; Janhunen et al. 2013; Wright et al. 2013). Likewise, we initially tested using a high dose of SCH (3 mg/kg), because this dose is used to prevent death from an overdose of amphetamine (Derlet et al. 1990). However, over a 7-day test period animals generally did not tolerate this dose, and thus we could record NAc shell activity in only one animal as shown in Fig. 10. Consequently, we used SCH at 1.5 mg/kg for the 7-day treatment period.
As a control, we also tested whether these DA antagonists by themselves produce any behavioral effects. This was accomplished by using two additional control groups with each one receiving either only RAC0.5 or SCH1.5. Specifically, rats were introduced on the open-field arena over 30 min as baseline, followed by an intragastric injection of saline at 30 min, and at 60 min (instead of DEP20) they received an injection of either RAC0.5 or SCH1.5 (see Fig. 8C). Finally, at 90 min, they received a second infusion of saline.
Intra-NAc Shell Infusions of DA Antagonists
Surgery and intra-NAc injection.
Intra-NAc shell infusions of DA antagonists were performed to directly determine their effects on feeding and locomotion. Animals were implanted with an intragastric catheter, as described above, and placed in a rat stereotaxic apparatus. For the bilateral intra-NAc shell infusions, two holes were drilled at AP +1.4 mm, L ±1 mm relative to Bregma. The tips of a stainless steel guide cannula aimed at the NAc shell were bilaterally inserted at 6.5 mm DV from Bregma. Two screws served as anchors in the skull bone, and the whole assembly was cemented to the skull with dental acrylic. A stylus was inserted into the cannula (to prevent clogging) and was removed before each injection. Rats were allowed to recover from surgery for at least 7 days. Microinjections were given via a 30-gauge stainless steel injector 1.0 mm larger than the tip of the guide cannula, connected via Teflon tubing to a 10-μl glass microsyringe (Hamilton 80366) that was attached to a microinfusion pump (KDS200 series; KD Scientific). A total volume of 0.5 μl (0.33 μl/min) per hemisphere was infused daily across 7 days of treatment. The injector was left into the guide cannula 1 additional minute to allow complete effusion (Gutierrez et al. 2003).
Effect of D1 (SCH) and D2 (RAC) antagonists infused in the NAc shell on DEP20’s effect on weight loss, food intake, locomotion, and stereotypy.
To investigate whether D1 and/or D2 receptors in the NAc shell mediate some (or all) of the pharmacological effects induced by intragastric DEP20, we used 18 animals randomly sorted into 6 groups (n = 3) (see Fig. 9). After 2 days of habituation (data not shown), each animal was placed in the open field for 90 min, and their locomotor activity was analyzed as described above using an RV2 video processor. After the initial 20-min baseline period, animals were briefly removed from the open field and injected with 2.5 μg/0.5 μl of either SCH or RAC antagonists directly in the NAc shell (Baldo et al. 2002; van den Boss et al. 1988). After 30 min (see Fig. 9C), all rats received the corresponding systemic infusion of either saline or DEP20 via the intragastric catheter. These behavioral experiments were carried out between 0700 and 1900 (light phase). The rats' body weight and chow food intake was measured daily just before placement in the open field (see Fig. 9, A and B).
Electrophysiology
Surgery.
Surgical procedures for electrode implantation in the nucleus accumbens followed methods described previously (Tellez et al. 2012). Briefly, animals were anesthetized using an intraperitoneal injection of pentobarbital sodium (50 mg/kg) and 0.1 ml of atropine sulfate. A movable 4 × 4 microwire array composed of formvar-coated tungsten wires (35-μm diameter) was unilaterally implanted in the NAc shell (centered from Bregma at coordinates AP = 1.4 mm, L = ±1 mm, and DV = 7.5 mm). One stainless steel screw that was soldered to a silver wire (50 μm) at the surface of cerebellum served as ground.
Multichannel recordings from the NAc shell.
All recordings began 1 wk after the postsurgery recovery period. They were performed in an operant behavioral box that was enclosed in a ventilated and sound-attenuating cubicle equipped with a webcam around 0900 each day. Unless otherwise mentioned, each recording session lasted 3 h and was divided into three 1-h epochs: baseline, infusion of saline, and infusion of an appetite suppressant with or without a DA antagonist. Action potentials and LFPs were recorded using a Multichannel Acquisition Processor (Plexon, Dallas, TX). Only single neurons with action potentials with a signal-to-noise ratio larger than 3:1 were analyzed. Action potentials were identified online by means of voltage-time threshold windows and a three-principal component contour template algorithm (Gutierrez et al. 2010). Spikes were sorted using off-line sorter software (Plexon), and the stability of waveform shape across the 3-h recording session was confirmed by plotting the average shape during baseline, saline, and appetite suppressant epochs.
LFPs recorded from the NAc shell were amplified 1,000 times, filtered at 0.7–300 Hz, and digitized at 1 kHz using a digital acquisition card (National Instruments, Austin, TX). For each LFP, the power spectral density (PSD) was estimated with the use of Welch's method, using a 2-s Hanning window with 50% overlap. Channels with exceptionally large noise levels, based on visual inspection, were excluded from analyses. From the remaining channels, when an artifact event occurred (e.g., saturations and abrupt changes in voltage), the segments 100 ms before to 300 ms after the event were eliminated. The PSD of each LFP was averaged across all channels to generate a single PSD plot per experiment. We also report the average PSDs at delta (1–4 Hz), theta (6–9 Hz), and beta (15–30 Hz) bands (Halje et al. 2012). For comparison across experiments, PSDs were normalized to their z score by subtracting their mean and dividing them by the standard deviation of the baseline period. To allow time for the drugs to achieve a steady pharmacological state, the LFPs 5 min before and 10 min after the start of each epoch were not analyzed.
Weight loss, food intake, and locomotion for intragastric infusions of DEP, PHEN, and BUP during multichannel recordings of the NAc shell activity.
For these experiments we used 10 animals with electrode arrays implanted in their NAc shell [DEP20 (n = 4), PHEN20 (n = 4), and BUP30 (n = 2)]. Drug infusions were made via intragastric injections. The animal's body weight and 24 h food intake were measured daily across 7 days for 20 min before a recording session was started. Pilot experiments with BUP indicated that a dose of 15 mg/kg was insufficient to induce decreases in weight or food intake or changes in locomotion or NAc shell oscillations (data not shown). Consequently, based on rat studies showing that 30 mg/kg of BUP reduced food intake (Janhunen et al. 2013), we used this dosage. For PHEN and DEP we used 20 mg/kg, based on previous studies and pilot experiments (Roth and Rowland 1999; see Fig. 1 for DEP20).
It is important to note that in our pilot study (see Supplementary Video 1), despite having food available, rats practically did not eat or drink anything during 2–3 h after drug administration, when these drugs exert their maximal locomotor effects (see Fig. 7B). Given this information, we decided not to have food available during the multichannel recording sessions since rats would simply ignore it, and also because the delivery of food pellets would interfere with our ability to accurately decode locomotion from the videos.
Modulation of NAc shell LFP oscillations induced by the gastric malaise agent LiCl.
To understand whether DEP20-induced LFP oscillations are related to any aversive-malaise brain state, we evaluated whether a prototypical gastric malaise agent, LiCl, was able to alter LFP's oscillations in the same way as DEP20 did (see Fig. 4). We used two animals with a microarray of electrodes implanted in their NAc shell and with an intragastric catheter (see above). A total of five recording sessions were successfully performed (we allowed at least 2 days of rest between each recording session to permit recovery from the sickness induced by previous injection of LiCl). To reduce animal distress, we did not inject LiCl more than three times per animal. Each recording session contained four 1-h epochs: baseline, saline, LiCl (0.4 M), and DEP20, respectively. LFP oscillations and locomotion were analyzed and displayed in Fig. 6, B–D. From these experiments, we could only record a few single-unit neurons (n = 12). Given this small sample size and because none of these neurons were significantly modulated by LiCl (data not shown), we could not make any conclusions from these data.
Intragastric NAc shell D1R and D2R antagonists' modulation of NAc shell activity.
To evaluate the effects of DA receptor antagonists on modulating DEP20’s effects on NAc shell activity, we implanted 10 animals with multichannel electrodes in their NAc shell [DEP20+RAC0.5 (n = 2 rats, 81 neurons), DEP20+SCH1.5 (n = 2 rats, 112 neurons), DEP20+SCH3 (n = 1, 34 neurons), RAC0.5 (n = 2, 93 neurons), and SCH (n = 3, 114 neurons)]. Experiments were performed in an operant chamber, and each epoch was 30 min long. To increase the statistical power, we pooled together the baseline and saline epochs (which were not significantly different; data not shown) and compared these data with the firing rate in the DEP20 and antagonist epochs using a Kruskal-Wallis test. The normalized z-score peristimulus time histogram (PSTH) of significantly modulated neurons was obtained, as were the normalized PSDs, at beta, theta, and delta bands (see Fig. 10, B and E). We tracked the animal's locomotion based on the center mass point method described above. Finally, to compare the global effect of DEP20 under chronic treatment of the above-mentioned D1R or D2R antagonists, the population firing rates of all recorded neurons (modulated or not by DEP) were plotted by aligning the response to time 0 as the moment of DEP20 infusion and normalizing the firing rate by using the 30-min saline period as a baseline (see Fig. 10, C and F).
Histology
At the end of the experiments rats were injected with pentobarbital sodium (150 mg/kg) and perfused with PBS, followed by 4% paraformaldehyde (PFH). Their brains were removed and placed in 10% sucrose-PFH (vol/vol) solution for 24 h, followed by sequential increases in sucrose-PFH concentration until reaching 30% at 72 h. To establish the placement of the electrodes, brains were sectioned (50 μm) and stained with cresyl violet.
Data Analyses
All analyses were performed using MATLAB toolboxes and homemade custom scripts.
Putative neuron-type classification.
Neurons were classified into putative cell types according to four features: firing rate, coefficient of variation 2 (CV2), valley-to-amplitude ratio (VAR), and valley-to-peak width (V-P width) (Table 1; see Tellez et. al. 2012). The firing rate was calculated as the number of spikes divided by the duration of baseline and/or saline periods (which were not significantly different; see Table 2). CV2 was calculated for each adjacent pair of interspike intervals (ISIs), and the average CV2_ISI was used in the assignment of neuron type. The two-ISI coefficient of variation was computed as CV2_ISI = |2(ISI2 − ISI1)/(ISI2 + ISI1)| (Holt et al. 1996). The VAR was calculated as the absolute value of the first valley in the waveform divided by the difference between its minimum value and the following maximum (see Fig. 3A, inset, and Yarom and Cohen 2011). For computation of the V-P width, the time between the minimum value and the following maximum was calculated (see black waveform in Fig. 3A, pFSI). For each neuron recorded, these four features were computed and classified into four clusters by using a fuzzy cluster algorithm and visualized by using principal component analysis (PCA) as seed, followed by the fuzzy Sammon's mapping plot as described in the MATLAB Fuzzy Clustering & Data Analysis Toolbox.
Table 1.
