Abstract
Patient: Female, 70
Final Diagnosis: Follicular variant of papillary thyroid cancer with renal metastases
Symptoms: Bleeding per vaginum
Medication: —
Clinical Procedure: Total thyroidectomy
Specialty: Oncology
Objective:
Rare disease
Background:
Follicular variant of papillary thyroid cancer (FV-PTC) is the second most common subtype of papillary thyroid cancer (PTC) after classic PTC. FV-PTC is characterized by nuclear features consistent with classic PTC but has a follicular architecture that lacks classic papillary morphology. Thyroid cancer rarely metastasizes to the kidney. Only 6 cases of FV-PTC metastasizing to the kidney have been reported in the English literature. We are reporting a case of FV-PTC with bilateral renal metastases discovered incidentally during work-up of primary endometrial cancer.
Case Report:
A 70-year-old woman presented with post-menopausal bleeding secondary to endometrial cancer. Staging work-up showed multiple bilateral lung nodules, bilateral soft tissue kidney masses, and multinodular goiter. The pathological and immnohistochemical profile of the lung biopsy was consistent with primary well-differentiated lung adenocarcinoma. Follow-up computerized tomography scan showed stable lung nodules and enlarging renal masses, which was suggestive of bilateral renal cancer. While the histologic features of the renal biopsy were not typical, the immunohistochemical staining of renal biopsy was positive for Paired box 8, thyroid transcription factor-1, thyroglobulin, and cytokeratin 7, suggesting the thyroid as the primary cancer site. The final histopathology on surgical specimen of total thyroidectomy revealed follicular variant of papillary thyroid cancer.
Conclusions:
The presence of pulmonary nodules and kidney masses does not always suggest the lung or the kidney as primary tumor sites. The clinician should be aware of the possibility of metastasis and look for the primary source, which in the present case was FV-PTC. Immunohistochemistry plays an important role in determining the primary site of origin. In case of multiple-organ metastases, each metastatic lesion should be biopsied as soon as possible for definitive diagnosis and appropriate treatment.
MeSH Keywords: Endometrial Neoplasms, Kidney Neoplasms, Lung Neoplasms, Thyroid Neoplasms
Background
Papillary thyroid cancer (PTC) is the most common type (80%) of thyroid cancer. Classic-PTC (c PTC) is the most common subtype of PTC followed by follicular variant of papillary thyroid cancer (FV-PTC). FV-PTC accounts for 10–20% of all PTC tumors and has 3 subtypes; completely encapsulated, well-circumscribed, and infiltrative, the last being the most aggressive. FV-PTC has a follicular architecture in which follicles are lined by cells that have nuclear features of PTC. Crile and Hazard first described FV-PTC in 1953 [1], which was later confirmed by Lindsay in 1960 [2]. Here, we report a case of bilateral renal metastases from FV-PTC found incidentally in a patient who presented with postmenopausal bleeding due to primary endometrial cancer. This is the first clinically detected case of FV-PTC with bilateral renal metastases reported in the English literature.
Case Report
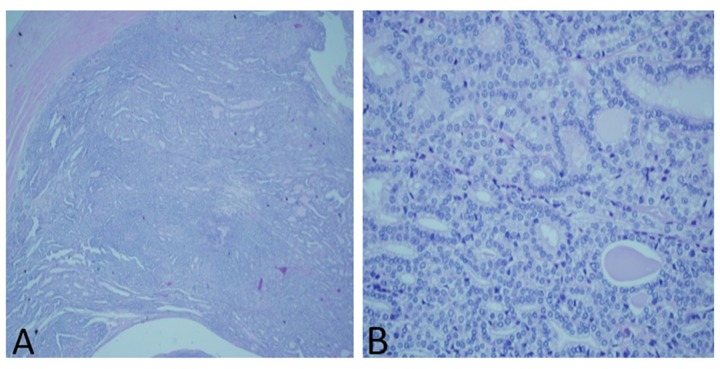
A 70-year-old Guyanese woman presented in March of 2014 with complaints of occasional postmenopausal bleeding of 2 months duration. There was no history of hematuria, flank pain, weight loss, thyroid cancer, or thyroid surgery. Laboratory tests showed normal urine analysis, normal complete blood count, and basic metabolic panel. Endometrial biopsy showed endometrial complex glandular hyperplasia with atypia and focal well-differentiated adenocarcinoma, endometrioid type (Figure 1). A staging computerized tomography (CT) scan done in May 2014 revealed multiple bilateral lung nodules (Figure 2A) and bilateral soft tissue kidney masses (Figure 2B), with right mass measuring 5.6×2.7 cm and left mass measuring 6.4×5.5 cm. The right and left lobes of the thyroid gland were enlarged, with multiple calcific foci, consistent with multinodular goiter. There was no evidence of significant adenopathy. No bone lesions were seen.
