Abstract
Nonhuman primates, compared with humans and rodents, have historically been far less used for studies of age-related hearing loss, primarily because of their long life span and high cost of maintenance. Strong similarities in genetics, anatomy, and neurophysiology of the auditory nervous system between humans and monkeys, however, could provide fruitful opportunities to enhance our understanding of hearing loss. The present study used a common, noninvasive technique for testing hearing sensitivity in humans, the auditory brainstem response (ABR), to assess the hearing of 48 rhesus macaques from 6 to 35 yr of age to clicks and tone stimuli between 0.5 and 16.0 kHz. Old monkeys, particularly those above 21.5 yr of age, had missing ABR waveforms at high frequencies. Regression analyses revealed that ABR threshold increased as a function of age at peaks II and IV simultaneously. In the suprathreshold hearing condition (70 dB peak sound pressure level), ABR-based audiograms similarly varied as a function of age such that old monkeys had smaller peak amplitudes and delayed latencies at low, middle, and high frequencies. Peripheral hearing differences remained a major influence associated with age-related changes in audiometric functions of old monkeys at a comparable sensation level across animals. The present findings suggest that hearing loss occurs in old monkeys across a wide range of frequencies and that these deficits increase in severity with age. Parallel to prior studies in monkeys, we found weak effects of sex on hearing, and future investigations are necessary to clarify its role in age-related hearing loss.
Keywords: rhesus macaque, hearing sensitivity, aging, geriatric, presbycusis, auditory brainstem response
age-related hearing loss (ARHL), or presbycusis, is the most common form of hearing deficit and is a pervasive sensorineural impairment associated with aging. ARHL is both peripheral and central in origin. It can impair the spectral, temporal, and spatial processing of sounds, as well as language perception and music appreciation (Gates and Mills 2005; Humes et al. 2012). It has been associated with pathological degenerations in the cochlea, such as atrophy of the stria vascularis, loss of hair cells, and loss of spiral ganglion cells (Bao and Ohlemiller 2010; Chisolm et al. 2003; Engle et al. 2013; Fetoni at al. 2011; Hawkins et al. 1986; Nelson and Hinojosa 2006; Schuknecht and Gacek 1993; Syka 2010). It is also linked to an imbalance between excitatory and inhibitory mechanisms within the central auditory system (Caspary et al. 2008). Hearing sensitivity of aged subjects is also strongly related to environmental noise exposure, which has been shown to be one of the primary factors for progressive hair-cell loss in the cochlea (Boettcher 2002; Gates and Mills 2005; Ohlemiller 2006).
The auditory brainstem response (ABR) is a noninvasive electroencephalography technique that measures peripheral hearing sensitivity and serves as a clinical screening tool to evaluate various types of hearing loss. It has been widely used in rodents to study ARHL (Boettcher 2002; Boettcher et al. 1993a, 1993b; Gates and Mills 2005; Syka 2010), which has provided important insights into how morphological and neurochemical changes in the peripheral and central auditory systems modulate ABRs in aged subjects. The macaque monkey has further been developed as an alternative animal model of human ARHL. Recent studies have shown that aging is correlated with the loss of hair cells and the loss of spiral ganglion cells in the macaque cochlea (Engle et al. 2013), as well as with different histochemical profiles in the auditory brainstem (Gray et al. 2013a, 2014), midbrain (Engle et al. 2014), and thalamus (Gray et al. 2013b). Previous studies in macaques have also shown that there are age-related changes in the ABR to click stimuli (Fowler et al. 2002; Harada et al. 1999; Laughlin et al. 1999; Torre and Fowler 2000; Torre et al. 2004), tone pips (8, 16, and 32 kHz; Fowler et al. 2010), and in detection thresholds measured behaviorally (Behar et al. 1965; Bennett et al. 1983). However, the responses to tone stimuli and age-related changes in ABR-based audiograms have not been well defined in rhesus macaques. The present study aims to evaluate the macaque as an animal model of ARHL by assessing ABR waveforms from young to old rhesus monkeys. We examined ABR waveforms at both threshold and suprathreshold levels, where most of the old monkeys had observable ABR waveforms. Peak amplitudes and latencies of ABR waveforms were used as additional measures to assess hearing sensitivity. It was hypothesized that old monkeys would have higher thresholds with lower amplitudes at longer peak latencies, compared with young and middle-aged monkeys, similar to those observed in humans and rodents (Boettcher 2002; Gates and Mills 2005). The study also examined whether there was an effect of sex associated with age-related changes in the ABR, as such an effect was recently discovered in rhesus monkeys to click stimuli (Fowler et al. 2010).
MATERIALS AND METHODS
Subjects
The subjects were 48 rhesus macaques (Macaca mulatta; 23 males, 25 females) from the California National Primate Research Center at the University of California at Davis. The subjects were from 6 to 35 yr of age (approximately equivalent to 18 to 105 yr of age for humans; see Davis and Leathers 1985). Table 1 shows the demographic information of all monkeys used in the present study. There was no significant age difference between the sexes (t-test, P > 0.1). All animals were maintained on ad libitum food and free access to water. No animal had a history of loud noise exposure, ear trauma, or ototoxic drug treatment. All experimental procedures adhered to the National Institutes of Health guidelines and were approved by the UC Davis Institutional Animal Care and Use Committee.
Table 1.
Demographic information of the 48 monkeys
| Sex | N | Age Range, yr | Mean | Median |
|---|---|---|---|---|
| Female | 15 | 21.6–35.3 | 25.2 ± 1.0 | 23.63 |
| 10 | 6.0–21.3 | 11.9 ± 1.7 | 9.38 | |
| Male | 12 | 22.3–25.4 | 24.0 ± 0.3 | 25.50 |
| 11 | 7.3–21.4 | 12.2 ± 1.3 | 10.29 |
Applicable values are means ± SE.
ABR Procedures
The ABR recording procedures were the same as those described in our previous studies (Engle et al. 2013, 2014; Gray et al. 2013a, 2013b, 2014), so they are only briefly summarized here. Experiments were conducted in an electrically and acoustically quiet room or a double-walled acoustic chamber, depending on the housing location of the animals. No difference was noted in recording quality between these two conditions. Each animal was anesthetized with ketamine (10 mg/kg im) and medatomidine (0.3 ml/10.0 kg im) and placed in a prone position with its head slightly elevated. The skin was cleaned with alcohol, and sterile 22-gauge stainless steel skin electrodes were placed subcutaneously behind both ears, on the forehead, and on the back of the neck (Allen and Starr 1978; Fowler et al. 2010; Torre and Fowler 2000; Torre et al. 2004). Auditory brainstem responses were collected using an Intelligent Hearing System (Smart EP Win USB, v. 3.97) controlled by a laptop computer. Electrode impedance was checked after placement into the skin and was below 1 kΩ for all recordings. Evoked responses were amplified 100,000 times, and the physiological filters were set to pass at 100–1,500 Hz. Auditory stimuli consisted of clicks and tone bursts (10-ms durations; 2-ms rise/fall trapezoidal envelope; 0.5, 1.0, 2.0, 4.0, 8.0, 12.0, and 16.0 kHz) presented binaurally via soft insert earphones (etymotic ER3A transducers) at a rate of 50 stimuli/s. Each stimulus was repeated a minimum of 2,000 times to obtain reliable average ABR recordings. For most subjects and stimuli, the initial sound level tested was 70 dB peak sound pressure level (pSPL). In those subjects with no observable ABR at this level, they were then tested at 80 dB pSPL. The sound level of stimuli presentation was then decreased in 10-dB pSPL increments until the ABR response was no longer observable. The sound level was then increased by 5-dB pSPL steps to determine whether the waveform would return.
Thus threshold in this case was defined as in linear interpolation between the sound level where a reliable waveform was produced and the level where it was absent. Upon completion of testing, the animals were given atipamezole (0.3 ml/10.0 kg im) to reverse the effects of the medatomidine and returned to their home cages to recover.
Data Analysis
Two independent observers blind to the age and sex of the animal scored the amplitudes and latencies of peaks II and IV of all ABR waveforms with an interobserver agreement of 95% or greater. Instances where differences exceeded 5 dB were rechecked and resolved in every case. Peaks II and IV were the most reliable ABR waveforms measured in the 48 monkeys, and thus these two peaks were the most prominent features of ABRs available for in-depth analyses. The peak amplitude of each wave was defined as the evoked potential from the summit of the peak to the lowest point of the ensuing trough. The peak latency for each peak was defined as the time from stimulus presentation onset to the summit of the peak. Instances where no observable waveform existed at any level for a click or tone stimulus suggested that a sound level higher than 80 dB pSPL was required to elicit the waveform. The traditional method for handling missing data is to exclude variables associated with those subjects, and thus the statistical power is reduced during group comparisons. Instead, we scored the threshold of the missing ABR as 85 dB pSPL, which is likely a considerable underestimate of the level necessary to elicit that particular peak waveform for that individual monkey. The corresponding ABR amplitude was estimated from spontaneous potentials within a 1-ms time window, according to the progression and temporal relationship of the missing peak with other observable peaks, stimulus types, and sound levels. This rationale takes into consideration the severity of hearing deficits from those subjects without observable ABRs at certain stimuli, particularly for geriatric monkeys. The present method therefore enabled us to include most subjects and to improve the statistical power of our data analysis.
The stepwise multiple-regression analysis (SPSS 19) was conducted separately for each parameter (threshold, amplitude, and latency) to assess the effects of age on peaks II and IV at the threshold levels that elicited those waveforms. Each parameter was the dependent variable. Regressions were conducted hierarchically with age (as a continuous variable) and sex entered to account for between-subject variability. Peak (II and IV; 2 levels) and stimulus type (clicks and tone bursts of 0.5, 1.0, 2.0, 4.0, 8.0, 12.0, and 16.0 kHz; 8 levels) were then entered to account for within-subject variability. Two-way, three-way, and four-way interactions were then orderly entered into the model. Predictor variables with a P value of 0.1 or above were removed from the model. We were also interested in analyzing the ABRs when the sounds were presented at a suprathreshold level of 70 dB pSPL. This level was tested in most monkeys, and the majority of subjects had observable peaks II and IV present although some old monkeys had no observable peak II and/or peak IV waveforms at some stimulus frequencies even at this level. The same regression analysis was used for ABR peak amplitudes and latencies during the 70-dB pSPL condition, as previously described. Latency differences between peaks II and IV were examined by the stepwise multiple-regression analysis for the threshold and the 70-dB pSPL conditions. The corresponding analysis only included data in which observable peaks II and IV were both present for a given auditory stimulus. Pearson correlations (SPSS 19) were used to assess the relationships between age and/or other factors on ABR peak parameters. Pairwise comparisons were achieved by Student's t statistics for two-level factors (i.e., sex and peak) and post hoc least-significant difference (LSD) tests for stimulus type and interaction effects. The critical probability level was set at 0.05 for all analyses and was adjusted by the number of pairwise comparisons conducted accordingly (Bonferroni correction).