Characteristics of putative cell types of NAc Shell
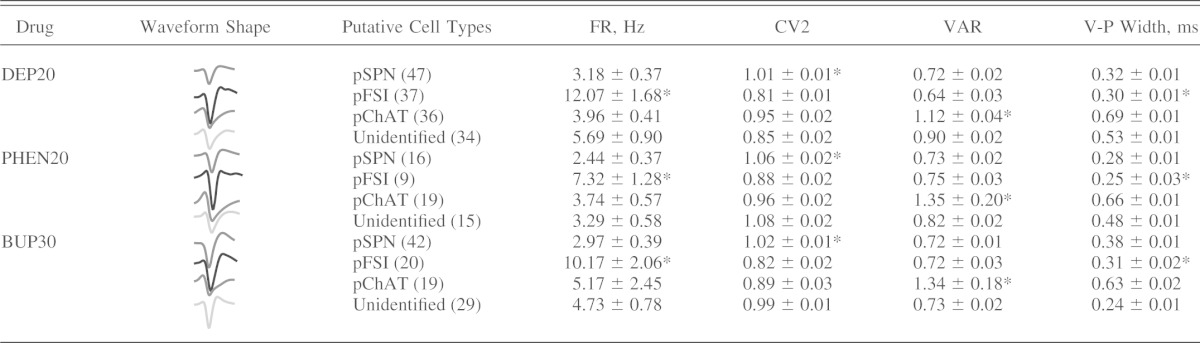
Values are means ± SE of firing rate (FR), coefficient of variation 2 (CV2), valley-to-amplitude ratio (VAR), and valley-to-peak (V-P) width in response to 20 mg/kg diethylpropion (DEP20), 20 mg/kg phentemine (PHEN20), and 30 mg/kg bupropion (BUP30), measured in putative cell types in the nucleus accumbens (NAc) shell: putative medium spiny projection neurons (pSPN), putative fast-spiking interneurons (pFSI), putative choline acetyltransferase interneurons (pChAT), or unidentified neurons, with no. of neurons given in parentheses
P < 0.05, comparison across putative cell types.
Table 2.
Firing rates as a function of drug
| Firing Rate, spikes/s per 60-min epoch |
||||
|---|---|---|---|---|
| Baseline | Saline | Drug | ||
| DEP20 | Days 1–14 | |||
| Activated (14) | 3.1 ± 0.01 | 3.1 ± 0.04 | 7.9 ± 0.18* | |
| Inhibited (104) | 5.3 ± 0.01 | 5.6 ± 0.01 | 1.8 ± 0.04* | |
| Days 1–7 | ||||
| Activated (14) | 3.1 ± 0.01 | 3.1 ± 0.04 | 7.9 ± 0.18* | |
| Inhibited (82) | 5.7 ± 0.01 | 6.0 ± 0.03 | 2.0 ± 0.04* | |
| PHEN20 | Days 1–7 | |||
| Activated (3) | 2.8 ± 0.01 | 2.6 ± 0.02 | 6.2 ± 0.08* | |
| Inhibited (38) | 3.9 ± 0.01 | 3.8 ± 0.01 | 1.6 ± 0.02* | |
| BUP30 | Days 1–7 | |||
| Activated (16) | 5.9 ± 0.01 | 6.3 ± 0.02 | 10.8 ± 0.17* | |
| Inhibited (48) | 3.6 ± 0.01 | 3.6 ± 0.01 | 1.8 ± 0.04* | |
Values are means ± SE, with no. of neurons given in parentheses.
P < 0.05, comparison across epochs.
Hypnograms: the LFP's brain state map.
For hypnograms (see Fig. 3A, top right) behavioral states were assigned using information obtained from the LFPs as outlined by Gervasoni et al. (2004) and Tellez et al. (2012). In brief, after elimination of segments with amplitude saturation, a sliding window Fourier transform was applied to each LFP signal to calculate two spectral amplitude ratios (0.5–20/0.5–55 Hz and 0.5–4.5/0.5–9 Hz for ratios 1 and 2, respectively). PCA was then applied to these ratios obtained from all LFP channels, and the PCs were used as the overall ratios measure. These measures obtained for each second of data were further smoothed with a Hanning window (20-s length). Finally, the two PCs of the spectral ratios were plotted against each other to construct a two-dimensional (2-D) state space (Fig. 3A, top right) where the density of points reflects the relative abundance of the different brain states. Rapid eye movement (REM) sleep was not included in this analysis because the percentage of time the animals spent in REM was <2% (unpublished observation). The final 2-D brain state map was selected and validated after visual inspection of the animal's behavior in the video taped with three behavioral states: quiet wake, slow-wave sleep (SWS), and active wake (Tellez et al. 2012).
Active and inactive neurons: after DEP, PHEN, and BUP administration.
Neuronal firing modulations such as those shown in Figs. 3B and 7C were identified using a Kruskal-Wallis test for α < 0.05. Firing rates were pooled across baseline and saline epochs because differences between them were not significantly different (Table 2). Moreover, after DEP, PHEN, and BUP infusions, because rats remained >80% of the time in the active awake state (Table 3), we compared the firing rates of the baseline-saline period during the quiet wake times (computed from the hypnogram) against the firing rates during active awake (from the time interval 2:10 to 3 h; see Figs. 3B and 7C) after the infusion of the appetite suppressants.
Table 3.
Time spent in each behavioral state across 60-min epochs
| Time, min |
||||
|---|---|---|---|---|
| Drug | Behavioral State | Baseline | Saline | Drug |
| DEP20 | qW | 17.7 ± 1.9 | 18.9 ± 1.6 | 6.7 ± 1.1* |
| SWS | 36.2 ± 2.3 | 33.9 ± 2.2 | 0.7 ± 0.2* | |
| aW | 4.6 ± 0.6 | 5.5 ± 0.8 | 50.6 ± 1.3* | |
| PHEN20 | qW | 16.0 ± 3.6 | 22.1 ± 3.9 | 9.7 ± 2.2# |
| SWS | 35.8 ± 4.1 | 30.1 ± 4.3 | 7.2 ± 2.1* | |
| aW | 7.1 ± 1.4 | 6.4 ± 1.4 | 41.1 ± 2.8* | |
| BUP30 | qW | 8.4 ± 1.5 | 11.2 ± 2.2 | 4.8 ± 2.3# |
| SWS | 36.0 ± 3.0 | 28.3 ± 1.8 | 2.1 ± 0.7* | |
| aW | 13.9 ± 2.7 | 18.3 ± 2.2 | 52.3 ± 2.3* | |
Values are means ± SE of time spent in quiet wake (qW), slow-wave sleep (SWS), or active awake (aW) state; n = 32 recording sessions for DEP20, 15 for PHEN20, and 12 for BUP30.
P < 0.05, significantly different from both baseline and saline epochs.
P < 0.05, significantly different from saline epoch.
Statistical Analyses
Unless otherwise mentioned, data are means ± SE. Statistical differences between groups were assessed by one-way ANOVA or repeated-measures ANOVA (RM ANOVA), followed by Fisher's post hoc analysis. For a detailed description of all P values and complete statistical analysis, please see Supplemental Table S1.
RESULTS
Behavior
Effect of DEP on weight loss and appetite suppression.
Preliminary experiments regarding the effects of DEP, PHEN, and BUP on appetite suppression revealed that DEP gave the greatest response (see below and Figs. 1 and 7A), and therefore its effects were initially explored to determine an optimal concentration. Figure 1A shows a graph of the change in body weight across 14 days of daily intraperitoneal injections of saline or 10, 20, or 40 mg/kg of DEP (noted as DEP10, 20, and 40, respectively). This was followed by a 7-day withdrawal of treatment (to day 21). With saline injections (control), the animals progressively gained weight until the trial ended on day 21. In contrast, increasing the DEP concentration caused an initial dose-dependent increase in weight loss, an effect that decreased across the treatment period. Specifically, for DEP10, after day 7 the animals gained weight, and from day 12 onward their weight approached that seen with saline injections. For DEP20, after the initial weight loss there was a recovery such that on day 12 their weight was the same as that on day 1 (although it remained significantly below that with saline). After the withdrawal of treatment on day 15, the animals rapidly gained weight to equal that of animals treated with saline (day 21). For DEP40, there was a monotonic decrease in weight until day 7 that was maintained across the entire treatment period. Withdrawal of DEP40 also produced a weight gain, but the animal's weight remained below that with saline. The overall mean (±SE) change in body weight across the 14 treatment days was 14.7 ± 1.2, 4.9 ± 3.6, −4.9 ± 2.8, and −14 ± 3.3 g for saline, DEP10, DEP20, and DEP40, respectively [RM ANOVA; main effect of dose: F(3,19) = 20.2, P < 0.0001; effect of days: F(3,13) = 25.6, P < 0.0001; and dose × days interaction: F(39,247) = 13.5, P < 0.0001]. Post hoc comparisons, relative to saline, indicated that DEP40 induced the greatest weight loss (P < 0.0001), followed by DEP20 (P = 0.0001) and DEP10 (P = 0.038). In summary, DEP induces a dose-dependent decrease in body weight.
In the same group of animals we also measured food intake over 24 h (Fig. 1B). Over the 14-day treatment period, the percent change of food intake from the day before the treatment started was 4.1 ± 4.1%, −14.4 ± 5.2%, −15.1 ± 5.7%, and −28.7 ± 4.8% for saline, DEP10, DEP20, and DEP40, respectively [RM ANOVA; main effect of dose: F(3,19) = 16.1, P <0.0001; effect of days: F(3,13) = 14.5, P <0.0001; and significant dose × days interaction: F(39,247) = 3.59, P < 0.0001]. Although over the 21-day treatment and withdrawal periods the saline-injected animals exhibited a relatively constant food intake, the animals under treatment initially decreased food intake in a dose-dependent manner (reflected at day 2 for measurements made 24 h later) with DEP40 showing the greatest reduction (post hoc: P <0.0001), followed by DEP20 (P = 0.0007) and DEP10 (P = 0.0013). Interestingly, DEP10 and DEP20 induced a similar suppression of appetite (P = 0.78, not significant, ns) in that after 7 days, rats treated with either dose exhibited a gradual recovery of food intake (Fig. 1B). For DEP40, after the large decrease in food intake on day 2, the animals increased their intake, but to levels significantly below that of the other treatments. Finally, 7 days after drug withdrawal (Fig. 1B, day 21) the food intake of all DEP-treated groups achieved similar (or slightly higher) levels to that of the saline group. These results show that over the treatment period, the animals developed tolerance to the anorectic effects of DEP but consistently consumed 5–10% less chow food than animals in the saline group.
Effect of DEP on locomotion and stereotypy.
Previous studies with rats reported that acute administration of DEP alters their locomotion and produces stereotypy (Reimer et al. 1995). In the present study we asked how a dose-dependent chronic treatment affects locomotion and stereotypic behavior (head weavings). In a new cohort of animals (n = 12) we evaluated the effects of 7-day intraperitoneal injections of different doses of DEP on their locomotion (Fig. 1C). Relative to the initial 1.5-h baseline period, when the animals were free to move in an open field, during the 3 days of saline injections (S1–S3) they did not display marked changes in locomotion (Fig. 1C). Throughout the treatment period the increase in locomotion was DEP10 > DEP20 > DEP40. Moreover, throughout the treatment period the responses to DEP10 and DEP20 remained elevated, whereas the responses to DEP40 showed a nonsignificant trend to decrease across days. Seven days of DEP treatment showed a main effect of dose [RM ANOVA; D1–D7: F(2,9) = 30.0, P = 0.0001] and nonsignificant effect of days [D1–D7: F(2,6) = 1.48, P = 0.20], with nonsignificant interaction [F(12,54) = 0.96, P = 0.49]. A Fisher's post hoc analysis showed that DEP10 induced more locomotion than DEP20 (P = 0.008), and in turn, DEP20 induced more locomotion than DEP40 (P < 0.002). Moreover, 1 day after treatment stopped (S8), DEP's effect approached baseline values. Thus the recovery from locomotion is faster than for food intake and weight loss.