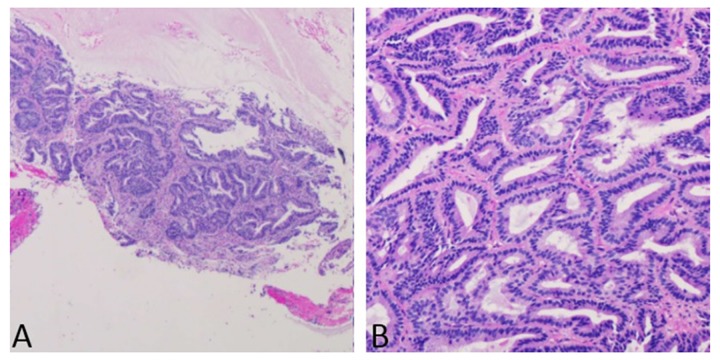
Figure 1.
Endometrial biopsy showing endometrial complex glandular hyperplasia with atypia and focal well-differentiated adenocarcinoma.
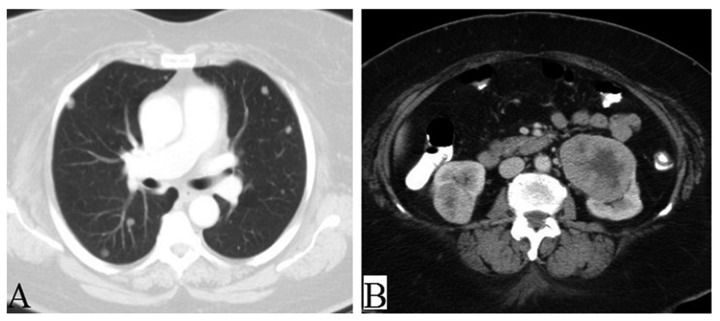
Figure 2.
Axial CT images demonstrates (A) multiple bilateral pulmonary nodules and (B) bilateral exophytic, enhancing, renal masses.
Presumptive diagnosis of primary renal cancer with lung metastases was considered. A positron emission tomography (PET) scan showed nonspecific findings of localized low-grade heterogeneous uptake in the enlarged partially calcified left thyroid lobe [standardized uptake value (SUV) max: 3.6] (Figure 3A, 3B), bilateral avid exophytic renal masses [left kidney SUVmax: 6.1, right kidney SUVmax: 4.3] with central photopenia in the left renal mass suggesting necrosis (Figure 4A, 4B), and bilateral mildly avid pulmonary nodules [SUVmax: 3.0] suspicious for lung metastases (Figure 5A, 5B). Furthermore, we detected a new osteolytic lesion suspicious for metastasis at the base of the greater trochanter with SUVmax of 3.0 (Figure 6A, 6B). PET scan also showed low-grade uptake localized to the endometrium [SUVmax: 4.5]. To confirm the origin of the tumor, a CT-guided lung biopsy was performed in July 2014. Pathology showed a well-differentiated adenocarcinoma (Figure 7). Immunohistochemistry (IHC) staining was positive for cytokeratin 7 (CK 7) thyroid transcription factor-1 (TTF-1) and negative for CD 10, S 100, estrogen receptor (ER), and cancer antigen 125 (CA-125). The pathology and IHC profile were consistent with primary lung cancer.