RESULTS
Effects of Age on ABR at Threshold Levels
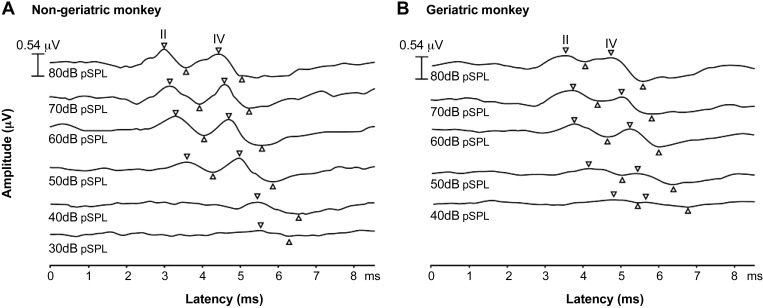
We studied 48 adult rhesus monkeys ranging from 6 to 35 yr of age. The most prominent peaks were II and IV although in many animals other peaks could be reliably observed as well. Figure 1 shows example waveforms from one nongeriatric monkey (Fig. 1A; 10.4 yr of age) and one geriatric monkey (Fig. 1B; 23.8 yr of age) to a click stimulus. In this example, peaks II and IV are clearly demarcated in both monkeys at the higher sound levels (upper traces). A common finding was that, as the stimulus level decreased, the amplitude of the peaks steadily decreased and the latency of those peaks steadily increased. This was clearly seen by comparing the size and latency of the peaks in each trace from top to bottom (80 to 30 or 40 dB pSPL). The second feature that we observed was that the geriatric monkeys did not show reliable waveforms at low sound levels, and thus they had higher ABR thresholds than young animals. Table 2 shows the percentage of female and male monkeys that had no observable peaks II and/or IV for at least one level across all of the sound stimuli presented. For monkeys below 21.5 yr of age, the absence of clear ABRs was mainly found at the two highest frequencies tested (12 and 16 kHz) at peak II. For monkeys above 21.5 yr of age, their hearing sensitivity was worst for high frequencies and spanned to some low and middle frequencies (e.g., 2 and 8 kHz at peaks II and IV). Table 3 presents the summary statistics of ABR audiograms of the 48 monkeys.
Fig. 1.
Auditory brainstem response (ABR) in a geriatric and a nongeriatric monkey. Representative examples of ABR waveforms are shown from a nongeriatric monkey (A, 10.4 yr of age) and a geriatric monkey (B, 23.8 yr of age). These waveforms were evoked by click stimuli across various sound levels (decibel peak sound pressure level, dB pSPL), starting at the highest level at the upper trace. The nongeriatric monkey had ABR thresholds at 30 dB pSPL for peak IV and at 40 dB pSPL for peak II, whereas the geriatric monkey had ABR thresholds at 40 dB pSPL for both peaks II and IV.
Table 2.
High-frequency hearing loss in old monkeys above 21.5 yr of age
| > 21.5 yr |
< 21.5 yr |
||||
|---|---|---|---|---|---|
| Stimulus Type | Female, % | Male, % | Female, % | Male, % | |
| Peak II | Clicks | 0.0 | 8.3 | 0.0 | 0.0 |
| 0.5 kHz | 6.6 | 0.0 | 10.0 | 9.1 | |
| 1.0 kHz | 13.3 | 8.3 | 10.0 | 9.1 | |
| 2.0 kHz | 33.3 | 33.3 | 10.0 | 18.2 | |
| 4.0 kHz | 13.3 | 0.0 | 0.0 | 9.1 | |
| 8.0 kHz | 33.3 | 33.3 | 0.0 | 9.1 | |
| 12.0 kHz | 60.0 | 66.6 | 40.0 | 54.5 | |
| 16.0 kHz | 66.6 | 83.3 | 40.0 | 54.5 | |
| Peak IV | Clicks | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.5 kHz | 0.0 | 0.0 | 0.0 | 0.0 | |
| 1.0 kHz | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2.0 kHz | 6.6 | 8.3 | 0.0 | 0.0 | |
| 4.0 kHz | 13.3 | 0.0 | 0.0 | 0.0 | |
| 8.0 kHz | 13.3 | 0.0 | 0.0 | 0.0 | |
| 12.0 kHz | 26.6 | 8.3 | 0.0 | 0.0 | |
| 16.0 kHz | 40.0 | 58.3 | 20.0 | 27.3 | |
Percentage of monkeys without observable auditory brainstem response (ABR) waveforms at peaks II and IV at the highest level tested. Note that monkeys above 21.5 yr of age also had hearing deficits extended to some low and middle frequencies (e.g., 2.0, 4.0, and 8.0 kHz).
Table 3.
Summary statistics of ABR audiograms from the 48 monkeys during stimulus threshold levels
| Subject | Age, yr | Thresholds, dB pSPL | Peak Amplitudes, μV | Peak Latencies, ms | |
|---|---|---|---|---|---|
| Female | 1 | 35.3 | 73.8 (3.3) | 0.11 (0.03) | 5.5 (0.4) |
| 2 | 30.4 | 63.1 (3.9) | 0.13 (0.02) | 5.5 (0.3) | |
| 3 | 29.3 | 54.7 (4.2) | 0.14 (0.03) | 5.5 (0.2) | |
| 4 | 26.2 | 67.2 (3.3) | 0.14 (0.02) | 5.4 (0.5) | |
| 5 | 26.2 | 63.8 (3.8) | 0.17 (0.03) | 5.1 (0.3) | |
| 6 | 24.0 | 66.6 (3.0) | 0.23 (0.04) | 5.0 (0.4) | |
| 7 | 23.8 | 78.4 (3.4) | 0.06 (0.02) | 4.8 (0.6) | |
| 8 | 23.4 | 62.5 (3.9) | 0.11 (0.02) | 5.0 (0.3) | |
| 9 | 23.3 | 47.2 (4.6) | 0.08 (0.02) | 5.8 (0.3) | |
| 10 | 23.3 | 57.5 (3.0) | 0.12 (0.02) | 5.5 (0.3) | |
| 11 | 23.3 | 38.4 (4.7) | 0.10 (0.02) | 6.2 (0.3) | |
| 12 | 23.0 | 52.2 (5.7) | 0.16 (0.03) | 5.5 (0.3) | |
| 13 | 22.3 | 64.1 (2.6) | 0.19 (0.03) | 5.0 (0.3) | |
| 14 | 22.3 | 54.1 (3.8) | 0.09 (0.02) | 5.1 (0.3) | |
| 15 | 21.6 | 52.8 (4.5) | 0.08 (0.02) | 5.9 (0.3) | |
| 16 | 21.3 | 56.3 (3.7) | 0.07 (0.01) | 5.7 (0.3) | |
| 17 | 20.3 | 50.6 (5.1) | 0.08 (0.01) | 5.9 (0.4) | |
| 18 | 16.3 | 28.4 (3.6) | 0.17 (0.04) | 4.9 (0.3) | |
| 19 | 10.4 | 34.4 (5.1) | 0.18 (0.03) | 4.6 (0.4) | |
| 20 | 9.4 | 37.2 (3.9) | 0.18 (0.02) | 4.5 (0.3) | |
| 21 | 9.3 | 49.1 (5.2) | 0.12 (0.02) | 5.4 (0.3) | |
| 22 | 9.3 | 32.5 (5.1) | 0.13 (0.01) | 4.7 (0.4) | |
| 23 | 9.3 | 36.9 (6.7) | 0.16 (0.04) | 5.1 (0.3) | |
| 24 | 7.4 | 33.1 (6.4) | 0.13 (0.02) | 5.3 (0.3) | |
| 25 | 6.0 | 55.3 (4.6) | 0.12 (0.02) | 5.6 (0.4) | |
| Male | 1 | 25.6 | 75.3 (2.0) | 0.20 (0.04) | 4.9 (0.3) |
| 2 | 25.4 | 46.3 (4.4) | 0.27 (0.04) | 5.0 (0.2) | |
| 3 | 25.2 | 68.1 (3.5) | 0.05 (0.01) | 5.9 (0.3) | |
| 4 | 24.4 | 71.9 (2.5) | 0.15 (0.05) | 5.1 (0.3) | |
| 5 | 23.9 | 56.9 (5.5) | 0.06 (0.01) | 5.5 (0.3) | |
| 6 | 23.8 | 50.9 (3.0) | 0.26 (0.04) | 4.3 (0.3) | |
| 7 | 23.6 | 48.8 (5.4) | 0.25 (0.03) | 4.9 (0.3) | |
| 8 | 23.5 | 62.5 (4.5) | 0.15 (0.04) | 5.1 (0.3) | |
| 9 | 23.3 | 54.7 (5.2) | 0.12 (0.01) | 5.6 (0.4) | |
| 10 | 23.3 | 52.2 (4.5) | 0.07 (0.01) | 5.4 (0.3) | |
| 11 | 22.8 | 63.1 (3.8) | 0.09 (0.02) | 5.2 (0.3) | |
| 12 | 22.3 | 50.3 (5.1) | 0.12 (0.03) | 5.5 (0.3) | |
| 13 | 21.4 | 57.2 (4.8) | 0.12 (0.02) | 5.5 (0.4) | |
| 14 | 15.4 | 43.1 (5.2) | 0.06 (0.01) | 5.4 (0.3) | |
| 15 | 15.4 | 30.9 (5.7) | 0.12 (0.03) | 4.9 (0.4) | |
| 16 | 14.3 | 55.9 (4.0) | 0.25 (0.03) | 4.5 (0.3) | |
| 17 | 13.3 | 35.3 (3.9) | 0.11 (0.01) | 4.8 (0.3) | |
| 18 | 10.3 | 40.6 (6.2) | 0.15 (0.02) | 5.1 (0.3) | |
| 19 | 10.3 | 22.2 (3.4) | 0.09 (0.01) | 5.5 (0.3) | |
| 20 | 9.3 | 53.1 (4.8) | 0.11 (0.02) | 5.7 (0.4) | |
| 21 | 8.4 | 39.7 (5.1) | 0.15 (0.02) | 4.8 (0.3) | |
| 22 | 8.3 | 44.1 (5.0) | 0.08 (0.01) | 6.1 (0.3) | |
| 23 | 7.3 | 51.9 (6.6) | 0.10 (0.02) | 5.5 (0.2) |
Values are shown as means (SE) of a subject after collapsing peaks and stimulus types. Note that these values were collected at a subject's threshold level, which was different among monkeys according to their hearing sensitivities. dB pSPL, decibels peak sound pressure level.
ABR thresholds increased with age.
We compared the ABR thresholds of all monkeys across the eight different stimuli that were tested. Age contributed to 16% of the variance in the ABR thresholds [F(1,766) = 145.56, P < 0.05, R2 change = 16%], and ABR thresholds were positively correlated with age (r = 0.40, P < 0.001). The regression model revealed a rate of 1.19 dB increase per year for ABR thresholds [b = 1.19, t(765) = 12.07, P < 0.001]. An interaction between age and peak significantly accounted for 1.3% of the variance [F(1,757) = 16.0, P < 0.05, R2 change = 1.3%]. Figure 2 illustrates that ABR thresholds increased with age at peak II (r = 0.30, P < 0.001) and peak IV (r = 0.52, P < 0.001), respectively.
Fig. 2.