With respect to DEP-induced changes in stereotypy (Fig. 1D), results after 3 days of saline injections (S1–S3) were not significantly different from baseline. However, at D1–D2 the increase in stereotypy was DEP20 = DEP40 > DEP10 [RM ANOVA; significant main effect of doses: F(2,9) = 8.60, P = 0.008, nonsignificant effect of days: F(2,1) = 0.15, P = 0.7; and no dose × days interaction: F(2,9) = 1.21, P = 0.34]. However, with treatment the order changed, because DEP10-induced stereotypy increased linearly to day 4 [for D1–D4, no significant differences between doses: F(2,9) = 0.92, P = 0.43; but significant effect of days: F(2,3) = 4.42, P = 0.012 and dose × days interaction: F(6,27) = 3.71, P = 0.008], whereas the others did not markedly change, so from D4 to D7 the responses to all concentrations were not statistically different. These data show that 7 treatment days of the three tested DEP concentrations induced stereotypy, but only DEP10 tended to sensitize across repeated injections. After treatment, a single saline injection (S8; Fig. 1D) returned stereotypy to baseline levels. It follows that with respect to locomotion and stereotypy, DEP does not produce long-term effects. In summary, since DEP20 provided significant but not extreme changes in weight loss, food intake, and motor effects relative to DEP10 or DEP40, in all subsequent experiments we chose to use the 20 mg/kg concentration.
A dose of DEP that suppresses food intake is not sufficient to delay gastric emptiness.
We also explored whether DEP20's anorectic effect could be mediated by a peripheral vagal satiety pathway such as that induced by CCK-8, a peptide that delays gastric emptiness (Chang et al. 2012). To test whether DEP20 can produce satiety by a similar mechanism, we performed a gastric emptiness assay, using CCK-8 as a positive control (Fig. 2). Briefly, after infusion of an oral Ensure gavage, gastric emptiness was 5.01 ± 0.24, 5.18 ± 0.12, and 6.18 ± 0.33 g for saline, DEP20, and CCK-8, respectively. Importantly, the differences were significant [1-way ANOVA; F(2,24) = 6.67, P = 0.005]. A post hoc analysis showed that a single intraperitoneal injection of CCK-8 delayed gastric emptiness relative to both saline (P = 0.003) and DEP20 (P = 0.008), whereas DEP20 and saline were not significantly different (P = 0.64). Hence, unlike CCK-8, which delays gastric emptiness, DEP20-induced anorexia does not seem to be mediated via peripheral vagal satiety signals.
Electrophysiology
To relate the behavioral studies to neural activity, we recorded the responses to DEP20 in the NAc shell, a brain region involved in reward, feeding, and motor activity (Brown et al. 2011; Kelley et al. 2005; Li et al. 2012). A total of 154 well-isolated single neurons were recorded and classified into putative medium spiny projection neurons (pSPNs; n = 47), putative fast-spiking interneurons (pFSIs; n = 37), putative choline acetyltransferase interneurons (pChATs; n = 36), and unidentified neurons (U; n = 34) (see Table 1 for data regarding firing rates and waveform shapes for each putative cell type).
DEP strongly modulates NAc shell neuronal activity.
Unless otherwise stated, all electrophysiological recordings followed the same protocol. That is, after a 1-h baseline period when the animals were free to move, the rats received a daily intragastric infusion of saline (at 1 h) and DEP20 at 2 h. Recordings were usually terminated after 3 h. Figure 3A shows a graph plotting 154 classified neurons (pSPN, pFSI, pChAT, and U) that were recorded before and after the infusion of DEP20. Relative to the not significantly different baseline and saline epochs (Table 2), two types of modulatory responses were observed: neurons whose activity decreased [inhibited by the appetite suppressant; blue dots (68%, 104/154)] and those whose activity increased [activated by the appetite suppressant; red dots (9%, 14/154); χ2(1) = 51.9, P < 0.001]. A representative activated response from a pSPN neuron (bottom left) and a representative inhibited response from a pChAT neuron (bottom right) are illustrated, as well as their locations on the modulation map (arrows; Fig. 3A). As noted, most responses were inhibited, and all neuronal types were equally affected [χ2(3) = 2.2, P = 0.53, ns; Table 4]. In contrast, for the activated responses, DEP20 significantly modulated more pSPNs than other cell types [χ2(3) = 9.8, P = 0.019; Table 4]. We note that these activated pSPNs could be either D1+ or D2+ expressing cells and that they might have different sensitivities to appetite suppressants (MacAskill et al. 2014). The remaining 36 neurons were unaffected.
Table 4.
Neurons with a significant firing rate modulation after drug infusion as a function of cell types
| No. of Neurons |
|||||
|---|---|---|---|---|---|
| Drug | Type of Response | pSPN | pFSI | pChAT | Unidentified |
| DEP20 | Days 1–14 | ||||
| Activated (14/154, 9%) | 10/47 (21%)# | 1/37 (3%) | 1/36 (3%) | 2/34 (6%) | |
| Inhibited (104/154, 68%)* | 24/47 (51%) | 24/37 (65%) | 28/36 (78%) | 28/34 (82%) | |
| Days 1–7 | |||||
| Activated (14/131, 11%) | 10/41 (24%)# | 1/34 (3%) | 1/27 (4%) | 2/29 (7%) | |
| Inhibited (82/131, 63%)* | 18/41 (44%) | 21/34 (62%) | 20/27 (74%) | 23/29 (79%) | |
| PHEN20 | Days 1–7 | ||||
| Activated (3/59, 5%) | 1/16 (6%) | 0/9 (0%) | 0/19 (0%) | 2/15 (13%) | |
| Inhibited 38 (38/59, 64%)* | 11/16 (69%) | 5/9 (55%) | 16/19 (84%) | 6/15 (40%) | |
| BUP30 | Days 1–7 | ||||
| Activated (16/110, 15%) | 8/42 (19%) | 4/20 (20%) | 0/19 (0%) | 4/29 (14%) | |
| Inhibited (48/110, 44%)* | 13/42 (31%) | 6/20 (30%) | 14/19 (74%) | 15/29 (52%) | |
Values are no. of neurons per indicated population within each cell type, with percentages given in parentheses.
P < 0.05, comparison between activated vs. inhibited neurons.
P < 0.05, comparison across all 4 putative cell types.
Also shown in these two examples is that in the baseline and saline epochs, the animals were in either a quiet awake (blue), active awake (green), or SWS (gray) states (Fig. 3A, see below PSTHs, and Table 3). These three states were obtained from analysis of LFPs (Fig. 3A, right, and Fig. 4A). In these experiments during the DEP20 epoch the animals were essentially in the active awake state (2–3 h) for the entire epoch (50.6 min; Table 3). In this regard, after DEP20 infusion, the amount of SWS significantly decreased from 36.2 and 33.9 min (ns) in the baseline and saline epochs, respectively, to 0.7 min in the DEP20 epoch [Table 3; n = 32 sessions; 1-way ANOVA; main effect epochs: F(2,93) = 113.7, P < 0.0001].
With respect to changes in firing rates, except for the abrupt increases caused by the transitions between SWS and awake states (see pSPN neuron, Fig. 3A, bottom left), there was not a marked change in spiking activity from baseline to saline (firing rates: pSPN, baseline = 0.57 and saline = 1.1 spikes/s; pChAT, baseline = 9.1 and saline = 8.9 spikes/s). In contrast, about 5 min after the infusion of DEP20 there was a marked change in inhibition and/or activation that lasted more than 1 h (pSPN, 3.4 spikes/s after DEP20 and pChAT, 3.1 spikes/s after DEP20; Fig. 3A). These changes are representative of the population responses (Table 2). The normalized individual and population activity changes (black traces) for 118 (104 inhibited and 14 activated) of the 154 neurons that significantly changed their firing rate in response to DEP20 are shown in Fig. 3B. These data clearly show that NAc shell activity is strongly modulated by DEP20.
DEP induces LFP oscillations in the NAc shell.
In addition to the recording of single-unit activity, we simultaneously recorded LFPs. Figure 4A shows 5-s representative LFP recordings when the animals were in three distinct behavioral states. The quiet awake state is characterized by low amplitude and fast oscillations (blue traces), whereas during SWS the recordings show their characteristic high-amplitude, low-frequency delta (1–4 Hz) oscillations (gray traces). The DEP20-induced active awake state is also characterized by high-amplitude, low-frequency delta oscillations that become larger over time (green traces). Figure 4B shows a spectrogram (1–30 Hz) of the LFPs over a single session that encompasses the baseline, saline, and DEP20 epochs. It is readily seen that after DEP20 infusion, the power at delta, beta, and theta frequency bands greatly increased. Also shown is the animal's locomotion (overlapped white line) that increased upon DEP20 application. The panel below the hypnogram displays the animal's behavior during the three epochs. Again, the data clearly show that in the DEP20 epoch, the animal did not exhibit SWS and was primarily in the active awake state (Table 3). Figure 4C displays the average of 32 recording sessions of the normalized PSD of the LFPs for beta (15–30 Hz), theta (6–9 Hz), and delta (1–4 Hz) oscillations. Interestingly, there were no significant changes during the baseline or saline epochs, a result that could arise from an averaging of the quiet awake and SWS states that occur at different times across experiments. Nevertheless, after DEP20 infusion, large and rapid (∼5 min) changes were revealed as a decreased z-score PSD at beta [Kruskal-Wallis; H(2) = 23,800, P < 0.0001] and theta [H(2) = 11,122, P < 0.0001] oscillations and an increase in delta oscillations [H(2) = 5,090, P < 0.0001; Table 5].
Table 5.
Z-score PSD of NAc Shell LFP as a function of drug
|
Z-Score PSD of NAc Shell LFP |
||||
|---|---|---|---|---|
| Baseline | Saline | Drug | ||
| DEP20 | Days 1–14 | |||
| Beta | −0.010 ± 0.003 | −0.001 ± 0.006 | −0.864 ± 0.032* | |
| Theta | 0.019 ± 0.002 | 0.060 ± 0.003# | −0.559 ± 0.006* | |
| Delta | 0.017 ± 0.003 | 0.029 ± 0.003 | 0.515 ± 0.026* | |
| Days 1–7 | ||||
| Beta | −0.012 ± 0.003 | −0.002 ± 0.007 | −0.333 ± 0.034* | |
| Theta | 0.015 ± 0.003 | 0.088 ± 0.004# | 0.126 ± 0.007* | |
| Delta | 0.013 ± 0.003 | 0.069 ± 0.004# | 0.979 ± 0.025* | |
| PHEN20 | Days 1–7 | |||
| Beta | 0.002 ± 0.006 | 0.106 ± 0.007# | 0.913 ± 0.029* | |
| Theta | 0.030 ± 0.004 | 0.051 ± 0.005 | 0.863 ± 0.007* | |
| Delta | 0.018 ± 0.004 | −0.085 ± 0.006# | 1.121 ± 0.030* | |
| BUP30 | Days 1–7 | |||
| Beta | −0.024 ± 0.005 | 0.002 ± 0.006 | 0.290 ± 0.031* | |
| Theta | 0.068 ± 0.003 | −0.143 ± 0.004 | 0.098 ± 0.007* | |
| Delta | 0.047 ± 0.004 | −0.257 ± 0.004# | 0.177 ± 0.031* | |
Values are means ± SE of z-score power spectral density (PSD) of NAc shell local field potential (LFP).
P < 0.05, significantly different from both baseline and saline epochs.
P < 0.05 significantly different from baseline epoch.
The mean locomotion responses (Fig. 4D) show that compared with the saline epoch (390 ± 12 cm/min), after DEP20 infusion there was a rapid (onset: 5.43 ± 0.44 min; n = 22) increase in locomotion (1,502 ± 20 cm/min) [Kruskal-Wallis; H(1) = 89.26, P < 0.0001]. In summary, these data show that DEP20 produces insomnia, increases locomotion, and alters oscillations in the NAc shell.
DEP20-induced changes in NAc shell activity across treatment period.
Having shown that repeated intraperitoneal injections of DEP20 produce transient changes in weight loss and food intake (Fig. 1), we investigated whether activity changes measured in the NAc shell would correlate with these behavioral changes. To achieve this, over 14 days we gave daily intragastric infusions of saline and DEP20 while measuring the NAc shell activity. Animals given the intragastric treatment, like those given the intraperitoneal treatment, showed a transient increase in weight loss and decrease in food intake that adapted as the treatment progressed (Fig. 5A). To obtain a sufficient number of neurons for statistical analysis, the treatment was divided into three periods (Fig. 5A): day 1, days 2–7, and days 8–14. The means of the population firing rates and normalized firing rates for day 1 (purple), days 2–7 (orange), and days 8–14 (brown) over the baseline, saline, and DEP20 epochs are shown in Fig. 5B and given in Table 6. Throughout the three epochs, the greatest activity (baseline and evoked) occurred in days 2–7 and the least in days 8–14. The normalized responses revealed no differences between the baseline and saline epochs and that the greatest inhibitory effect occurred during days 8–14 (Table 6).
Table 6.
Behavioral and neuronal changes in NAc shell activity after repeated use of appetite suppressants
| Relative to Day Before Treatment |
||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Period | Change in bodyweight, g | 24-h Chow intake, % | Activity | Baseline | Saline | Drugs | |
| DEP20 | Day 1 | −9.5 ± 2.5 | −45.4 ± 13.8 | Firing rate, Hz | All (22) | 5.1 ± 0.02 | 4.9 ± 0.01 | 3.0 ± 0.02 |
| Inhibited (19) | 5.4 ± 0.01 | 5.4 ± 0.01 | 1.9 ± 0.04 | |||||
| Activated (3) | 2.4 ± 0.01 | 2.8 ± 0.01 | 4.6 ± 0.03 | |||||
| z-score (PSD) | Beta (15–30 Hz) | 0.030 ± 0.009 | 0.033 ± 0.008 | 0.638 ± 0.009 | ||||
| Theta (6–9 Hz) | 0.042 ± 0.008 | 0.187 ± 0.008 | 1.063 ± 0.012 | |||||
| Delta (1–4 Hz) | 0.020 ± 0.009 | 0.088 ± 0.008 | 1.519 ± 0.007 | |||||
| Distance, cm/min | 555 ± 24 | 383 ± 26 | 2088 ± 64 | |||||
| Days 2–7 | −12.3 ± 1.9 | −29.4 ± 4.2 | Firing rate, Hz | All (109) | 6.3 ± 0.01 | 6.6 ± 0.01 | 4.5 ± 0.03 | |
| Inhibited (87) | 5.6 ± 0.01 | 6.0 ± 0.01 | 2.0 ± 0.04 | |||||
| Activated (22) | 3.1 ± 0.01 | 3.0 ± 0.04 | 7.9 ± 0.18 | |||||
| z-score (PSD) | Beta | −0.017 ± 0.003 | −0.007 ± 0.008 | −0.455 ± 0.035* | ||||
| Theta | 0.012 ± 0.003 | 0.066 ± 0.004 | 0.008 ± 0.008* | |||||
| Delta | 0.013 ± 0.003 | 0.063 ± 0.004 | 0.918 ± 0.026* | |||||
| Distance, cm/min | 452 ± 19 | 448 ± 16 | 1382 ± 25 | |||||
| Days 8–14 | −1.8 ± 2.1* | −9.6 ± 2.9* | Firing rate, Hz | All (23) | 3.8 ± 0.003 | 3.7 ± 0.006 | 1.1 ± 0.003 | |
| Inhibited (23) | 3.8 ± 0.003 | 3.7 ± 0.006 | 1.1 ± 0.003 | |||||
| Activated (0) | ||||||||
| z-score (PSD) | Beta | −0.007 ± 0.005 | 0.001 ± 0.006 | −1.574 ± 0.019* | ||||
| Theta | 0.028 ± 0.004 | −0.008 ± 0.005 | −1.459 ± 0.008* | |||||
| Delta | 0.029 ± 0.004 | −0.069 ± 0.005 | −0.047 ± 0.017* | |||||
| Distance, cm/min | 280 ± 12 | 294 ± 14 | 1449 ± 24 | |||||
| PHEN20 | Day 1 | −1.7 ± 1.8 | −27.7 ± 3.5 | Firing rate, Hz | All (20) | 3.6 ± 0.02 | 3.3 ± 0.02 | 2.8 ± 0.01 |
| Inhibited (11) | 3.1 ± 0.002 | 2.6 ± 0.003 | 1.1 ± 0.01 | |||||
| Activated (1) | 0.9 ± 0.001 | 0.9 ± 0.001 | 2.0 ± 0.01 | |||||
| z-score (PSD) | Beta | 0.013 ± 0.008 | −0.061 ± 0.006 | 0.627 ± 0.022 | ||||
| Theta | 0.035 ± 0.006 | 0.017 ± 0.006 | 0.869 ± 0.009 | |||||
| Delta | 0.029 ± 0.006 | −0.089 ± 0.006 | 1.172 ± 0.022 | |||||
| Distance, cm/min | 240 ± 20 | 195 ± 11 | 572 ± 23 | |||||
| Days 2–7 | −0.5 ± 2.9 | −20.3 ± 3.8 | Firing rate, Hz | All (39) | 4.0 ± 0.01 | 4.1 ± 0.01 | 2.6 ± 0.01 | |
| Inhibited (27) | 4.4 ± 0.01 | 4.3 ± 0.01 | 1.9 ± 0.03 | |||||
| Activated (2) | 3.7 ± 0.01 | 3.4 ± 0.02 | 8.2 ± 0.06 | |||||
| z-score (PSD) | Beta | −0.004 ± 0.005 | 0.191 ± 0.008 | 1.051 ± 0.026* | ||||
| Theta | 0.027 ± 0.005 | 0.068 ± 0.006 | 0.848 ± 0.008 | |||||
| Delta | 0.012 ± 0.004 | −0.083 ± 0.007 | 1.079 ± 0.028 | |||||
| Distance, cm/min | 155 ± 12 | 218 ± 8 | 836 ± 43 | |||||
| BUP30 | Day 1 | −2.2 ± 1.1 | −15.7 ± 1.8 | Firing rate, Hz | All (18) | 5.8 ± 0.03 | 6.4 ± 0.03 | 6.7 ± 0.03 |
| Inhibited (9) | 3.5 ± 0.01 | 3.4 ± 0.01 | 1.6 ± 0.01 | |||||
| Activated (2) | 10.5 ± 0.03 | 12.6 ± 0.04 | 20.2 ± 0.05 | |||||
| z-score (PSD) | Beta | 0.011 ± 0.008 | 0.013 ± 0.007 | −0.245 ± 0.011 | ||||
| Theta | 0.055 ± 0.007 | 0.044 ± 0.007 | 0.297 ± 0.009 | |||||
| Delta | 0.037 ± 0.007 | −0.039 ± 0.007 | 0.508 ± 0.012 | |||||
| Distance, cm/min | 146 ± 10 | 102 ± 6 | 487 ± 28 | |||||
| Days 2–7 | 7.9 ± 4.1* | −12.8 ± 5.5 | Firing rate, Hz | All (92) | 5.1 ± 0.02 | 5.0 ± 0.02 | 5.1 ± 0.02 | |
| Inhibited (39) | 3.6 ± 0.01 | 3.7 ± 0.007 | 1.9 ± 0.03 | |||||
| Activated (14) | 5.3 ± 0.02 | 5.3 ± 0.02 | 9.4 ± 0.16 | |||||
| z-score (PSD) | Beta | −0.031 ± 0.005 | −0.0003 ± 0.006 | 0.383 ± 0.028* | ||||
| Theta | 0.071 ± 0.004 | −0.180 ± 0.004 | 0.060 ± 0.007* | |||||
| Delta | 0.049 ± 0.004 | −0.300 ± 0.004 | 0.116 ± 0.028* | |||||
| Distance, cm/min | 230 ± 10 | 188 ± 10 | 651 ± 24 | |||||
Values are means ± SE; no. of neurons is given in parentheses.
P < 0.05, comparison across periods.
Analysis of the LFP oscillations over these three periods shows dramatic changes in DEP20 responses (Fig. 5C, Table 6). Specifically, on day 1 there were statistically significant increases at beta, theta, and delta oscillations. On days 2–7 all three oscillations decreased in magnitude, and on days 8–14 the beta and theta oscillations became largely negative and the delta oscillations decreased to saline levels. Thus the changes in weight loss and food intake are best correlated with increases in the oscillations on day 1, and the adaptation (tolerance) to the treatment is better correlated with the diminution of the amplitude of the oscillations over the succeeding treatment days.
DEP20 induces taste aversion to a novel tastant (similar to LiCl), but the NAc shell LFP oscillations induced by DEP20 are different from those evoked by LiCl.
To explore whether DEP20 has a potential to induce taste aversion, which may contribute to its anorectic effects, we performed a conditioned taste aversion experiment. We used DEP20 or LiCl (0.4 M) as the unconditioned stimulus (US) and a novel tastant (saccharin) as the conditioned stimulus (CS). Figure 6A displays the 15-min intake of water and the CS across the experiment; note that during 3 days of baseline (W1–W3), water intake gradually increased as rats habituated to drink their entire daily allotment of fluids within 15 min. On the acquisition day (ACQ), the animals were presented with a novel 0.1% saccharin solution, and they consumed less saccharin than water on W3. Surprisingly, in the Test session, both DEP20 and LiCl groups rejected saccharin to the same extent [1-way ANOVA; F(1,10) = 1. 3, P = 0.28], suggesting that they were able to associate a gastric distress induced by a single injection of DEP20 with the consumption of a novel tastant. This is reflected by the smaller intake of saccharin in the Test session compared with that on ACQ. Nevertheless, as measured by the faster extinction of DEP20 relative to the LiCl group, the strength of the aversive memory induced by DEP20 was weaker compared with the one produced by LiCl [RM ANOVA; EXT1–EXT3 main effect; F(1,10) = 5.41, P = 0.042; effect across days: F(1,2) = 30.91, P < 0.0001]. In summary, both DEP20 and LiCl reduced the consumption of a novel tastant.
In light of this result, we tested whether DEP20-induced LFP oscillations could reflect any kind of aversive or gastric malaise-induced brain state. We recorded LFPs in the NAc shell while the same rat received saline, LiCl, and finally DEP20 in the same session. Figure 6B shows the spectrogram of a representative experiment, and Fig. 6C shows the normalized PSD LFP changes. The injection of LiCl, despite the presence of a transit peak, showed a nonsignificant decrease in delta and theta oscillations, but DEP20 significantly increased beta, theta, and delta oscillations [Kruskal-Wallis; H(2) = 13,124, P < 0.0001; H(2) = 4,146, P < 0.0001; H(2) = 118, P < 0.0001, respectively]. The hypnogram is shown in Fig. 6B, bottom. Note that after DEP20, rats are primarily in the active awake state (green) with increased LFP oscillations, whereas LiCl infusion prevents SWS (gray) by inducing gastric malaise but without increasing the LFP oscillations. The average locomotion (Fig. 6D) reveals that LiCl showed a “spike-patterned” locomotion profile that is likely caused by the distress, and not because LiCl increased exploratory locomotion as did DEP20. In summary, these data clearly show that the LFP oscillations in the NAc shell evoked by LiCl, a prototypical gastric malaise agent, and DEP20 are significantly distinct.
Appetite suppressants PHEN and BUP also decrease food intake, increase locomotion and stereotypy, and modulate NAc shell activity.