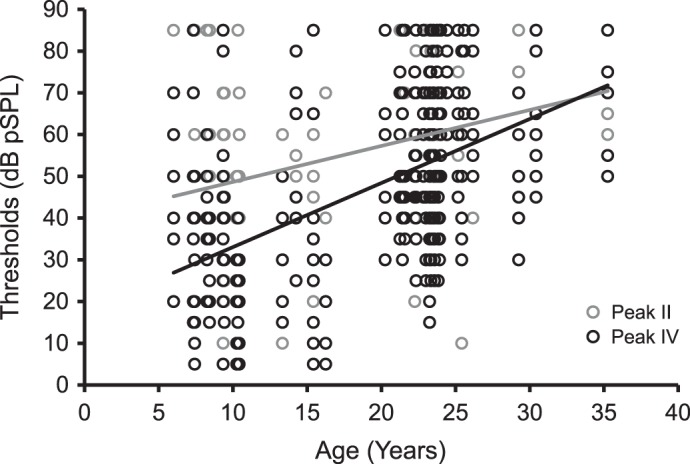
ABR threshold increased as a function of age at both peaks II and IV. The graph shows ABR thresholds of the 48 monkeys (8 data points per monkey per peak) at peak II (gray circles) and peak IV (black circles). Monkeys sometimes had the same ABR thresholds for more than 1 stimulus type or peak. Age was positively correlated with ABR thresholds at peak II (gray trend line: r = 0.30, P < 0.001) and peak IV (black trend line: r = 0.52, P < 0.001).
Peak [F(1,765) = 45.7, P < 0.05, R2 change = 4.7%] and stimulus type [F(7,758) = 32.88, P < 0.05, R2 change = 18.5%] contributed to 4.7 and 18.5% of the variance, respectively. ABR peak thresholds at peak II were higher than those at peak IV [t(383) = 6.76, 2-tailed, P < 0.001]. For stimulus type, ABR thresholds with 12- and 16-kHz tone bursts were higher than the rest of other stimulus types (clicks, 0.5-, 1.0-, 2.0-, 4.0-, and 8.0-kHz tone bursts; post hoc LSD tests, P < 0.001). An interaction between peak and stimulus type was present [F(7,750) = 4.30, P < 0.05, R2 change = 2.3%], which indicated that monkeys had higher ABR thresholds with 0.5-, 1.0-, 2.0-, 12.0-, and 16.0-kHz tone bursts at peak II, relative to peak IV. Last, there were neither main effects nor interactions associated with sex on ABR thresholds (P > 0.41).
ABR peak amplitudes at threshold levels varied with peak and stimulus type only.
Our analysis revealed that peak [F(1,765) = 16.24, P < 0.05, R2 change = 2.1%] and stimulus type [F(7,758) = 13.08, P < 0.05, R2 change = 10.5%] contributed to the variance in the ABR peak amplitudes but not by age or sex. Results showed that peak amplitudes at high frequencies (12 and 16 kHz) were smaller than those at some low and middle frequencies (for details, see Table 4).
Table 4.
Reduced amplitudes and shortened latencies of ABRs at high frequencies during stimulus threshold and suprathreshold (70 dB pSPL) levels
| Stimulus Level | ABR | Results | Pairwise Comparisons |
|---|---|---|---|
| Threshold | Peak Amplitudes | Stimulus | clicks, 0.5, 1.0, 2.0, 8.0 kHz >12.0 or 16.0 kHz† |
| Peak | IV > II† | ||
| Peak Latencies | Stimulus | 1.0, 2.0 kHz >16.0 kHz* | |
| Peak × Stimulus | II: 1.0, 2.0, 4.0 kHz >12.0 or 16.0 kHz* | ||
| IV: 0.5, 1.0, 2.0, 4.0 kHz >16.0 kHz† | |||
| 70 dB pSPL | Peak Amplitudes | Stimulus | clicks, 0.5, 1.0, 2.0, 4.0, 8.0 kHz >12.0 or 16.0 kHz* |
| Peak | IV > II† | ||
| Peak Latencies | Stimulus | 0.5, 1.0 kHz > clicks, 2.0, 8.0, 12.0, 16.0 kHz* | |
| Sex × Stimulus | n.s. |
Monkeys had lower peak amplitudes with shorter latencies at high frequencies (12.0 and 16.0 kHz) than clicks, low, and middle frequencies. Pairwise comparisons were conducted by t statistics and post hoc least significant difference tests.
P < 0.005,
P < 0.001. n.s., not significant.
ABR peak latencies increased with a combined influence of age, sex, and stimulus type.
The stepwise multiple-regression analysis of ABR peak latencies showed that a significant interaction between age and sex accounted for 0.5% of the variance in the ABR peak latencies [F(1,638) = 6.68, P < 0.05, R2 change = 0.5%]. Figure 3 shows a significant correlation between age and peak latencies present in female monkeys only (black squares; r = 0.13, P = 0.016). An interaction between age and stimulus type accounted for 2.3% of the variance [F(7,624) = 4.55, P < 0.05, R2 change = 2.3%]. However, there were only trends toward significant correlations between age and peak latencies at 4 kHz (r = 0.21, P = 0.07) and 12 kHz (r = 0.26, P = 0.06). Stimulus type [F(7,639) = 23.49, P < 0.05, R2 change = 12.7%] and a peak × stimulus interaction [F(7,617) = 2.97, P < 0.05, R2 change = 1.5%] accounted for 12.7 and 1.5% of the variance, respectively. Peak latencies at high frequencies (12 and 16 kHz) were generally shorter than those at low and middle ones (for details, see Table 4).
Fig. 3.
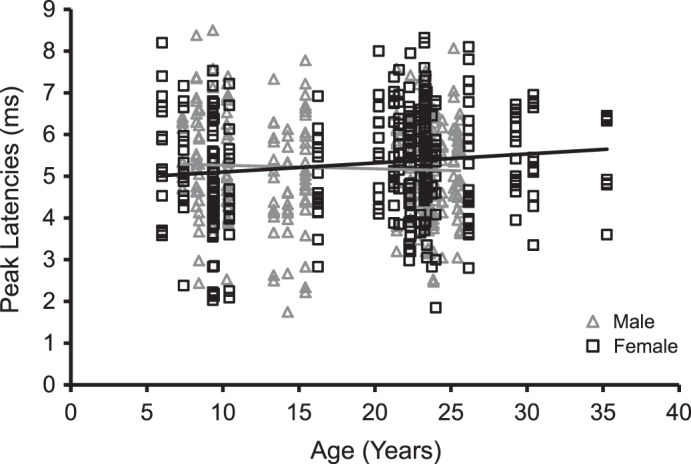
ABR peak latencies at threshold increased as a function of age shown in female monkeys. The graph shows the peak latencies of the 48 monkeys, in females (black squares) and males (gray triangles), after collapsing data from peaks II and IV. A weak, positive correlation between age and peak latencies was present in female monkeys (black trend line: r = 0.13, P = 0.016) but not male monkeys (gray trend line: r = −0.04, P > 0.1). There was no sex difference on peak latencies at threshold levels.
No age-related change in interpeak latency differences at threshold levels.
The analysis of interpeak latency differences revealed 15.1% of the variance contributed by stimulus type [F(7,285) = 7.22, P < 0.05, R2 change = 15.1%] but not by any other factor. Pairwise comparisons indicated that interpeak latency differences for 12-kHz tone bursts were larger than those for clicks and 1-, 4-, and 8-kHz tones (post hoc LSD tests, P < 0.005; data not shown). There was a trend toward significance that interpeak latency differences for 16-kHz tone bursts were larger than those for clicks and 1-, 4-, and 8-kHz tone bursts (P values ranging from 0.02 to 0.05). The latter, nonsignificant findings may be due to the fact that observable waveforms of peak II or peak IV were often missing at 16-kHz tone bursts, and therefore the decreased sample size reduced the statistical power of the present analysis. Nevertheless, the present findings suggest that the latency differences between peaks II and IV would increase for high-frequency stimuli at threshold levels, compared with low- and middle-frequency stimuli.
Effects of Age on the ABR Measured Above Threshold
ABR amplitudes decreased with age.
To compare the two ABR peaks across monkeys with different ages at a suprathreshold sound level, we analyzed the responses at 70 dB pSPL across all of the stimuli that were tested. This value was chosen because this was the highest sound level tested for the majority of monkeys studied (some geriatric monkeys were only tested at 80 dB pSPL, and no waveform was noted). Table 5 presents the summary statistics from the 48 monkeys. Age accounted for 13.1% of the variance at the suprathreshold level [F(1,766) = 115.58, P < 0.05, R2 change = 13.1%], where the peak amplitudes were negatively correlated with age (r = −0.36, P < 0.001). Age also interacted with peak and stimulus type, respectively, to account for 0.03 and 2.50% of the variance [age × peak: F(1,757) = 4.14, P < 0.05, R2 change = 0.03%; age × stimulus: F(7,740) = 4.52, P < 0.05, R2 change = 2.5%]. Figure 4A demonstrates that peak amplitudes decreased with age for both peak II (r = −0.34, P < 0.001) and peak IV (r = −0.40, P < 0.001). Peak amplitudes also decreased with age for clicks and all tone frequencies (Pearson's r ranging between −0.30 and −0.49, P < 0.005; see Table 6). Similar to the threshold condition, peak [F(1,765) = 42.82, P < 0.05, R2 change = 4.6%] and stimulus type [F(7,758) = 30.50, P < 0.05, R2 change = 18.1%] contributed to the variance of the statistic model. Peak amplitudes were lower at high frequencies (12 and 16 kHz) compared with the low and middle frequencies (for details, see Table 4).
Table 5.