In addition to DEP20, we tested two other commonly used appetite suppressants, PHEN20 and BUP30, in a new cohort of animals both to determine their effects and to compare them with each other and to effects of DEP20. Relative to saline infusions, both PHEN20 and BUP30 caused a reduction in body weight, albeit to a lesser extent than DEP20 (Fig. 7A, top). Specifically, across 7 days of treatment the change in body weight was saline, 8.1 ± 1.2 g; DEP20, −11.6 ± 2.3 g; PHEN20, −0.6 ± 2.4 g; and BUP30, 5.7 ± 3.2 g. RM ANOVA showed a significant effect between groups [main effect of treatment: F(3,21) = 18.0, P < 0.0001], a significant effect across 7 days [D2–D8: F(3,6) = 13.7, P < 0.0001], and significant interaction between treatment and days [F(18,126) = 3.7, P < 0.0001]. Furthermore, PHEN20 and BUP30 also decreased food intake: saline, −0.7 ± 3.5%; DEP20, −26.6 ± 5.5%; PHEN20, −18.7 ± 3.3%; and BUP30, −11.5 ± 4.4% [RM ANOVA; main effect of treatment: F(3,21) = 9.2, P = 0.0004; effect across days: F(3,6) = 10.9, P < 0.0001; interaction between groups and days: F(18,126) = 2.52, P = 0.0015]. Compared with saline, DEP20 (P < 0.0001) and PHEN20 (P = 0.002) significantly reduced food intake (post hoc test), whereas BUP30 across days showed a nonsignificant trend (P = 0.064), probably because it only significantly decreased food intake during D5–D8 (post hoc; P = 0.04; Fig. 7A, bottom).
We also measured during 30-min epochs of baseline and saline and for 1.5 h after anorexic drug delivery how these three appetite suppressants affected locomotion (Fig. 7B, top) and stereotypy (bottom). In the initial 20 min of the baseline epoch, in all groups the animals' normal arousal and accompanying locomotion gradually decreased, and thus there was a small but significant decrease in locomotion between the baseline and saline epochs [RM ANOVA; main effect: F(3,80) = 7.8, P < 0.0001]. In the drug epoch, the saline response remained consistently low, whereas the response greatly increased with DEP20, PHEN20, and BUP30. DEP20 induced a slightly greater locomotion (3,925 ± 288 cm/min) than PHEN20 (3,515 ± 199 cm/min) and BUP30 (3,325 ± 233 cm/min) with a significant increase of locomotion compared with saline (843 ± 158 cm/min) [main effect across treatment drug epoch: F(3,78) = 90.02, P < 0.0001; effect across time: F (3,17) = 15.5, P < 0.0001; treatment × time interaction: F(51,1326) = 5.69, P < 0.0001]. The significant interaction indicates that BUP30-induced locomotion rapidly tends to return to baseline (also see Supplementary Video 1).
For stereotypy measurements, there was a small decrease from the baseline to the saline epoch [RM ANOVA; F(3,80) = 3.4, P = 0.02] that was followed by rapid increase after DEP20, PHEN20, and BUP30 infusions. DEP20 induced almost twice the number of head weavings (86 ± 7 counts/5 min) as PHEN20 (45 ± 5 counts/5 min) or BUP30 (37 ± 5 counts/5 min). RM ANOVA showed a significant difference across treatment groups [main effect: F(3,78) = 75.0, P < 0.0001; effect across time: F(3,29) = 61.3, P < 0.0001] and interaction between treatment and time [F(87,2262) = 16.1, P < 0.0001]. Furthermore, post hoc analysis revealed that DEP20 significantly induced more stereotypy than either PHEN20 (P < 0.0001) or BUP30 (P < 0.0001).
From some of the same animals shown in Fig. 7A, we recorded NAc shell activity during the baseline, saline, PHEN20 (Fig. 7C, left) or BUP30 epochs (Fig. 7C, right). As with DEP20, both these compounds evoked both decreases and increases in firing rate. PHEN20 was most similar to DEP20 in that it produced 64% (38/59) inhibited responses and only 5% (3/59) activated responses [χ2(1) = 23, P < 0.0001; compared with 63% (P = 0.91, ns) and 11%, respectively, for DEP20 (P = 0.24, ns); Table 4]. With PHEN20, all cell types were equally inhibited [Table 4; χ2(3) = 2.6, P = 0.44], whereas only three neurons were activated (1 pSPN and 2 unidentified; Table 4).
The application of BUP30 also induced a greater number of inhibited (44%, 48/110) than activated (15%, 16/110) responses [χ2(1) = 12.5, P < 0.0001]. The proportion of inhibited responses induced by BUP30 was not significantly different than for DEP20 [over 7 days: χ2(1) = 2.6, P = 0.1, ns] or for PHEN20 [χ2(1) = 2.08, P = 0.14]. Activated responses were also not significantly different among drugs [BUP30, 15%; PHEN20, 5%; and DEP20, 11%; χ2(2) = 2.8, P = 0.23]. BUP30 showed a nonsignificant trend to inhibit a greater percentage of pChAT than pSPN neurons [χ2(1) = 3.4, P = 0.064; see Table 4]. In other words, over a 7-day treatment period, all three compounds produced essentially the same effect. Information about the average firing rate of activated and inhibited neurons by the three appetite suppressants are shown in Table 2.
With regard to the changes in LFPs evoked by PHEN20 and/or BUP30, we found both similarities and differences compared with those evoked by DEP20. Whereas over the entire 14-day treatment period DEP20 decreased beta and theta oscillations and increased delta oscillations, all three oscillations were increased by PHEN20 and BUP30 (over a 7-day period; Figs. 5C and 7D, Tables 5 and 6). With regard to locomotion, we found that DEP20 infusions caused a greater increase in locomotion than either PHEN20 or BUP30 infusions [F(2,48) = 16.3, P < 0.0001]. Furthermore, the onset of locomotion induced by DEP20 [5.43 ± 0.44 min; F(2,49) = 9.26, P = 0.0004] was significantly shorter than that for PHEN20 (19.1 ± 3.6 min; P = 0.0003) or BUP30 (16.7 ± 2.3 min; P < 0.0001), which did not differ (P = 0.28).
In summary, repeated applications of these three appetite suppressants produce weight loss, decrease food intake, increase locomotion, evoke a greater percentage of inhibited than activated NAc shell responses, and evoke appetite suppressant -dependent changes in LFP oscillations in the NAc.
DEP-induced locomotion, stereotypy, weight loss, and food intake are attenuated by intragastric infusion of D1R and D2R antagonists.
Since the NAc shell receives dopaminergic input from various sources (Haber et al. 1985), we determined if the effects evoked by DEP20 would be modulated by intragastric infusion of either/or the D2R antagonist RAC0.5 and/or the D1R antagonist SCH1.5. Figure 8A shows a plot of the change in body weight in naive rats over 7 days of daily treatment (n = 3 per group). As controls, we found that relative to saline, infusions of RAC0.5 (positive gain 18.4 ± 5.4 g) or SCH1.5 (11.8 ± 2.7 g) alone did not induce a significant weight loss, although intragastric infusions of DEP20 produced a large weight loss (−15.3 ± 3.1 g). This weight loss could be reversed by intragastric infusions of either DEP20+RAC0.5 (positive gain 1.4 ± 1.9 g) or DEP20+SCH1.5 (−4.4 ± 5.4 g). RM ANOVA showed a significant main effect of group [D2–D8: F(5,12) = 11.4, P = 0.0003] and across days [F(5,6) = 36.4, P <0.0001] and significant interaction between groups and days [F(30,72) = 4.9, P <0.0001]. A post hoc analysis showed that compared with DEP20, both DEP20+RAC0.5 and DEP20+SCH1.5 (only for D3–D8) significantly attenuated the weight loss induced by DEP20 (all P < 0.05).
For these same animals, the percent change in 24-h food intake was also measured (Fig. 8B). Relative to that on day 1, the food intake over the treatment was saline, 4.9 ± 9.8%; RAC0.5, 5.7 ± 8.3%; SCH1.5, 2.54 ± 5.7%; DEP20, −28.7 ± 3.8%; DEP20+SCH1.5, −16.9 ± 7.3%; and DEP20+RAC0.5, −7.13 ± 6.8%. RM ANOVA showed a significant main effect of group [F(5,12) = 4.8, P = 0.011] and across days [F(5,6) = 5, P = 0.0002] and significant interaction between groups and days [F(30,72) = 2.9, P = 0.0001]. The DEP20+SCH1.5 group ate slightly more food than the DEP20 group, although the differences were not significant (P = 0.21), whereas RAC0.5 significantly reversed food intake suppression induced by DEP20 (P = 0.032; compare DEP20 vs. DEP20+RAC0.5). These data reveal that DEP's effect on weight loss and food intake is markedly affected by inhibition of D1 and D2 receptors. Below we show that some of these effects are the result of inhibiting DA receptors in the NAc shell.
We also measured the animals changes in locomotion and stereotypy during a 2-h period (Fig. 8C, each epoch 30 min: baseline, saline, DEP20, and antagonist). For locomotion, infusion of RAC0.5 (812 ± 137 cm/5 min) or SCH1.5 (1,161 ± 152 cm/5 min) alone did not increase locomotion relative to saline (1,008 ± 181 cm/5 min). In all cases the animal's locomotion decreased from the baseline to the saline epoch and increased on the infusion of DEP20 (note that the responses to DEP20 are not identical, likely because they were affected by the previous treatment days). The DEP20-induced locomotion [3,957 ± 293 cm/5 min; RM ANOVA from 1 to 120 min; main effect of groups: F(5,118) = 45.1, P < 0.0001] was attenuated by subsequent administrations of either SCH1.5 (3,193 ± 198 cm/5 min; P < 0.0001; group DEP20+SCH1.5) or RAC0.5 (2,675 ± 233 cm/5 min; P < 0.0001; group DEP20+RAC0.5). Locomotion was not significantly different between DEP20+RAC0.5 and DEP20+SCH1.5 (P = 0.06).
Similar effects to those reported above were also found for stereotypy (Fig. 8D). Again, application of RAC0.5 or SCH1.5 alone did not evoke head-weaving stereotypy (Fig. 8D). However, DEP20 evoked stereotypy [97 ± 7 counts/5 min; RM ANOVA from 1 to 120 min; main effect of groups: F(5,118) = 64.8, P < 0.0001] was significantly reduced by either DEP20+RAC0.5 (54 ± 12 counts/5 min; post hoc, P = 0.0004) or DEP20+SCH1.5 (40 ± 8 counts/5 min; P < 0.0001). Note that DEP20-induced stereotypy remained at high levels for times longer than 1 h, whereas stereotypy declined within 30 min in the presence of the antagonists. These results provide evidence for the involvement of DA receptors in the DEP20-induced locomotion and stereotypy as well as on its anorectic effects.
Blockade of D1R and D2R directly in the NAc shell diminishes the pharmacological effects of DEP20.
To directly address the question of whether DA receptors in the NAc shell mediate DEP's behavioral effects, we injected either D1R (SCH) or D2R antagonists (RAC) directly into the NAc shell, whereas rats in the above-described experiment received intragastric infusion of DEP20. Figure 9A shows a plot of the change in body weight across 7 days of daily treatment. Briefly, all groups received two injections: one directly in the NAc shell [saline (Sal), SCH, or RAC] and a second one intragastrically (Sal or DEP20). RM ANOVA showed a significant main effect of group [D2–D8: F(5,12) = 20.4, P <0.0001] and across days [F(5,6) = 87.2, P <0.0001] and significant interaction between groups and days [F(30,72) = 2.7, P = 0.0004]. A post hoc analysis showed that relative to the control group Sal+Sal (10 ± 1.1 g), neither the group RAC+Sal (9.2 ± 1.6 g; P = 0.74) nor SCH+Sal (13.9 ± 0.9 g; P = 0.13) was significantly different, indicating that infusion of these DA antagonists in the NAc shell alone did not induce a significant weight loss. The weight loss produced by intragastric infusions of DEP20 (Sal+DEP20) was −6.4 ± 1.9 g (P < 0.0001), and it was significantly attenuated by intra-NAc shell infusions of either SCH+DEP20 (positive gain 2.1 ± 2.5 g; P = 0.004) or RAC+DEP20 (−0.8 ± 1.7 g; P = 0.04). Thus D1 and D2 NAc shell receptors are clearly involved in DEP20’s induction of weight loss.
For the same animals, food intake was also measured (Fig. 9B). Specifically, RM ANOVA for D2 to D8 showed a significant main effect of group [F(5,12) = 7.5, P = 0.002], a significant effect across days [F(5,6) = 14.7, P < 0.0001], and a significant interaction between groups and days [F(30,72) = 3.2, P < 0.0001]. Overall, across 7 days of treatment (D2–D8), post hoc analysis revealed that the RAC+DEP20 group showed a nonsignificant trend to consume more chow food relative to the Sal+DEP20 group (P = 0.075) but largely reduced chow intake in D2 and D3 [1-way ANOVA; F(1,10) = 10.61, P = 0.0086; Sal+DEP20 vs. RAC+DEP20]. Likewise, the SCH+DEP20 group ate significantly more food than the Sal+DEP20 group (P = 0.009). No differences were found between the SCH+DEP20 and RAC+DEP20 groups (P = 0.28). These data show that DEP20’s effects on food intake were partially, but significantly, reduced by NAc shell blockade of D1 and D2 receptors.
Locomotion and stereotypy were also measured under these conditions (Fig. 9C; see materials and methods). For locomotion, we found that relative to Sal+Sal (381 ± 79 cm/5 min), NAc shell infusion of RAC+Sal (404 ± 90 cm/5 min) or SCH+Sal (347 ± 71 cm/5 min) did not alter locomotion. In contrast, Sal+DEP20 induced a rapid onset (5 min) and robust locomotion [3,303 ± 368 cm/5 min; RM ANOVA; main effect of groups: F(5,118) = 159.7, P < 0.0001]. For the RAC+DEP20 experiments, the onset of locomotion induced by DEP20 was delayed by 15 min and the magnitude of locomotion was attenuated (2,094 ± 199 cm/5 min; P < 0.0001). Similar results were found for the SCH+DEP20 experiments, where the onset of locomotion induced by DEP20 was further delayed by 35 min, as well as its magnitude (881 ± 47 cm/5 min; P < 0.0001).
Similar effects to those found for locomotion were replicated for head-weaving stereotypy (Fig. 9D). As controls, we found that RAC or SCH by themselves did not evoke head-weaving stereotypy (Fig. 9D). Whereas DEP20 evoked a robust stereotypy [72 ± 2 counts/5 min; RM ANOVA; main effect of groups: F(5,118) = 212, P < 0.0001], this effect was significantly diminished (but not eliminated) by either RAC+DEP20 (40 ± 2 counts/5 min; post hoc P < 0.0001) or SCH+DEP20 (7 ± 1 counts/5 min; P < 0.0001). Importantly, both groups, RAC+DEP20 (P < 0.0001) and especially SCH+DEP20 (P < 0.0001), almost completely attenuated the DEP20-induced stereotypy. These results provide evidence for the involvement of NAc shell dopamine receptors in the ability of DEP20 to trigger and to maintain locomotion and stereotypy.
DEP20-induced LFP oscillations and spiking inhibition in the NAc shell are mediated by D1R and D2R receptors.
Having shown that both intragastric and intra-NAc shell infusions affect behavior for technical reasons (to avoid drift in waveform stability induced by intra-NAc microinjections), we next determined whether intragastric infusion of the two tested DA antagonists would similarly affect DEP20-induced NAc shell activity. We recorded the electrophysiological responses over 7 days of an intragastric infusion of RAC or SCH 30 min after the infusion of DEP20 (see below for controls). Figure 10A is a plot of the normalized color-coded PSTHs of all neurons (over 7 days) modulated by DEP20 during the baseline, saline, DEP20, and RAC0.5 epochs. In the presence of RAC0.5 (DEP20+RAC0.5), the extreme bias toward the inhibition seen with DEP20 alone (only neurons recorded over 7 days of DEP20) were diminished, since now only 30% (39/81) were inhibited and 48% (25/81) were activated [χ2(1) = 2.2, P = 0.13, ns]. The averaged normalized DEP20-induced z-score PSD for the beta, theta, and delta oscillations over the treatment period were seen to decrease to baseline levels with RAC0.5 (Fig. 10B). Finally, RAC0.5 alone did not induce changes in the LFP oscillations at beta, theta, and delta bands over the 7 days of treatment (see Fig. 10B, thin traces), and neither induced locomotion during single-unit recordings (Fig. 10B, bottom, pink trace).
RAC0.5 also attenuated and delayed the DEP20-induced increase in locomotion (Fig. 10B, bottom; cyan trace indicates locomotion measured in the operant chamber) [Kruskal-Wallis; H(2) = 53, P < 0.0001] and delayed the onset of DEP20-induced locomotion by 11.8 ± 2.2 min, which was significantly slower than that normally evoked by DEP20 alone [6.2 ± 1.36 min; F(1,21) = 5.05 P = 0.035; over 7 days of treatment].
Figure 10C shows the global population firing rate of all 81 neurons recorded with DEP20+RAC0.5 (cyan trace). It is seen that the global inhibitory imbalance induced by DEP20 is largely abolished by repeated administrations of RAC0.5. Nevertheless, RAC0.5 alone modulated NAc shell single-unit activity, although the percentage of neurons inhibited (n = 29/93, 31%) was similar to the percentage of those activated (n = 26/93, 28%) (P = 0.72, ns; data not shown). In summary, during the infusion of RAC0.5 alone, the average population activity of all 93 neurons recorded showed a slight but transient activation that rapidly returned to baseline (<4 min; see Fig. 10C, pink trace). It follows that RAC0.5 alone does not induce an inhibitory imbalance in the NAc shell population activity.
We also tested SCH1.5 alone and found that it modulated single-unit activity to about the same extent, inducing nearly the same amount of inhibition (n = 34/114 neurons, 29.8%) as activation (n = 35/114 neurons, 30%; data not shown). Accordingly, SCH1.5 alone did not produce any inhibitory imbalance at the population activity level (see Fig. 10F, black trace). In this regard, SCH1.5 did not restore the DEP20-induced inhibitory imbalance such that with SCH1.5 there were 63% (71/112) inhibited and 17.8% (20/112) activated neurons [χ2= 20.8, P < 0.0001; Fig. 10D]. This result was unexpected given the effect of SCH1.5 on the behavioral data (Fig. 8, DEP20+SCH1.5). Consequently, we determined whether SCH3 would restore the DEP20-induced imbalance. Indeed, under this condition, 41% (14/34) were inactivated and 21% (7/34) activated (the proportions of inactive to active responses were not significantly different; χ2= 1.7, P = 0.18). Thus SCH3 effectively rebalanced the DEP20-induced imbalance (inhibited/activated) by decreasing the number of neurons with inhibited responses and slightly increasing the number of active responses.
With regard to the DEP20-induced beta, theta, and delta oscillations over the treatment period, the onset of DEP20-induced oscillations was markedly delayed by SCH1.5 and even more by SCH3 (Fig. 10E). As a control, we found that SCH1.5 alone did not modulate beta, theta, or delta LFP oscillations (Fig. 10E, thinnest traces). In addition, after the infusion of SCH1.5, the magnitude of the LFP PSD slightly changed (from 3.0 ± 0.08 in the DEP20 epoch to 2.59 ± 0.08 in the SCH epoch). Likewise, for SCH3, it also marginally changed from 1.18 ± 0.06 in the DEP20 epoch to 0.92 ± 0.07 in the SCH1.5 epoch. Nevertheless, SCH markedly attenuated DEP20-induced beta, theta, and delta oscillations in a dose-dependent manner. Likewise, SCH decreased the locomotion in a dose-dependent manner. It also delayed the onset of DEP20-induced locomotion from 6.2 ± 1.4 to 11.5 ± 2.5 (SCH1.5) and 24.9 ± 4.5 min (SCH3) [F(2,33) = 11.64, P = 0.0001; Fig. 10E, bottom, green trace].
Finally, to determine at the population firing rate level whether SCH would reverse the inhibition induced by DEP20, we plotted in Fig. 10F the population activity of all the recorded neurons (modulated or not by DEP20). SCH reversed the strong inhibition evoked by DEP20 in a dose-dependent manner at the population NAc shell activity level.
DISCUSSION
The most common recommendations for persons wanting to lose weight are dietary changes, exercise, and, in many cases, the short-term use of appetite suppressants. Many prescribed suppressants, such as diethylpropion (DEP), phentermine (PHEN), and bupropion (BUP), are mild psychostimulants and, as such, increase wakefulness, increase psychomotor activity, cause changes in mood, and produce weight loss (Silverstone 1992). To cause all these changes, these compounds must affect many brain areas, in part by increasing the concentration of neurotransmitters such as serotonin (to reduce craving), norepinephrine (to increase activity and metabolism), and dopamine (for reward and motor activity) (Rothman et al. 2002). Nevertheless, despite their extensive usage, their evoked behavioral changes and their corresponding neuronal correlates remain poorly understood. For this reason, in rats we investigated behavioral and concurrently neural responses in the NAc shell to repeated intragastric treatments of DEP, PHEN, and BUP.
In this study we found that all three anorexic compounds increased weight loss, decreased food intake, and increased locomotion and stereotypy, although the animals developed tolerance to these drugs with increasing treatments. Recordings of responses from the NAc shell revealed that these appetite suppressants evoked a global inhibitory imbalance involving all putative neuronal types that correlated with the onset of locomotion and stereotypy. Analysis of the LFPs revealed that these compounds initially increased delta, theta, and beta oscillations, which correlated with the animal's initial weight loss and decrease in food intake and also with the subsequent tolerance developed during treatment. In addition, we identified roles for dopamine D1-like and D2-like receptors in the DEP20-induced effect on weight loss, food intake, locomotion, stereotypy, global inhibition of NAc shell spiking activity, and changes in LFP oscillations. Thus activation of D1-like and D2-like receptors by DEP (and presumably similar to other appetite suppressants; Janhunen et al. 2013) are important factors in the ability of this class of anorectic compounds to induce weight loss and corresponding behavioral changes.
Comparison of the Appetite Suppressants
Previous studies in humans revealed that at appropriate concentrations, DEP, PHEN, and BUP produce weight loss (Hendricks et al. 2009) with the order DEP ≥ PHEN > BUP (Cercato et al. 2009; Suplicy et al. 2014). In the present study, using rats, we found that DEP20 was slightly more potent than PHEN20 with regard to weight loss (Fig. 7A), locomotion, and stereotypy (Fig. 7B), although for food intake it was equipotent (suppression). Nevertheless, our data show that these three drugs are in fact more similar than different. For example, the efficacy of the drugs was not significantly different with respect to times in behavioral states (Table 3), putative cell types of NAc shell neurons activated or inhibited (Tables 1 and 4), changes in firing rates (inhibited/activated; Table 2), and initial effects on LFPs (Figs. 4, 5, and 7). Thus, in the NAc shell, these appetite suppressants evoke a similar neuronal signature. In this regard, similarities were expected because these drugs are all mild congeners of amphetamine.
The effects of these appetite suppressants on weight loss and food intake are of clinical interest because after the initial decrease of weight and food intake, the animals developed tolerance to them that resulted in increasing their food intake and regaining weight (Fig. 7A). These results parallel findings in humans taking these same appetite suppressants (Fernstrom and Choi 2008). Although humans develop tolerance to the weight loss effects induced by appetite suppressants on a different time scale (in months, not weeks), they follow the same pattern as rats. For humans, after 5–6 mo of treatment their weight loss plateaus. From this point their body weight is either maintained or is slowly increased (Cercato et al. 2009). Although the mechanisms explaining why appetite suppressants develop tolerance are largely unknown, we found that LFP oscillations in the NAc shell can be used as a biomarker to determine when tolerance to DEP20 develops.