Summary statistics of ABR audiograms from the 48 monkeys during the suprathreshold stimulus level
| Subject | Age, yr | Peak Amplitudes, μV | Peak Latencies, ms | |
|---|---|---|---|---|
| Female | 1 | 35.3 | 0.14 (0.04) | 4.9 (0.3) |
| 2 | 30.4 | 0.14 (0.03) | 5.1 (0.3) | |
| 3 | 29.3 | 0.32 (0.06) | 4.7 (0.3) | |
| 4 | 26.2 | 0.14 (0.03) | 4.6 (0.3) | |
| 5 | 26.2 | 0.19 (0.04) | 4.7 (0.4) | |
| 6 | 24.0 | 0.23 (0.05) | 4.6 (0.3) | |
| 7 | 23.8 | 0.07 (0.03) | 4.4 (0.7) | |
| 8 | 23.4 | 0.15 (0.02) | 4.7 (0.3) | |
| 9 | 23.3 | 0.25 (0.06) | 4.8 (0.2) | |
| 10 | 23.3 | 0.21 (0.03) | 4.8 (0.4) | |
| 11 | 23.3 | 0.28 (0.04) | 5.2 (0.4) | |
| 12 | 23.0 | 0.28 (0.07) | 4.6 (0.3) | |
| 13 | 22.3 | 0.26 (0.04) | 4.8 (0.4) | |
| 14 | 22.3 | 0.13 (0.03) | 4.4 (0.4) | |
| 15 | 21.6 | 0.25 (0.04) | 5.1 (0.3) | |
| 16 | 21.3 | 0.16 (0.04) | 4.8 (0.3) | |
| 17 | 20.3 | 0.22 (0.03) | 5.1 (0.4) | |
| 18 | 16.3 | 0.71 (0.09) | 4.1 (0.2) | |
| 19 | 10.4 | 0.60 (0.10) | 3.9 (0.3) | |
| 20 | 9.4 | 0.71 (0.12) | 3.9 (0.2) | |
| 21 | 9.3 | 0.46 (0.09) | 4.5 (0.2) | |
| 22 | 9.3 | 0.59 (0.08) | 3.9 (0.3) | |
| 23 | 9.3 | 0.48 (0.06) | 3.8 (0.2) | |
| 24 | 7.4 | 0.63 (0.09) | 4.0 (0.2) | |
| 25 | 6.0 | 0.24 (0.05) | 4.8 (0.3) | |
| Male | 1 | 25.6 | 0.19 (0.04) | 5.1 (0.3) |
| 2 | 25.4 | 0.45 (0.05) | 4.5 (0.2) | |
| 3 | 25.2 | 0.05 (0.01) | 5.9 (0.4) | |
| 4 | 24.4 | 0.12 (0.03) | 5.1 (0.3) | |
| 5 | 23.9 | 0.15 (0.03) | 4.5 (0.3) | |
| 6 | 23.8 | 0.41 (0.05) | 4.0 (0.3) | |
| 7 | 23.6 | 0.53 (0.10) | 4.3 (0.3) | |
| 8 | 23.5 | 0.22 (0.05) | 4.7 (0.3) | |
| 9 | 23.3 | 0.21 (0.04) | 4.8 (0.2) | |
| 10 | 23.3 | 0.20 (0.03) | 4.7 (0.3) | |
| 11 | 22.8 | 0.12 (0.03) | 5.0 (0.3) | |
| 12 | 22.3 | 0.24 (0.05) | 4.5 (0.3) | |
| 13 | 21.4 | 0.17 (0.04) | 4.9 (0.3) | |
| 14 | 15.4 | 0.20 (0.04) | 4.4 (0.3) | |
| 15 | 15.4 | 0.60 (0.06) | 3.9 (0.3) | |
| 16 | 14.3 | 0.31 (0.04) | 4.3 (0.2) | |
| 17 | 13.3 | 0.48 (0.10) | 4.1 (0.3) | |
| 18 | 10.3 | 0.46 (0.06) | 4.1 (0.2) | |
| 19 | 10.3 | 0.52 (0.09) | 4.3 (0.3) | |
| 20 | 9.3 | 0.19 (0.04) | 5.0 (0.4) | |
| 21 | 8.4 | 0.33 (0.04) | 4.0 (0.3) | |
| 22 | 8.3 | 0.31 (0.05) | 5.0 (0.3) | |
| 23 | 7.3 | 0.35 (0.06) | 4.7 (0.3) |
Values are means (SE) of a subject after collapsing peaks and stimulus types. Note that these values were collected at the same sound level of 70 dB pSPL among all monkeys in the present study.
Fig. 4.
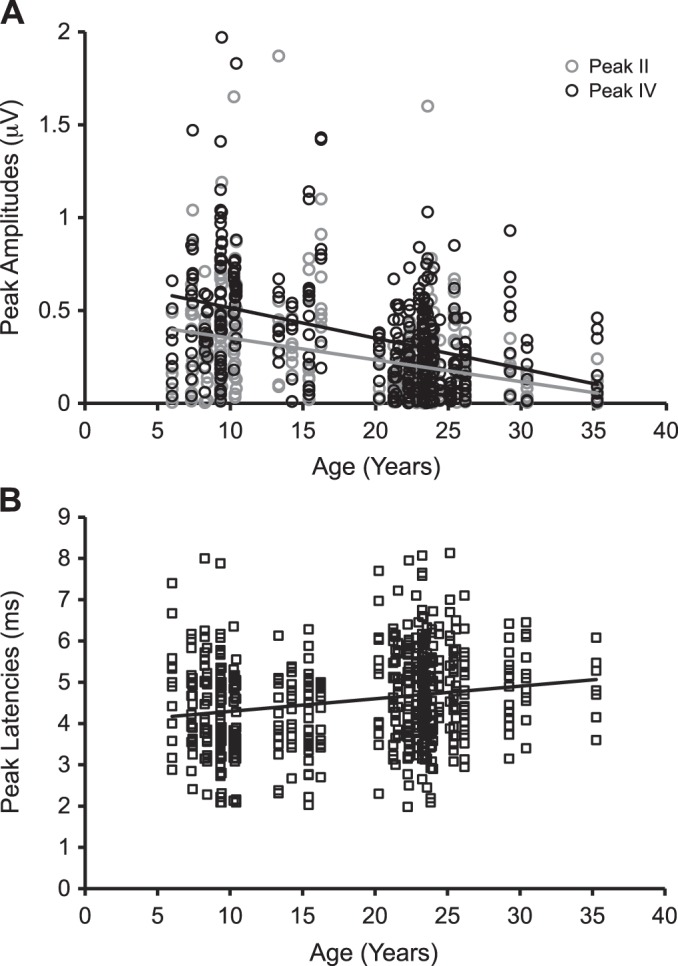
ABR peak amplitudes and latencies varied as a function of age during the suprathreshold condition at 70 dB pSPL. Old monkeys had weak amplitudes (A) at both peak II (gray circles and trend line: r = −0.34, P < 0.001) and peak IV (black circles and trend line: r = −0.40, P < 0.001), accompanied by long latencies (r = 0.20, P < 0.001) (B; data were collapsed between peaks II and IV).
Table 6.
Peak amplitudes and latencies varied as a function of age during the suprathreshold level of 70 dB pSPL
| Pearson's r |
||
|---|---|---|
| Stimulus Type | Peak Amplitudes | Peak Latencies |
| Clicks | −0.29† | 0.08 |
| 0.5 kHz | −0.46‡ | 0.21 |
| 1.0 kHz | −0.49‡ | 0.17 |
| 2.0 kHz | −0.48‡ | 0.39† |
| 4.0 kHz | −0.48‡ | 0.63‡ |
| 8.0 kHz | −0.40‡ | 0.24 |
| 12.0 kHz | −0.36‡ | 0.39* |
| 16.0 kHz | −0.33‡ | 0.27 |
Values presented here were correlation coefficients between age and ABR parameters at the eight stimulus types, after combining data from peaks II and IV. Peak amplitudes decreased with age at clicks and all frequencies, whereas peak latencies increased with age at some low, middle, and high frequencies.
P < 0.05,
P < 0.005,
P < 0.001.
ABR peak latencies increased with age.
We also noted an increase in ABR peak latencies at some frequencies for stimuli at 70 dB pSPL. Age accounted for 3.9% of the variance at the suprathreshold level [F(1,603) = 24.61, P < 0.05, R2 change = 3.9%], where peak latencies increased with age (r = 0.20, P < 0.001; Fig. 4B). Age also interacted with stimulus type to account for 2.1% of the variance [age × stimulus: F(7,580) = 4.24, P < 0.05, R2 change = 2.1%]. Peak latencies increased with age at 2, 4, and 12 kHz (see Table 6). There was also a significant three-way interaction between age, sex, and stimulus type, which contributed to 1.9% of the variance [F(7,573) = 3.97, P < 0.05, R2 change = 1.9%]. It was revealed that peak latencies increased with age at some low, middle, and high frequencies in female and male monkeys (see Table 7). For factors besides age, stimulus type [F(7,595) = 24.69, P < 0.05, R2 change = 13.1%] and a sex × stimulus type interaction [F(7,587) = 2.78, P < 0.05, R2 change = 1.4%] accounted for the variance. Peak latencies at high frequencies (12 and 16 kHz) were generally shorter than those at low frequencies, but post hoc tests did not reveal significant effects of sex on ABR latencies at any stimulus type (for details, see Table 4).
Table 7.
Peak latencies varied as a function of age in female and male monkeys at 70 dB pSPL
| Pearson's r |
||
|---|---|---|
| Stimulus Type | Female | Male |
| Clicks | 0.07 | 0.17 |
| 0.5 kHz | 0.51* | −0.15 |
| 1.0 kHz | 0.28 | 0.08 |
| 2.0 kHz | 0.29 | 0.56† |
| 4.0 kHz | 0.63† | 0.65‡ |
| 8.0 kHz | 0.31 | 0.16 |
| 12.0 kHz | 0.58* | 0.26 |
| 16.0 kHz | 0.53 | −0.31 |
Values presented here were correlation coefficients between age and ABR latencies after collapsing data from peaks II and IV. A significant interaction between age, sex, and stimulus type revealed that peak latencies above thresholds increased with age at different frequencies between sexes. There was no sex difference for any stimulus type on peak latencies in the regression model.
P < 0.05,
P < 0.005,
P < 0.001.
No age-related change in interpeak latency differences at suprathreshold levels.
The analysis of interpeak latency differences only revealed a main effect of stimulus type [F(7,256) = 8.89, P < 0.05, R2 change = 19.6%]. Pairwise comparisons indicated that interpeak latency differences at 1.0-kHz tones were smaller than those at 0.5-, 2.0-, 8.0-, and 12.0-kHz tones (post hoc LSD tests, P < 0.005; data not shown). Similar to the threshold condition, there was no age-related change for interpeak latency differences at the level of 70 dB pSPL.
Age-Related Changes in ABR-Based Audiograms Were Not Cofounded by Three Elderly Female Monkeys
The present findings indicate the possibility of a sex-related influence on ABR latencies at threshold stimulus levels and at the level of 70 dB pSPL. Three of the female monkeys were the oldest of our geriatric subjects (29.3, 30.4, and 35.3 yr of age compared with the others between 21.6 and 26.2 yr of age), and they could have skewed the current results. To test whether this was indeed the case, we repeated the statistical analyses for peak II and IV latencies at threshold and at 70 dB pSPL, after omitting the data from the three oldest female monkeys. The results showed that first there was, again, no significant difference of age between females (mean = 23.5 ± 0.4 yr) and males (mean = 23.9 ± 0.3 yr) within monkeys above 21.5 yr of age (P = 0.98). At the stimulus threshold levels, the modified analysis yielded a significant interaction between age and sex [F(1,603) = 5.67, P < 0.05, R2 change = 0.5%] in that peak latencies still increased with age in female monkeys only (r = 0.13, P = 0.024). At 70 dB pSPL, the three-way interaction between age, sex, and stimulus type remained significant [F(7,541) = 2.10, P < 0.05, R2 change = 1.1%], and the correlations were still present at the same frequencies for female monkeys, as before. The interaction of sex and stimulus type remained significant at 70 dB pSPL, but pairwise comparison revealed no significant effect of sex on peak latencies at any stimulus type (P > 0.1). Therefore, the ABR-based audiograms can be differently modulated by the sex of the individuals, and the results cannot be explained solely by the presence of the three oldest female monkeys included in the present study.