Locomotion and Stereotypy
Locomotion involves any type of forward nonrepetitive movement, whereas stereotypy behaviors are repetitive motor patterns, such as head oscillations, with no obvious function (Mason 1991). Although all three DEP concentrations tested increased locomotion (Fig. 1C; Reimer et al. 1995), DEP10 produced the greatest increase in locomotion, a result that contrasts with the food intake studies, where the DEP40 had the greatest effect (Fig. 1A). DEP also induces stereotypy (Safta et al. 1976), and as with locomotion, after treatment is stopped for 1 day, the stereotypic response returns to baseline, a result similar to that observed after chronic treatment with amphetamine (Borison et al. 1977). This is the first report showing that, like DEP and amphetamine (Safta et al. 1976), PHEN20 and BUP30 also induce head-weaving stereotypy (Fig. 7B), suggesting this behavior is a hallmark of this class of appetite suppressants. The rapid onset of locomotor activity suggests that DEP can be rapidly metabolized and cross the blood-brain barrier (Carlsson and Johansson 1978; Cox and Maickel 1972).
Behavioral Mechanisms of Appetite Suppressants Hypophagia
In this study we used information showing that amphetamine delayed the onset of eating and increased the inter-meal interval (Blundell et al. 1976). These data rule out an effect on satiety signals but support the idea that amphetamine-induced anorexia evokes motor stimulatory effects that compete (interfere) with feeding (Wolgin 2000). In agreement with this idea, our data indicate that, unlike the anorexic effect of the short-term satiety peptide CCK-8, which delays gastric emptying, DEP20-induced anorexia is not mediated by a mechanism that involves delaying gastric emptiness (Fig. 2). This result is also consistent with our data showing that rats on appetite suppressants completely neglect chow food during the initial 2 h after injection (see Supplementary Video 1; see also Ghosh and Parvathy 1976). In other words, the data suggest that the greater the locomotion and stereotypy, the greater the food suppression (Fig. 7B).
Another mechanism of appetite suppressants to induce anorexia is their potential to induce gastric malaise. It is known that amphetamine can induce taste aversion (CTA; see Carey 1973). In the present study we show that DEP20, and presumably other similar appetite suppressants, can also induce taste aversion to a novel palatable tastant (Fenu and Di Chiara 2003). We suggest that DEP20’s ability to induce taste aversion can partially contribute to its anorectic effects by decreasing the consumption of novel foods and/or by reducing the probability of trying novel foods. However, DEP20’s anorectic effect induced on a highly familiar food cannot readily be attributed to taste aversion, because it is much more difficult to acquire aversion to familiar foods (Garcia et al. 1974). Moreover, because the animals increased their food intake across injection days (Fig. 1A), it is unlikely this can be attributed to conditioned taste aversion, which shows little tolerance (Garcia et al. 1974). Thus not all anorectic effects of appetite suppressants can be accounted for by their potential to induce gastric malaise. Additional evidence against the possibility that animals developed a strong taste aversion to chow is the finding that rats dramatically increased food intake the very first day after drug withdrawal (data not shown), and this overconsumption of chow (relative to saline group) still persisted even 7 days after drug withdrawal (see Fig. 1A, day 21).
In summary, the behavioral effects induced by these appetite suppressants reflect the strong interconnection among different brain networks controlling weight loss, feeding, sleep, locomotion, and stereotypy (Costa et al. 2007; Nicola 2010; Seiden et al. 1993; Tellez et al. 2012). As outlined below, dopamine plays important roles in relating all these behaviors (de Araujo et al. 2012; Dzirasa et al. 2006).
Neuronal Modulation of the NAc Shell Single-Unit Activity
To determine possible neuronal correlates underlying these behavioral effects, we performed recordings in the NAc shell, a brain region that integrates inputs from limbic brain regions and transmits them to motor and feeding regions (Hajnal and Norgren 2004; Mogenson et al. 1980). For this reason appetite suppressants are likely acting in multiple brain regions, and the NAc shell is not the only area important for the action of these appetite suppressants (Shi et al. 2000). However, in this study we found that DA receptors in the NAc shell contribute to most, if not all, of the pharmacological effects induced by DEP20 (Fig. 9). Furthermore, the NAc shell is a critical region for amphetamine's effects because direct infusions of large doses of amphetamine into the NAc shell can suppress food intake and stimulate locomotion (Carr and White 1986; Kelley et al. 1989). In contrast, lower doses of amphetamine can promote food intake (Evans and Vaccarino 1986, 1990; Wise et al. 1989), suggesting that the level of dopamine stimulation in the NAc can have a bidirectional control on food intake. In the same vein, it has been shown that pharmacological inactivation of the NAc shell using low doses of the GABAA agonist muscimol promotes feeding, although higher doses suppressed feeding and induced locomotion (Kelley et al. 1989; Stratford and Kelley 1997).
This biphasic effect has been substantiated by the finding of a moderate inhibitory imbalance in the population NAc shell produced by rats eating hedonically positive foods (Krause et al. 2010; Tellez et al. 2012), whereas Krause et al. 2010 showed that electrical stimulation of the NAc shell stops feeding, revealing that NAc activity can have a bidirectional control on feeding. In the present study we found a large inhibitory imbalance in the rat NAc activity obtained with appetite suppressants, which decreases food intake and increases locomotion and stereotypy. We are aware that these data add a degree of complexity to both the “gating feeding hypothesis,” which states that inhibition in the NAc allows feeding behavior (Krause et al. 2010), and the “NAc decreased (reward)/increased (aversion) activity hypothesis” (Carlezon and Thomas 2009). Thus the strength of the inhibitory imbalance of the population NAc shell activity can correlate with multiple and sometimes conflicting behavioral outputs, such as food intake and appetite suppressant-induced anorexia.
An important result of this study is that all three appetite suppressants evoked a large inhibitory imbalance in the NAc shell that involved all putative cell types and that matched the onset and continuance of locomotion and stereotypy (Fig. 7; Tables 1 and 4). These locomotor behaviors are incompatible with both food intake and sleep. These data also show that pSPNs are the neurons most likely to be activated by DEP20 (Table 4) and that BUP30 inhibited a greater percentage of pChAT neurons than the other appetite suppressants (Table 4), a finding consistent with its antinicotinic activity (Arias 2009). In summary, we have identified a correlation between changes in NAc shell single-unit activity and the onset of motor changes.
LFP Oscillations in the NAc Shell
Whereas appetite suppressants cause a rapid imbalance in the firing rate of NAc shell neurons that correlates with the onset of locomotion and stereotypy, changes in LFPs correlate with the longer term behaviors involving food intake and the accompanying weight loss. This behavior is illustrated in experiments with DEP20 over 14 days (Fig. 5 and Table 6). However, for all the tested compounds, by 1 day after treatment, beta, theta, and delta oscillations were large and positive, and this change is associated with the largest decrease in food intake (Fig. 5C and Table 6). However, on subsequent treatment days, the magnitude of the three oscillations decreased and food intake increased. This is the first report regarding the measurement of the temporal changes evoked by appetite suppressants in the NAc shell and their relation to food intake. We are aware that these oscillations are also involved in motor and other behaviors (Jenkinson and Brown 2011) and that, in rats, cortical EEG studies that have shown that DEP evokes robust cortical delta oscillations (Safta et al. 1976). We hypothesize that amplification of delta oscillations during awake states over those seen in SWS (Steriade et al. 2001) is related to conditions were DA levels are outside of their physiological dynamic range (Costa et al. 2006; Lemaire et al. 2012) and situations where energy consumption needs to be reduced (Dworak et al. 2011). Likewise, beta oscillations are normally high during tonic muscle rigidity in preparation to voluntary movement, but they are amplified in Parkinson disease patients, whose DA levels are lower than its physiological range (Jenkinson and Brown 2011). It is possible that the decrease of DEP20-induced beta oscillations might be related to a DA alteration after chronic use, whereas theta oscillations induced by appetite suppressants might be related to locomotion and exploratory head-weaving behaviors (Buzsaki 2005).
DEP20-induced LFP Oscillations Do Not Reflect an Aversive Gastric Malaise Brain State
Fenu and Di Chiara (2003) reported that the D1 receptor antagonist SCH39166, administered before amphetamine either systematically or directly in the NAc shell, prevented the facilitation of conditioned taste aversion induced by amphetamine. They concluded that amphetamine facilitates taste aversion learning via D1 receptors of the NAc shell. This result highlights an important role of dopamine release in the NAc shell and the acquisition of taste aversion induced by amphetamine. Although the neuronal correlates of the aversive potential of appetite suppressants is beyond the scope of this article, we provide evidence indicating that DEP20-induced LFP oscillations do not correlate with an aversive gastric malaise-induced brain state. This is because a prototypical gastric malaise agent, LiCl, did not alter NAc shell LFP oscillations (Fig. 6C). Indeed, no LiCl-induced LFP oscillations occurred even when LiCl induced insomnia or distress movements (e.g., lying on the belly), although it has been shown that LiCl can also increase dopamine release in the NAc shell (Ventura et al. 2007).
Dopamine Receptors and DEP's Effect
The three appetite suppressants tested induce the release of norepinephrine, serotonin, and dopamine (Opacka-Juffry et al. 2014). In the present study we have showed that their appetite-suppressing and motor effects, as well as changes in NAc shell activity, can be accounted for in part by their ability to release dopamine. In this regard, amphetamine causes DA to be released in the rat NAc (Daberkow et al. 2013) and also alters their feeding and motor behaviors (stereotypies) (Seiden et al. 1993). Indeed, a general dopaminergic tone appears to be necessary to express the full magnitude of locomotor activity and stereotypy induced by amphetamine (Sotnikova et al. 2005), DEP (Samanin and Garattini 1993), PHEN (Offermeier and Potgieter 1972), or BUP (Janhunen et al. 2013). What has not been tested is how these drugs affect the NAc shell activity and whether DA antagonists can reverse their effects. In this study we showed that DEP's effects on locomotion and stereotypy were attenuated by both systemic and intra-NAc shell infusions of D1R and D2R antagonists (Figs. 8 and 9). In agreement with the proposed role of these receptors, we found that SCH-23390 strongly delayed the onset and magnitude of locomotion and stereotypy, suggesting that activation of D1Rs is necessary for triggering these behaviors (Szczypka et al. 1999). Our data also indicate that D1Rs can be involved in food intake and weight loss. In agreement with the prominent role of D2Rs in mediating feeding, energy homeostasis (Kim et al. 2010), and obesity (Johnson and Kenny 2010), we observed that D2R antagonist reversed the anorectic and weight loss effects induced by DEP and (when infused intragastrically) reversed the net inhibitory imbalance in the NAc shell produced by DEP. Nevertheless, D2Rs seem to also play an important role in triggering locomotion and stereotypy. Our data favor the idea of a more cooperative action of both D1- and D2-like receptors in the brain and in the NAc mediating the pharmacological effects of this class of appetite suppressants.
In summary, these results demonstrate that appetite suppressants modulate weight loss, food intake, locomotion, and stereotypy. Finally, we found that both their induced behavioral and electrophysiological changes were largely reversed by D1R and D2R antagonists, thus providing a role for these receptors in the anorexia induced by these appetite suppressants.
GRANTS
This project was supported in part by Consejo Nacional de Ciencia y Tecnologia of Mexico Grants 179484, Salud2010-02-151001, ICYTDF-PICSA12-126, and Productos Medix 001275 (to R. Gutierrez).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.K., J.S., S.A.S., and R.G. conception and design of research; B.K., C.I.P., J.S., M.G.M., and R.G. performed experiments; B.K., C.I.P., A.L., D.E., and R.G. analyzed data; B.K., A.L., D.E., S.A.S., and R.G. interpreted results of experiments; B.K., C.I.P., A.L., S.A.S., and R.G. prepared figures; B.K., S.A.S., and R.G. drafted manuscript; B.K., C.I.P., A.L., J.S., M.G.M., D.E., S.A.S., and R.G. approved final version of manuscript; S.A.S. and R.G. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Isaac O. Perez, Dayana Buendia, and Sebastian Citlalan for invaluable technical support. We especially thank Andrei Vargas for building electrodes.