Effects of Age on ABR at a Comparable Sensation Level
Hearing deficits attributable to cochlear degeneration increase with aging and simultaneously affect the ABR parameters of some older animals (Boettcher 2002; Engle et al. 2013, Sergeyenko et al. 2013). One way to differentiate the impact of peripheral vs. central changes on ABRs is to assess the audiometric functions of monkeys at similar sensation levels, i.e., the same stimulus intensity relative to the hearing threshold as opposed to the absolute intensity. We therefore used a conservative estimate of ABR threshold shifts (30 dB SPL) that is used when defining mild impairment of hearing loss in humans (Engle et al. 2013) and rodents (Parthasarathy et al. 2014). For all cases where a monkey had an observable ABR at a given frequency, we measured the peak amplitudes and latencies for peaks II and IV at 30 dB pSPL above their threshold level for that stimulus. This was necessarily restricted to cases where the threshold was ≤40 dB pSPL, as 70 dB pSPL was generally the highest level tested (80 dB pSPL was only tested if no ABR waveforms were present at 70 dB pSPL). This method allowed us to include sufficient numbers of old monkeys for statistical analysis. Because of a large proportion of monkeys above 21.5 yr of age with missing ABRs at high frequencies (see Table 2), the sensation-level analysis was restricted by ABRs recorded to clicks and 0.5–8.0-kHz frequencies. For each peak, correlation analysis was based on the available data of 38 monkeys for ABR amplitudes and latencies with one or more stimulus types. This analysis only yielded a trend toward significance for peak amplitudes between age and peak II (r = −0.21, P = 0.06) and none for other conditions (Table 8). We further performed the analysis at each stimulus type per peak (clicks and 0.5, 1.0, 2.0, 4.0, and 8.0 kHz; <21.5 yr of age: n = 7–12; >21.5 yr of age: n = 6–8). Although ABR amplitudes of peak II decreased with age for click stimuli (r = −0.52, P = 0.04, before Bonferroni correction), there was only a trend toward significance for ABR latencies to 8 kHz at the same peak (r = 0.53, P = 0.06). Again, no significant result was associated with peak IV at any stimulus type for amplitudes and latencies (P > 0.1; data not shown). These additional analyses suggest that peripheral hearing functions remained a major influence on ABR audiograms of old monkeys.
Table 8.
Minimal effect of age on peak amplitudes and latencies at the sensation level comparable among young and old monkeys
| Conditions | Peak Amplitudes | Peak Latencies | < 21.5 yr | > 21.5 yr |
|---|---|---|---|---|
| Peak II | r = −0.21, P = 0.06 | r = −0.13, P > 0.1 | N = 20 | N = 18 |
| Peak IV | r = −0.10, P > 0.1 | r = 0.01, P > 0.1 | N = 20 | N = 15 |
Values presented here were correlation coefficients between age and ABR parameters at each peak condition, based on ABR audiograms of each monkey recorded at 30 dB pSPL above its given threshold. The statistical analysis was limited to those subjects with observable ABRs for 1 or more stimulus types. Effects of age on peak II were minimal or trending toward significance that peripheral hearing function remained a major influence on the central auditory system of geriatric monkeys.
In summary, monkeys above 21.5 yr of age had missing ABRs for some middle and high frequencies, relative to those below this age. Geriatric monkeys showed higher ABR thresholds. Their ABR waveforms were smaller in peak amplitudes and had longer peak latencies at suprathreshold levels compared with younger animals. Preliminary evidence suggested that the effects of age may be differently expressed on ABR latencies between male and female monkeys at threshold stimulus levels. Only female monkeys showed age-related changes on peak latencies at threshold but not male monkeys. Consistent with prior studies about the relationship of ABRs and sound frequencies in nonhuman primates (Harada et al. 1999; Lasky et al. 1995, 1999; Laughlin et al. 1999; Torre and Fowler 2000), high-frequency sounds were associated with lower amplitudes and shorter latencies than those from low- and middle-frequency sounds. The interpeak latency analysis did not reveal age-related differences in the present study.
DISCUSSION
Investigations of ARHL have been widely conducted in rodents and humans, and the few studies in nonhuman primates have shown similarities in ABRs to click stimuli between humans and monkeys (Lasky et al. 1995, 1999; Laughlin et al. 1999; Torre and Fowler 2000). The present study examined auditory brainstem responses across clicks and a variety of tones and evaluated how hearing deficits were expressed in nonhuman primates as a function of age. We found that monkeys above 21.5 yr of age required higher stimulus levels to elicit distinct peaks II and IV, particularly for high-stimulus frequencies. Furthermore, the stepwise multiple-regression analysis showed that the threshold stimulus level increased as a function of age for both peaks II and IV. Elevated ABR thresholds among geriatric monkeys in the present study are consistent with previous studies in nonhuman primates (Fowler et al. 2002, 2010; Harada et al. 1999; Lasky et al. 1999; Torre and Fowler 2000; Torre et al. 2004), rodents (Backoff and Caspary 1994; Boettcher 2002; McFadden et al. 1997; Parthasarathy and Bartlett 2012; Parthasarathy et al. 2014; Syka 2010), and humans (Demeester et al. 2009; Gates and Mills 2005; Jerger and Johnson 1988; Lavoie et al. 2008; Rowe 1978; Skoe et al. 2013; Wiley et al. 2008). At the suprathreshold level (70 dB pSPL), the amplitudes of peaks II and IV decreased, and their corresponding peak latencies increased as a function of age for clicks and tone frequencies. These results are in good agreement with other human and animal studies comparing ABR parameters between young and aged subjects (Alvarado et al. 2014; Boettcher et al. 1993a, 1993b; Harada et al. 1999; Harkins 1981; Jerger and Hall 1980; Jerger and Johnson 1988; Parthasarathy et al. 2014; Popelar et al. 2006; Rosenhall et al. 1986; Syka 2010; Torre and Fowler 2000; Torre et al. 2004).
The effects of aging on peak amplitudes and latencies were clear and substantial at the 70-dB pSPL condition compared with the threshold condition. This was because the actual stimulus level in the threshold ABR condition could vary between animals, whereas the suprathreshold values were at the same stimulus level for all animals that were tested. For example, many geriatric monkeys had no observable ABR peaks II, IV, or both for some middle and high frequencies below the suprathreshold level. These factors combine to result in a wide variance within the ABR data at threshold and thus did not yield a substantial relationship between age and peak amplitude compared with those revealed at 70 dB pSPL. Increased intervals between peaks II and IV are sometimes reported in aged humans and animals (Alvarado et al. 2014; Backoff and Caspary 1994; Jerger and Hall 1980; Rowe 1978; Torre and Fowler 2000), whereas the present study and others do not (Harkins 1981; Martini et al. 1991; Ottaviani et al. 1990). The results from the present study often showed an overall effect of age on ABR peak amplitudes and latencies and sometimes at both peaks II and IV simultaneously. These findings suggest an overall increase of ABR latencies at peak II and peak IV, and thus there was no age-related change in the latencies between the two peaks.
High-Frequency Hearing Loss is Prevalent in Geriatric Monkeys
The auditory sensitivity revealed in the present study is similar to behavioral pure-tone thresholds tested in rhesus macaques (Behar et al. 1965; Bennett et al. 1983). The ABR thresholds of our monkeys remained low and flat at peaks II and IV between 0.5 and 8.0 kHz and then increased rapidly at 12.0 and 16.0 kHz (Table 3). Our study shares an abrupt pattern of reduced hearing sensitivity at high frequencies with Bennett et al. (1983), which reported a rapid increase in behavioral thresholds beyond 16 or 20 kHz tested in rhesus macaques. Similarly, many of our geriatric monkeys above 21.5 yr of age did not have clear, observable ABR peak II, IV, or both at 12 and 16 kHz. These patterns of hearing impairments are similar to those reported by Bennett et al. (1983), in which three rhesus monkeys at 31 yr of age had difficulties in detecting tone frequencies at 16.000, 22.627, and 32.000 kHz. Fowler et al. (2010) reported that ABR threshold increased significantly with age at 16 kHz after the age of 21. Although there are differences in hearing test paradigms (free-field speakers vs. earphones) and behavioral states (awake vs. anesthetized), overall patterns of age-related changes in hearing sensitivity are similar for those old monkeys showing replicable ABRs. Approximately 47% and 24% of monkeys below 21.5 yr of age did not have an observable peak II or IV at high frequencies, respectively (see Table 2). Among the 11 monkeys with missing ABRs at high frequencies, five of them were in middle-age range (between 15.4 and 21.4 yr of age, approximately equivalent to 46.2 to 64.2 human yr of age; see Davis and Leathers 1985). The summary statistics indicate that some degree of hearing impairments at high frequencies may occur early in age in some monkeys, and the deficits at high frequencies get worse later in life. Fowler et al. (2010) reported that age-related decreases in ABR thresholds differed by frequencies (8, 16, and 32 kHz), whereas Bennett et al. (1983) reported that behavioral thresholds could be a “flat loss” at all levels (0.125 to 32.000 kHz). The present findings suggest that our geriatric monkeys had a tendency toward an overall decline in hearing sensitivity measured by the stimulus level threshold and an overall decrease in peak amplitude at stimulus levels above this threshold.
The present findings extend the results on age-related influences on hearing sensitivity reported by one of our previous studies (Engle et al. 2013). Engle et al. (2013) examined the relationship of ABR thresholds and morphological changes of cochleae in nine rhesus monkeys aged from 10 to 35 yr of age that were included in the present dataset. That study quantified audiograms based on the pattern of ABR threshold shifts by calculating the pure tone average (PTA), an average ABR threshold from the seven tone frequencies, and the PTA loss (PTAL), the average, age-normalized threshold, by subtracting the tone thresholds of the youngest monkey. Those results showed that old monkeys experienced audiometric hearing deficits and cochlear histopathologies that were similar to those seen in humans. The reported PTAL increased with a function of age, at a robust correlation of 0.88. Thus we compared similar metrics in the present study with those in Engle et al. (2013) although the present dataset used a much larger sample size with a more continuous age range. We found that the calculated PTA and age-normalized PTAL increased as a function of age (see Fig. 5 for details) and that there was a positive correlation between age and PTAL (r = 0.61, P < 0.001, collapsed peaks II and IV). Engle et al. (2013) estimated that ABR thresholds to click stimuli increased at an approximate rate of 1.8 dB/yr, whereas the present study is in good agreement with approximate increase rates of 1.13 and 1.12 dB/yr for click stimuli and tone stimuli, respectively. Our estimated rates of ABR threshold increase per year (1.19 dB/yr after collapsing the two peaks) are closer to the reported rate of 1.2 dB/yr by Fowler et al. (2002) and Torre et al. (2004), likely attributable to the larger number of animal subjects in the present study. These results concomitantly indicate that hearing deficits spanned across the low-, middle-, and high-frequency levels in the 48 rhesus monkeys as a function of age, similar to results found in human studies on hearing loss.
Fig. 5.
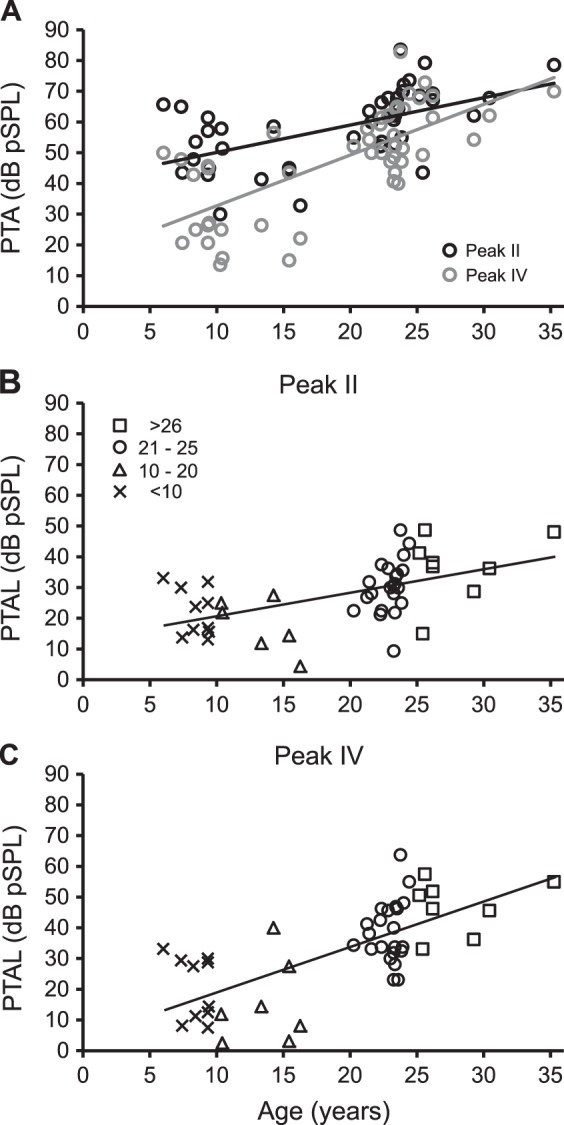
Hearing loss increases as a function of age. Pure tone average (PTA) and PTA loss (PTAL, age normalized with the lowest PTA threshold of a young monkey) of 48 monkeys are depicted in relation to age. A: PTA thresholds increased with age at both peaks II (black circles and trend line: r = 0.55, P < 0.001) and IV (gray circles and trend line: r = 0.71, P < 0.001). B and C: PTAL increased with age at peaks II (r = 0.54, P < 0.001) and IV (r = 0.70, P < 0.001) respectively. Data were organized into 4 age ranges for better visualization, <10 yr (Xs), 10–20 yr (triangles), 21–25 yr (circles), and >26 yr (squares).