REFERENCES
- Arias HR. Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions? Int J Biochem Cell Biol 41: 2098–2108, 2009. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res 137: 165–177, 2002. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse 36: 102–113, 2000. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Latham CJ, Leshem MB. Differences between the anorexic actions of amphetamine and fenfluramine–possible effects on hunger and satiety. J Pharm Pharmacol 28: 471–477, 1976. [DOI] [PubMed] [Google Scholar]
- Borison RL, Havdala HS, Diamond BI. Chronic phenylethylamine stereotypy in rats: a new animal model for schizophrenia? Life Sci 21: 117–122, 1977. [DOI] [PubMed] [Google Scholar]
- Broening HW, Morford LL, Vorhees CV. Interactions of dopamine D1 and D2 receptor antagonists with d-methamphetamine-induced hyperthermia and striatal dopamine and serotonin reductions. Synapse 56: 84–93, 2005. [DOI] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci 34: 1997–2006, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15: 827–840, 2005. [DOI] [PubMed] [Google Scholar]
- Carey RJ. Long-term aversion to a saccharin solution induced by repeated amphetamine injections. Pharmacol Biochem Behav 1: 265–270, 1973. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56, Suppl 1: 122–132, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson C, Johansson BB. Blood-brain barrier dysfunction after amphetamine administration in rats. Acta Neuropathol (Berl) 41: 125–129, 1978. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Contributions of dopamine terminal areas to amphetamine-induced anorexia and adipsia. Pharmacol Biochem Behav 25: 17–22, 1986. [DOI] [PubMed] [Google Scholar]
- Cercato C, Roizenblatt VA, Leanca CC, Segal A, Lopes Filho AP, Mancini MC, Halpern A. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond) 33: 857–865, 2009. [DOI] [PubMed] [Google Scholar]
- Chang FY, Lu CL, Chen TS, Wang PS. The role of cholecystokinin 1 receptor in prolactin inhibited gastric emptying of male rat. J Neurogastroenterol Motil 18: 385–390, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover KL, Collins SM, Weingarten HP. A comparison of cholecystokinin-induced changes in gastric emptying and feeding in rats. Am J Physiol Regul Integr Comp Physiol 255: R21–R26, 1988. [DOI] [PubMed] [Google Scholar]
- Costa RM, Gutierrez R, de Araujo IE, Coelho MR, Kloth AD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Dopamine levels modulate the updating of tastant values. Genes Brain Behav 6: 314–320, 2007. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 52: 359–369, 2006. [DOI] [PubMed] [Google Scholar]
- Cox RH Jr, Maickel RP. Comparison of anorexigenic and behavioral potency of phenylethylamines. J Pharmacol Exp Ther 181: 1–9, 1972. [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Garris PA, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci 33: 452–463, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Ferreira JG, Tellez LA, Ren X, Yeckel CW. The gut-brain dopamine axis: a regulatory system for caloric intake. Physiol Behav 106: 394–399, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derlet RW, Albertson TE, Rice P. The effect of SCH 23390 against toxic doses of cocaine, d-amphetamine and methamphetamine. Life Sci 47: 821–827, 1990. [DOI] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Basheer R. Delta oscillations induced by ketamine increase energy levels in sleep-wake related brain regions. Neuroscience 197: 72–79, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA. Dopaminergic control of sleep-wake states. J Neurosci 26: 10577–10589, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KR, Vaccarino FJ. Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev 14: 9–22, 1990. [DOI] [PubMed] [Google Scholar]
- Evans KR, Vaccarino FJ. Intra-nucleus accumbens amphetamine: dose-dependent effects on food intake. Pharmacol Biochem Behav 25: 1149–1151, 1986. [DOI] [PubMed] [Google Scholar]
- Fenu S, Di Chiara G. Facilitation of conditioned taste aversion learning by systemic amphetamine: role of nucleus accumbens shell dopamine D1 receptors. Eur J Neurosci 18: 2025–2030, 2003. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Choi S. The development of tolerance to drugs that suppress food intake. Pharmacol Ther 117: 105–122, 2008. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother 7: 17–24, 2007. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science 185: 824–831, 1974. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. Global forebrain dynamics predict rat behavioral states and their transitions. J Neurosci 24: 11137–11147, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MN, Parvathy S. Tolerance pattern of the anorexigenic action of amphetamines, fenfluramine, phenmetrazine and diethylpropion in rats. Br J Pharmacol 57: 479–485, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Carmena JM, Nicolelis MA, Simon SA. Orbitofrontal ensemble activity monitors licking and distinguishes among natural rewards. J Neurophysiol 95: 119–133, 2006. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Simon SA, Nicolelis MA. Licking-induced synchrony in the taste-reward circuit improves cue discrimination during learning. J Neurosci 30: 287–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Tellez LA, Bermudez-Rattoni F. Blockade of cortical muscarinic but not NMDA receptors prevents a novel taste from becoming familiar. Eur J Neurosci 17: 1556–1562, 2003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol 235: 322–335, 1985. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Sucrose sham feeding decreases accumbens norepinephrine in the rat. Physiol Behav 82: 43–47, 2004. [DOI] [PubMed] [Google Scholar]
- Halje P, Tamte M, Richter U, Mohammed M, Cenci MA, Petersson P. Levodopa-induced dyskinesia is strongly associated with resonant cortical oscillations. J Neurosci 32: 16541–16551, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp C, Kang EM, Borders-Hemphill V. Use of prescription antiobesity drugs in the United States. Pharmacotherapy 33: 1299–1307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 17: 1730–1735, 2009. [DOI] [PubMed] [Google Scholar]
- Holt GR, Softky WR, Koch C, Douglas RJ. Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J Neurophysiol 75: 1806–1814, 1996. [DOI] [PubMed] [Google Scholar]
- Janhunen SK, la Fleur SE, Adan RA. Blocking alpha2A adrenoceptors, but not dopamine receptors, augments bupropion-induced hypophagia in rats. Obesity (Silver Spring) 21: E700–E708, 2013. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci 34: 611–618, 2011. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13: 635–641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86: 773–795, 2005. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Gauthier AM, Lang CG. Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res 35: 27–39, 1989. [DOI] [PubMed] [Google Scholar]
- Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY, Shin SW, An JJ, Kim MS, Choi SY, Sun W, Baik JH. Enhanced hypothalamic leptin signaling in mice lacking dopamine D2 receptors. J Biol Chem 285: 8905–8917, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci 30: 4746–4756, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire N, Hernandez LF, Hu D, Kubota Y, Howe MW, Graybiel AM. Effects of dopamine depletion on LFP oscillations in striatum are task- and learning-dependent and selectively reversed by l-DOPA. Proc Natl Acad Sci USA 109: 18126–18131, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Chung S, Lu DP, Cho YK. Descending projections from the nucleus accumbens shell suppress activity of taste-responsive neurons in the hamster parabrachial nuclei. J Neurophysiol 108: 1288–1298, 2012. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Moreton JE. A technique for chronic intragastric drug administration in the rat. Life Sci 25: 593–600, 1979. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci 17: 1198–1207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GJ. Stereotypies and suffering. Behav Processes 25: 103–115, 1991. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron 78: 910–922, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson K. Reducing the global prevalence of overweight and obesity. Lancet 2014. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97, 1980. [DOI] [PubMed] [Google Scholar]
- Moyers SB. Medications as adjunct therapy for weight loss: approved and off-label agents in use. J Am Diet Assoc 105: 948–959, 2005. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci 30: 16585–16600, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermeier J, Potgieter B. The possible central stimulant mechanisms of some appetite suppressants. S Afr Med J 46: 72–77, 1972. [PubMed] [Google Scholar]
- Opacka-Juffry J, Pinnell T, Patel N, Bevan M, Meintel M, Davidson C. Stimulant mechanisms of cathinones–effects of mephedrone and other cathinones on basal and electrically evoked dopamine efflux in rat accumbens brain slices. Prog Neuropsychopharmacol Biol Psychiatry 54: 122–130, 2014. [DOI] [PubMed] [Google Scholar]
- Reimer AR, Martin-Iverson MT, Urichuk LJ, Coutts RT, Byrne A. Conditioned place preferences, conditioned locomotion, and behavioral sensitization occur in rats treated with diethylpropion. Pharmacol Biochem Behav 51: 89–96, 1995. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Yin HH. Elevated dopamine alters consummatory pattern generation and increases behavioral variability during learning. Front Integr Neurosci 9: 37, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JD, Rowland NE. Anorectic efficacy of the fenfluramine/phentermine combination in rats: additivity or synergy? Eur J Pharmacol 373: 127–134, 1999. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann NY Acad Sci 965: 109–126, 2002. [DOI] [PubMed] [Google Scholar]
- Safta L, Cuparencu B, Sirbu A, Secareanu A. Experimental observations on the effect of amphepramone on the behavior, locomotion, pentretrazol seizures and electroencephalogram. Psychopharmacology (Berl) 50: 165–169, 1976. [DOI] [PubMed] [Google Scholar]
- Samanin R, Garattini S. Neurochemical mechanism of action of anorectic drugs. Pharmacol Toxicol 73: 63–68, 1993. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol 33: 639–677, 1993. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Smith PL, Bunney BS. Endogenous DA-mediated feedback inhibition of DA neurons: involvement of both D(1)- and D(2)-like receptors. Synapse 35: 111–119, 2000. [DOI] [PubMed] [Google Scholar]
- Silverstone T. Appetite suppressants. A review. Drugs 43: 820–836, 1992. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Beaulieu JM, Barak LS, Wetsel WC, Caron MG, Gainetdinov RR. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol 3: e271, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17: 4434–4440, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suplicy H, Boguszewski CL, Dos Santos CM, do Desterro de Figueiredo M, Cunha DR, Radominski R. A comparative study of five centrally acting drugs on the pharmacological treatment of obesity. Int J Obes (Lond) 38: 1097–1103, 2014. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Rainey MA, Kim DS, Alaynick WA, Marck BT, Matsumoto AM, Palmiter RD. Feeding behavior in dopamine-deficient mice. Proc Natl Acad Sci USA 96: 12138–12143, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez LA, Perez IO, Simon SA, Gutierrez R. Transitions between sleep and feeding states in rat ventral striatum neurons. J Neurophysiol 108: 1739–1751, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno A, Lazaro R, Wang PY, Higashiyama R, Machida K, Tsukamoto H. Mouse intragastric infusion (iG) model. Nat Protoc 7: 771–781, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boss R, Cools AR, Ogren SO. Differential effects of the selective D2-antagonist raclopride in the nucleus accumbens of the rat on spontaneous and d-amphetamine-induced activity. Psychopharmacology (Berl) 95: 447–451, 1988. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci USA 104: 5181–5186, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Convit A, Logan J, Wong CT, Shumay E, Fowler JS, Volkow ND. BMI modulates calorie-dependent dopamine changes in accumbens from glucose intake. PLoS One 9: e101585, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med 3: 8–18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbert B, Mohundro BL, Shaw V, Andres A. Appetite suppressants as adjuncts for weight loss. Am Fam Physician 83: 1–2, 2011. [PubMed] [Google Scholar]
- Wise RA, Fotuhi M, Colle LM. Facilitation of feeding by nucleus accumbens amphetamine injections: latency and speed measures. Pharmacol Biochem Behav 32: 769–772, 1989. [DOI] [PubMed] [Google Scholar]
- Wolgin DL. Contingent tolerance to amphetamine hypophagia: new insights into the role of environmental context in the expression of stereotypy. Neurosci Biobehav Rev 24: 279–294, 2000. [DOI] [PubMed] [Google Scholar]
- Wright JM, Dobosiewicz MR, Clarke PB. The role of dopaminergic transmission through D1-like and D2-like receptors in amphetamine-induced rat ultrasonic vocalizations. Psychopharmacology (Berl) 225: 853–868, 2013. [DOI] [PubMed] [Google Scholar]
- Yarom O, Cohen D. Putative cholinergic interneurons in the ventral and dorsal regions of the striatum have distinct roles in a two choice alternative association task. Front Syst Neurosci 5: 36, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]