Peak amplitude and latency of ABR peaks II and IV estimate neural function of hearing to the level of the auditory brainstem. The cochlear nucleus and the contralateral lateral lemniscus are thought to contribute peaks II and IV in macaques, respectively (Allen and Starr 1978; Doyle et al. 1983; Lasky et al. 1995; Laughlin et al. 1999; Møller and Burgess 1986; Torre and Fowler 2000). The ABR peak amplitude is a function of the number and synchrony of neural units firing at a given recording site. The ABR peak latency reflects the neural transmission time of auditory signals, which represents the aggregate responses of the synchronous firing of auditory nerve fibers and auditory brainstem neurons. Engle et al. (2013) demonstrated that the loss of outer hair cells and spiral ganglion cells accounted for most of the decreases in ABR thresholds to low (1 and 2 kHz), middle (8 kHz), and high frequencies (12 and 16 kHz) in geriatric monkeys. The number of cochlear pathologies was highly correlated with age (r = 0.9) and with ABR thresholds to pure tone stimuli (r = 0.78). These factors have been associated with reduced endolymphic potentials and asynchronous activity of auditory nerve fibers, which contribute to the reduction of cochlear sensitivity and neural functions (Bao and Ohlemiller 2010; Boettcher 2002; Nelson and Hinojosa 2006; Popelar et al. 2006). These morphological changes in the macaque cochleae are certainly present in many of our geriatric monkeys, which had weakened and delayed ABR peaks to the suprathreshold stimuli. The sensation-level analysis examined the effect of age on hearing sensitivity of monkeys at a level 30 dB pSPL above threshold. This aimed to compensate for peripheral hearing differences among monkeys that varied in cochlear function attributable to aging. On the basis of the limited data of those monkeys with observable ABRs, there was only a minimal effect of age on ABRs at some stimulus types and only for peak II. These preliminary results imply that the age-related effects seen at peaks II and IV of ABRs, which are thought to be contributed by the cochlear nucleus and contralateral lateral lemniscus in rhesus monkeys, are due in large part to changes in the periphery, consistent with the loss of hair cells and/or spiral ganglion cells demonstrated in some of these same monkeys (Engle et al. 2013). Age-related pathological changes can concomitantly weaken neural signaling from the cochlea to the ascending central auditory system and contribute to the hearing loss present in geriatric monkeys.
Comments on Uses of ABRs to Measure Hearing Sensitivity in Old Monkeys
In the present study, several of our geriatric monkeys had missing ABR waveforms at high frequencies. Traditional statistical methods may consider this to be a biased sample of the monkey population, and ABR audiometrics of these subjects should be excluded from further analyses. We chose not to do this, as presumably either an ABR would have been elicited if we used a stimulus level >80 dB pSPL or in fact that animal did not have sufficient neural elements to elicit an ABR at any level. We reasoned that eliminating these data strongly biased the results in favor of no difference in peak thresholds as a function of age. By assigning a value of 85 dB pSPL, we are certainly underestimating many of the thresholds that would have been measured if we had used higher-intensity stimuli. The present study used alternative methods to handle missing data (see materials and methods for details), allowed us to include all available data from the 48 monkeys, and improved the statistical power of the present analysis.
The present study did not assess middle ear or cochlear function, and these factors may also interact with age and modulate ABR audiograms. Age-related changes in sound transmission and tone sensitivity in geriatric monkeys have been reported previously (Fowler et al. 2002, 2010; Torre and Fowler 2000; Torre et al. 2000, 2004). Our laboratory has systemically evaluated age-related changes in the peripheral and central auditory system from a subset of the same 48 monkeys as well as others in the cochlea (Engle et al. 2013), cochlear nucleus (Gray et al. 2014), superior olive complex (Gray et al. 2013a), inferior colliculus (Engle et al. 2014), and medial geniculate nucleus (Gray et al. 2013b). These studies demonstrate how ABR audiograms of peaks II and/or IV varied with pathological/neurochemical changes along the ascending auditory pathway and their implications on ARHL. The past and present findings equally suggest that ABR thresholds and other ABR parameters serve as useful measures to predict age-related hearing deficits in monkeys.
We chose to focus our studies on peaks II and IV of the ABRs, as it was the most prominent in the monkeys we tested using our stimulus parameters. The ABR audiometrics of wave 1 have been shown to be a useful predictor for age-related changes in the peripheral auditory system in CBA/CaJ mice, where peak amplitudes of wave 1 (equivalent to peak I in nonhuman primates) are strongly correlated with age and cochlear synaptopathy (Sergeyenko et al. 2013). Peak I measured in our monkeys was always weak and transient, particularly at low stimulus levels. Many of the 48 monkeys did not have clear and observable ABRs at peak I, whereas those at peaks II and IV of the same animal were robust and prominent for in-depth analysis (Lasky et al. 1995; Laughlin et al. 1999; Torre and Fowler 2000). Nevertheless, we did apply our analysis techniques to that limited dataset at 70 dB pSPL and found that peak I amplitudes and latencies were significantly correlated with age (see Fig. 6 and Table 9). These preliminary results should be interpreted with caution but are consistent with those revealed for peaks II and IV in the present study and also parallel to the findings of Sergeyenko et al. (2013).
Fig. 6.
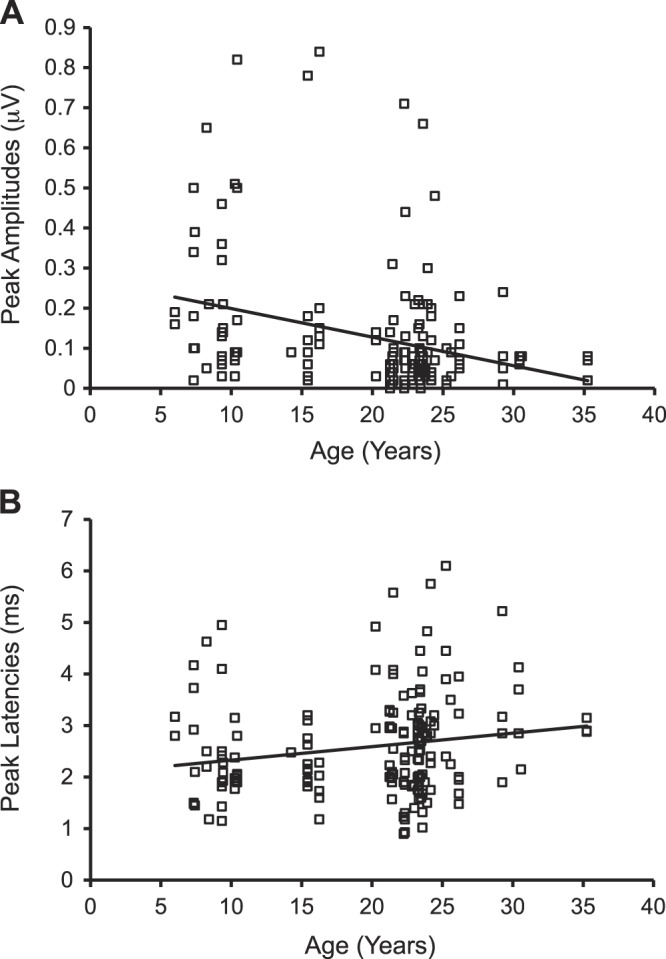
Peak I amplitudes and latencies varied as a function of age during the suprathreshold stimulus level. On the basis of limited data from 42 monkeys showing observable ABRs at peak I, old monkeys had weak peak amplitudes [A: F(1,164) = 16.88, P < 0.05, R2 change = 9.7%; r = −0.31, P < 0.001] and long latencies [B: F(1,164) = 5.57, P < 0.05, R2 change = 3.3%; r = 0.18, P < 0.05] at peak I. There were 6 monkeys with observable peak I for only 1–2 stimulus types, and thus they were not included in data analysis.
Table 9.
Summary statistics of ABR audiograms at peak I from 42 monkeys during the suprathreshold stimulus level of 70 dB pSPL
| Subject | Age, yr | Peak Amplitudes, μV | Peak Latencies, ms | |
|---|---|---|---|---|
| Female | 1 | 35.3 | 0.06 (0.02) | 3.0 (0.1) |
| 2 | 30.5 | 0.07 (0.01) | 3.2 (0.4) | |
| 3 | 29.3 | 0.07 (0.04) | 3.0 (0.5) | |
| 4 | 26.2 | 0.07 (0.02) | 1.6 (0.1) | |
| 5 | 26.2 | 0.14 (0.04) | 2.8 (0.5) | |
| 6 | 23.9 | 0.20 (0.06) | 3.0 (1.0) | |
| 7 | 23.8 | 0.04 (0.01) | 2.4 (0.5) | |
| 8 | 23.4 | 0.02 (0.01) | 3.3 (0.4) | |
| 9 | 23.3 | 0.05 (0.02) | 2.5 (0.3) | |
| 10 | 23.3 | 0.11 (0.04) | 2.4 (0.3) | |
| 11 | 23.3 | 0.05 (0.01) | 2.3 (0.3) | |
| 12 | 23.0 | 0.14 (0.08) | 1.6 (0.2) | |
| 13 | 22.3 | 0.22 (0.08) | 1.5 (0.3) | |
| 14 | 22.3 | 0.16 (0.11) | 2.2 (0.4) | |
| 15 | 21.5 | 0.08 (0.02) | 3.6 (0.5) | |
| 16 | 21.3 | 0.05 (0.02) | 2.8 (0.2) | |
| 17 | 20.3 | 0.10 (0.03) | 4.0 (0.6) | |
| 18 | 16.3 | 0.29 (0.14) | 1.8 (0.2) | |
| 19 | 10.4 | 0.40 (0.17) | 2.2 (0.2) | |
| 20 | 9.4 | 0.17 (0.02) | 2.1 (0.1) | |
| 21 | 9.3 | 0.07 (0.01) | 2.1 (0.2) | |
| 22 | 9.3 | 0.27 (0.20) | 1.5 (0.4) | |
| 23 | 7.4 | 0.25 (0.15) | 1.8 (0.3) | |
| 24 | 6.0 | 0.18 (0.02) | 3.0 (0.2) | |
| Male | 1 | 25.6 | 0.06 (0.03) | 2.9 (0.6) |
| 2 | 25.3 | 0.04 (0.02) | 4.2 (0.8) | |
| 3 | 24.4 | 0.28 (0.21) | 3.1 (0.1) | |
| 4 | 24.2 | 0.10 (0.03) | 3.0 (0.6) | |
| 5 | 23.6 | 0.10 (0.03) | 2.4 (0.5) | |
| 6 | 23.5 | 0.06 (0.01) | 2.7 (0.4) | |
| 7 | 23.3 | 0.07 (0.03) | 2.5 (0.2) | |
| 8 | 23.3 | 0.07 (0.02) | 2.6 (0.2) | |
| 9 | 22.8 | 0.05 (0.01) | 2.8 (0.4) | |
| 10 | 22.3 | 0.05 (0.02) | 2.0 (0.4) | |
| 11 | 21.4 | 0.10 (0.05) | 2.1 (0.2) | |
| 12 | 15.4 | 0.03 (0.01) | 2.4 (0.2) | |
| 13 | 15.4 | 0.29 (0.16) | 2.3 (0.2) | |
| 14 | 10.3 | 0.16 (0.09) | 2.2 (0.2) | |
| 15 | 9.3 | 0.21 (0.08) | 3.2 (0.8) | |
| 16 | 8.4 | 0.21 (0.05) | 1.2 (0.5) | |
| 17 | 8.3 | 0.25 (0.20) | 3.1 (0.8) | |
| 18 | 7.3 | 0.23 (0.09) | 2.8 (0.6) |
Values are means (SE) after collapsing stimulus types. Note that these values were collected at the same sound level of 70 dB pSPL from monkeys with clear, observable peak I. There were 6 monkeys with observable peak I for only 1–2 stimulus types, and thus they were not included in data analysis.
The central auditory system also undergoes age-related changes in rodents and nonhuman primates. Neurochemical changes in the central auditory system have been linked to age, and excitatory and inhibitory mechanisms (e.g., glutamatergic, GABAgeric, and glycinergic pathways) on auditory neurons are weakened in aged rodents (Alvarado et al. 2014; Caspary et al. 2008; Fetoni et al. 2011; Syka 2010). Different changes regarding inhibitory mechanisms in the central auditory system have recently been revealed in aged monkeys by using protein markers such as calcium-binding protein paravalbumin (PV) and nitric oxide synthase NADPH-diaphorase (NADPHd). The densities of neurons coupled with these protein markers are shown to be associated with age, ABR thresholds, and other ABR parameters in the cochlear nucleus (Gray et al. 2014), medial superior olive (Gray et al. 2013a), inferior colliculus (Engle et al. 2014), and medial geniculate nucleus (Gray et al. 2013b). Whereas the present and previous ABR studies showed a decrease in ABR amplitudes among geriatric monkeys, PV- or NADPHd-stained cells within the central auditory system instead increased with the same ABR parameter in aged subjects. Although the exact nature of the increased PV/NADPHd-positive cells within the central auditory system is still unclear, the phenomenon is proposed as a compensation to enhance the efficacy of inhibitory mechanisms during normal aging (Caspary et al. 2008; Engle et al. 2014).
Sex-Related Influence on ARHL in Monkeys
When assessing auditory sensitivity, prior studies on rodents, monkeys, and humans have shown mixed results about sex differences on ABRs and other acoustic tests. In rats and mice, sex differences are often reported to be influenced by combined actions of aging and sex hormones (Fetoni et al. 2011; Willott 2009) although the direction of sex differences on ABR parameters varies across studies. In mice of the C57BL/6J (B6) strain, females with or without an ovariectomy showed higher ABR thresholds than males for tone stimuli of 8–32 kHz, whereas gonadectomy did not affect ABR thresholds of male or female controls (Willott 2009; Willott et al. 2008). Hearing loss at high frequencies (above 32 kHz of ABR and cochlear nerve envelope response) was also present to a higher degree in C57BL/6 females than males at 200 days of age and beyond, and these females exhibited more progressive and severe age-related hearing loss from low to high frequencies when compared with males (Henry 2002, 2004). Aged CBA female mice (24 mo old), in contrast, had lower ABR thresholds than males, but no sex differences were found in the ABR amplitudes for young and middle-aged CBA mice (Guimaraes et al. 2004). In rats, females had lower ABR thresholds than males, and they had higher amplitudes and shorter latencies at higher stimulus levels in the newborn/young (postnatal day 22 or 220) and middle-aged animals (postnatal day 520; Church et al. 2012). On the other hand, human studies have reported substantial evidence for sex differences on age-related influences on hearing sensitivity. Hearing loss progresses faster in men than women for both young and old individuals, from low to high frequencies (McFadden 1998; Pearson et al. 1995). For click-evoked ABRs, men generally have longer latencies and smaller amplitudes than women (Coleman et al. 1994; Durrant et al. 1990; Gates et al. 1999; Jerger and Hall 1980; Jerger and Johnson 1988; McFadden 1998; Michalewski et al. 1980; Watson 1996), and the same trend is also true for speech-evoked ABRs (Krizman et al. 2012). Wiley et al. (2008) illustrated that hearing loss between males and females also varied with stimulus frequencies. Above 50 yr of age, men showed a larger degree of hearing loss at higher frequencies (e.g., 8 kHz) than women, whereas both sexes showed changes in behavioral thresholds at a similar rate at low frequencies (e.g., 0.5–1.0 kHz).
In marmosets and rhesus monkeys, there is no reported sex difference in ABR-based audiograms, middle latency responses, or distortion product otoacoustic emissions (DPOAEs) (Harada et al. 1999; Lasky et al. 1999; Torre and Fowler 2000). One study examined the effects of aging and caloric restriction on hearing loss and reported that female rhesus monkeys had larger ABR peak amplitudes in shorter latencies than male monkeys (Fowler et al. 2002). A later study by the same research group revealed no sex difference on DPOAEs between age groups, but a rather weak sex-related effect showed that male monkeys had marginally higher ABR thresholds than female monkeys at 32 kHz (Fowler et al. 2010). The present findings illustrate no significant difference in ABR-based audiograms at or above threshold levels between male and female monkeys. The preliminary evidence suggests that ABR peak latencies did weakly increase as a function of age in female monkeys only. Results from the analysis of peaks elicited by above-threshold stimulus levels indicated that age-related influences on females and males were present at different frequencies. These findings remained consistent even when the three oldest female monkeys were excluded. Although the present results showed a noteworthy interaction between sex and stimulus type on ABR peak latencies above threshold levels, pairwise comparisons did not reveal a significant difference between male and female monkeys. It is still unknown why the age-related influences on peak latencies at threshold only appeared in female monkeys. Hormonal influences on ABR audiograms of geriatric subjects may be another important factor contributing to our results, as the current trend exhibited in female monkeys is somewhat similar to those found in C57BL/6J (B6) inbred female mice. Estrogen levels are known to effectively modulate hearing sensitivity in female humans and rodents (Hultcrantz et al. 2006). Auditory thresholds vary with the menstrual cycle in females, and hormonal changes before and after menopause may impact audiometric measures in geriatric women (Caruso et al. 2003; Hederstierna et al. 2010; Krizman et al. 2012; Lavoie et al. 2008; Serra et al. 2003; Tandon et al. 2001; Vander Werff and Burns 2011). Follow-up investigations in nonhuman primates are necessary to clarify how a variety of sex-related factors could modulate hearing sensitivity during aging. For example, female rhesus monkeys were reported to have shorter ABR latencies after estrogen treatments, relative to the control group (Hultcrantz et al. 2006). Experiments that systemically monitor and manipulate estrogen at different ages should allow an assessment of how the neuroendocrine system may influence the efficacy of peripheral and central auditory functions and their potential relationship with aging.
In summary, the present study included a large number of monkeys with different degrees of hearing sensitivity and assessed how age-related hearing deficits were expressed in ABRs at threshold and suprathreshold levels. Parallel to humans and rodents, there was a robust decline in hearing sensitivity with age across a stimulus spectrum of low, middle, high, and broadband frequencies. Old monkeys also had lower amplitudes and longer latencies at peaks II and IV compared with younger animals. These results can be explained by peripheral hearing differences between young and old monkeys in which effects of age became minimal after controlling hearing sensation levels across subjects. Although the nongeriatric monkeys (<21.5 yr of age) had lower thresholds (i.e., higher sensitivity) with well-formed ABR peaks, some of them did not have clear, reliable ABR peaks II and IV at high frequencies. This implies that some degree of hearing loss is present in young and middle-aged monkeys, which progressively gets worse with age. The present study also revealed a weak but significant relationship between age and peak latencies in females at threshold, but this finding was not the same as those reported in Fowler et al. (2010). Nevertheless, both studies are in agreement that the role of sex in age-related hearing loss is less well known in nonhuman primates. Future studies are necessary to examine how sex-related factors (e.g., sex hormones) interplay with aging and modulate hearing capability in monkeys.
GRANTS
This work was supported by National Institute on Aging grant no. R01 AG034137 (G. H. Recanzone).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.-W.N. analyzed data; C.-W.N. interpreted results of experiments; C.-W.N. prepared figures; C.-W.N. drafted manuscript; C.-W.N., X.N., and G.H.R. edited and revised manuscript; C.-W.N., X.N., J.R.E., and G.H.R. approved final version of manuscript; X.N. and J.R.E. performed experiments; J.R.E. and G.H.R. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Dina Juarez-Salinas and Misty Fletcher for help with this project and Shelly Lenz, Rhonda Oates-O'Brien, and Guy Martin for excellent animal care.
REFERENCES
- Allen AR, Starr A. Auditory brain stem potentials in monkey (M. mulatta) and man. Electroencephalogr Clin Neurophysiol 45: 53–63, 1978. [DOI] [PubMed] [Google Scholar]
- Alvarado JC, Fuentes-Santamaría V, Gabaldón-Ull MC, Blanco JL, Juiz JM. Wistar rats: a forgotten model of age-related hearing loss. Front Aging Neurosci 6: 29, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backoff PM, Caspary DM. Age-related changes in auditory brainstem responses in Fischer 344 rats: effects of rate and intensity. Hear Res 73: 163–72, 1994. [DOI] [PubMed] [Google Scholar]
- Bao J, Ohlemiller KK. Age-related loss of spiral ganglion neurons. Hear Res 264: 93–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar I, Cronholm JN, Loeb M. Auditory sensitivity of the rhesus monkey. J Comp Physiol Psychol 59: 426–428, 1965. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Davis RT, Miller JM. Demonstration of presbycusis across repeated measures in a nonhuman primate species. Behav Neurosci 97: 602–607, 1983. [DOI] [PubMed] [Google Scholar]
- Boettcher FA. Presbycusis and the auditory brainstem response. J Speech Lang Hear Res 45: 1249–1261, 2002. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Norton BL. Age-related changes in auditory evoked potentials of gerbils. I. Response amplitudes. Hear Res 71: 137–145, 1993a. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Norton BL, Schmiedt RA. Age-related changes in auditory evoked potentials of gerbils. II. Response latencies. Hear Res 71: 146–156, 1993b. [DOI] [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Rugolo S, Intelisano G, Farina M, Cocuzza S, Serra A. Auditory brainstem response in premenopausal women taking oral contraceptives. Hum Reprod 18: 85–89, 2003. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211: 1781–1791, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisolm TH, Willott JF, Lister JJ. The aging auditory system: anatomic and physiologic changes and implications for rehabilitation. Int J Audiol 42: S3–S10, 2003. [PubMed] [Google Scholar]
- Church MW, Hotra JW, Holmes PA, Anumba JI, Jackson DA, Adams BR. Auditory brainstem response (ABR) abnormalities across the life span of rats prenatally exposed to alcohol. Alcohol Clin Exp Res 36: 83–96, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res 80: 209–215, 1994. [DOI] [PubMed] [Google Scholar]
- Davis RT, Leathers CW. Behavior and Pathology of Aging in Rhesus Monkeys. New York, NY: Liss, 1985. [Google Scholar]
- Demeester K, van Wieringen A, Hendrickx JJ, Topsakal V, Fransen E, van Laer L, Van Camp G, Van de Heyning P. Audiometric shape and presbycusis. Int J Audiol 484: 222–232, 2009. [DOI] [PubMed] [Google Scholar]
- Doyle WJ, Saad MM, Fria TJ. Maturation of the auditory brain stem response in rhesus monkeys (Macaca mulatta). Electroencephalogr Clin Neurophysiol 56: 210–223, 1983. [DOI] [PubMed] [Google Scholar]
- Durrant JD, Sabo DL, Hyre RJ. Gender, head size, and ABRs examined in large clinical sample. Ear Hear 11: 210–214, 1990. [DOI] [PubMed] [Google Scholar]
- Engle JR, Gray DT, Turner H, Udell JB, Recanzone GH. Age-related neurochemical changes in the rhesus macaque inferior colliculus. Front Aging Neurosci 6: 73, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle JR, Tinling S, Recanzone GH. Age-related hearing loss in rhesus monkeys is correlated with cochlear histopathologies. PLoS One 8: e55092, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetoni AR, Picciotti PM, Paludetti G, Troiani D. Pathogenesis of presbycusis in animal models: a review. Exp Gerontol 46: 413–425, 2011. [DOI] [PubMed] [Google Scholar]
- Fowler CG, Torre P 3rd, Kemnitz JW. Effects of caloric restriction and aging on the auditory function of rhesus monkeys (Macaca mulatta): The University of Wisconsin Study. Hear Res 169: 24–35, 2002. [DOI] [PubMed] [Google Scholar]
- Fowler CG, Chiasson KB, Leslie TH, Thomas D, Beasley TM, Kemnitz JW, Weindruch R. Auditory function in rhesus monkeys: effects of aging and caloric restriction in the Wisconsin monkeys five years later. Hear Res 261: 75–81, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet 366: 1111–1120, 2005. [DOI] [PubMed] [Google Scholar]
- Gates GA, Couropmitree NN, Myers RH. Genetic associations in age-related hearing thresholds. Arch Otolaryngol Head Neck Surg 125: 654–659, 1999. [DOI] [PubMed] [Google Scholar]
- Gray DT, Engle JR, Recanzone GH. Age-related neurochemical changes in the rhesus macaque superior olivary complex. J Comp Neurol 522: 573–591, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DT, Rudolph ML, Engle JR, Recanzone GH. Parvalbumin increases in the medial and lateral geniculate nuclei of aged rhesus macaques. Front Aging Neurosci 5: 69, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DT, Engle JR, Recanzone GH. Age-related neurochemical changes in the rhesus macaque cochlear nucleus. J Comp Neurol 522: 1527–1541, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim SH, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res 192: 83–89, 2004. [DOI] [PubMed] [Google Scholar]
- Harada T, Tokuriki M, Tanioka Y. Age-related changes in the brainstem auditory evoked potentials of the marmoset. Hear Res 128: 119–124, 1999. [DOI] [PubMed] [Google Scholar]
- Harkins SW. Effects of age and interstimulus interval on the brainstem auditory evoked potential. Int J Neurosci 15: 107–118, 1981. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Miller JM, Rouse RC, Davis JA, Rarey K. Inner ear histopathology in aging rhesus monkeys (Macaca mulatta). In: Behavior and Pathology of Aging in Rhesus Monkeys, edited by Davis RT, Leathers CW. New York, NY: Liss, 1986. [Google Scholar]
- Hederstierna C, Hultcrantz M, Collins A, Rosenhall U. The menopause triggers hearing decline in healthy women. Hear Res 259: 31–35, 2010. [DOI] [PubMed] [Google Scholar]
- Henry KR. Sex- and age-related elevation of cochlear nerve envelope response (CNER) and auditory brainstem response (ABR) thresholds in C57BL/6 mice. Hear Res 170: 107–15, 2002. [DOI] [PubMed] [Google Scholar]
- Henry KR. Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hear Res 190: 141–148, 2004. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol (Stockh) 126: 10–14, 2006. [DOI] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH, Wingfield A. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol 23: 635–666, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol 106: 387–391, 1980. [DOI] [PubMed] [Google Scholar]
- Jerger J, Johnson K. Interactions of age, gender, and sensorineural hearing loss on ABR latency. Ear Hear 9: 168–176, 1988. [DOI] [PubMed] [Google Scholar]
- Krizman J, Skoe E, Kraus N. Sex differences in auditory subcortical function. Clin Neurophysiol 123: 590–597, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky RE, Maier MM, Snodgrass EB, Laughlin NK, Hecox KE. Auditory evoked brainstem and middle latency responses in Macaca mulatta and humans. Hear Res 89: 212–225, 1995. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Soto AA, Luck ML, Laughlin NK. Otoacoustic emission, evoked potential, and behavioral auditory thresholds in the rhesus monkey (Macaca mulatta). Hear Res 136: 35–43, 1999. [DOI] [PubMed] [Google Scholar]
- Laughlin NK, Hartup BK, Lasky RE, Meier MM, Hecox KE. The development of auditory event related potentials in the rhesus monkey (Macaca mulatta). Dev Psychobiol 34: 37–56, 1999. [PubMed] [Google Scholar]
- Lavoie BA, Mehta R, Thornton AR. Linear and nonlinear changes in the auditory brainstem response of aging humans. Clin Neurophysiol 119: 772–785, 2008. [DOI] [PubMed] [Google Scholar]
- Martini A, Comacchio F, Magnavita V. Auditory brainstem and middle latency evoked responses in the clinical evaluation of diabetes. Diabet Med 8: S74–S77, 1991. [DOI] [PubMed] [Google Scholar]
- McFadden D. Sex differences in the auditory system. Dev Neuropsychol 14: 261–298, 1998. [Google Scholar]
- McFadden SL, Campo P, Quaranta N, Henderson D. Age-related decline of auditory function in the chinchilla (Chinchilla laniger). Hear Res 111: 114–126, 1997. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Thompson LW, Patterson JV, Bowman TE, Litzelman D. Sex differences in the amplitudes and latencies of the human auditory brain stem potential. Electroencephalogr Clin Neurophysiol 48: 351–356, 1980. [DOI] [PubMed] [Google Scholar]
- Møller AR, Burgess J. Neural generators of the brain-stem auditory evoked potentials (BAEPs) in the rhesus monkey. Electroencephalogr Clin Neurophysiol 65: 361–372, 1986. [DOI] [PubMed] [Google Scholar]
- Nelson EG, Hinojosa R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope 116, Suppl 112: 1–12, 2006. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res 1091: 89–102, 2006. [DOI] [PubMed] [Google Scholar]
- Ottaviani F, Maurizi M, D'Alatri L, Almadori G. Auditory brainstem responses in the aged. Acta Otolaryngol Suppl Stockh 476: 110–113, 1990. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett E. Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hear Res 289: 52–62, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Datta J, Torres JA, Hopkins C, Bartlett EL. Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. J Assoc Res Otolaryngol 15: 649–661, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JD, Morrell CH, Gordon-Salant S, Brant LJ, Metter EJ, Klein LL, Fozard JL. Gender differences in a longitudinal study of age-associated hearing loss. J Acoust Soc Am 97: 1196–1205, 1995. [DOI] [PubMed] [Google Scholar]
- Popelar J, Groh D, Pelánová J, Canlon B, Syka J. Age-related changes in cochlear and brainstem auditory functions in Fischer 344 rats. Neurobiol Aging 27: 490–500, 2006. [DOI] [PubMed] [Google Scholar]
- Rosenhall U, Pedersen K, Dotevall M. Effects of presbycusis and other types of hearing loss on auditory brainstem responses. Scand Audiol 15: 179–185, 1986. [DOI] [PubMed] [Google Scholar]
- Rowe MJ., 3rd Normal variability of the brain-stem auditory evoked response in young and old adult subjects. Electroencephalogr Clin Neurophysiol 44: 459–470, 1978. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol 102: 1–16, 1993. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33: 13686–13694, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra A, Maiolino L, Agnello C, Messina A, Caruso S. Auditory brain stem response throughout the menstrual cycle. Ann Otol Rhinol Laryngol 112: 549–553, 2003. [DOI] [PubMed] [Google Scholar]
- Skoe E, Krizman J, Anderson S, Kraus N. Stability and plasticity of auditory brainstem function across the lifespan. Cereb Cortex 25: 1415–1426, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J. The Fischer 344 rat as a model of presbycusis. Hear Res 264: 70–78, 2010. [DOI] [PubMed] [Google Scholar]
- Tandon OP, Khaliq F, Goel N. Auditory evoked potential responses in menopausal women: a normative study. Indian J Physiol Pharmacol 45: 361–366, 2001. [PubMed] [Google Scholar]
- Torre P 3rd, Fowler CG. Age-related changes in auditory function of rhesus monkeys (Macaca mulatta). Hear Res 142: 131–140, 2000. [DOI] [PubMed] [Google Scholar]
- Torre P 3rd, Lasky RE, Fowler CG. Aging and middle ear function in rhesus monkeys (Macaca mulatta). Audiology 39: 300–304, 2000. [DOI] [PubMed] [Google Scholar]
- Torre P 3rd, Mattison JA, Fowler CG, Lane MA, Roth GS, Ingram DK. Assessment of auditory function in rhesus monkeys (Macaca mulatta): effects of age and calorie restriction. Neurobiol Aging 25: 945–954, 2004. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Burns KS. Brain stem responses to speech in younger and older adults. Ear Hear 32: 168–180, 2011. [DOI] [PubMed] [Google Scholar]
- Watson DR. The effects of cochlear hearing loss, age and sex on the auditory brainstem response. Audiology 35: 246–258, 1996. [DOI] [PubMed] [Google Scholar]
- Wiley TL, Chappell R, Carmichael L, Nondahl DM, Cruickshanks KJ. Changes in hearing thresholds over 10 years in older adults. J Am Acad Audiol 19: 281–292; quiz 371, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF. Effects of sex, gonadal hormones, and augmented acoustic environments on sensorineural hearing loss and the central auditory system: insights from research on C57BL/6J mice. Hear Res 252: 89–99, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Vanden Bosche J, Shimizu T, Ding DL, Salvi R. Effects of exposing C57BL/6J mice to high- and low-frequency augmented acoustic environments: auditory brainstem response thresholds, cytocochleograms, anterior cochlear nucleus morphology and the role of gonadal hormones. Hear Res 235: 60–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]