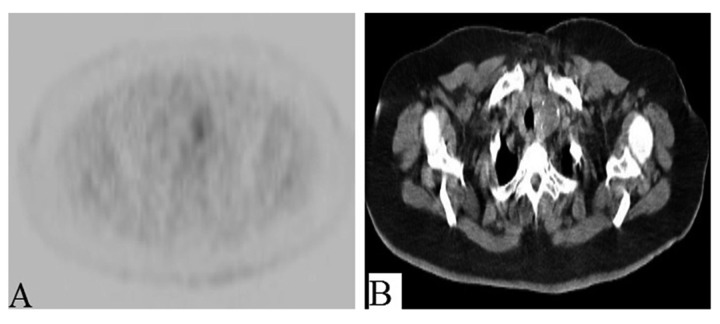
Figure 3.
(A, B) PET/CT demonstrates mild uptake in a partially calcified nodule in the left thyroid.
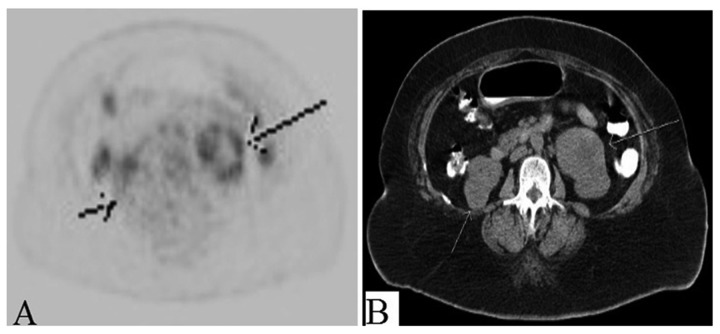
Figure 4.
(A, B) Bilateral exophytic renal masses are metabolically active. The left renal mass has a central low density that is not avid on PET and suggests necrosis/cystic degeneration.
Figure 5.
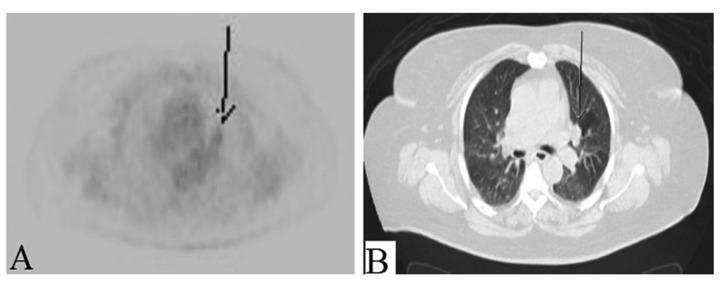
(A, B) Left perihilar nodule is mildly avid on PET and nearly isointense to mediastinal background.
Figure 6.
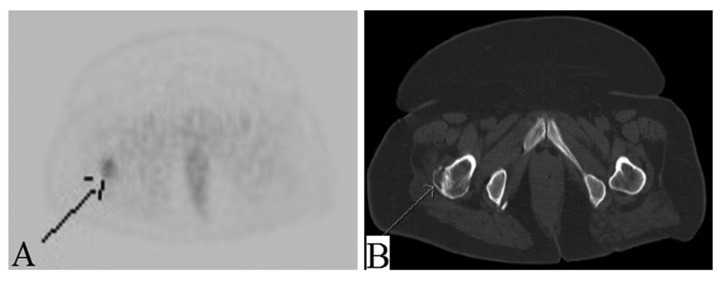
(A, B) Mildly avid lytic lesion on PET in right greater trochanter.
Figure 7.
(A, B) Lung biopsy showing neoplasm with clear cell features.
The right femoral lytic lesion was biopsied in August 2014 to obtain more tissue for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) status for the possible use of Erlotinib; however, the results came back negative. Fluorescence in situ hybridization for ROS 1 was also negative, so Crizotinib could not be used. The IHC profile of bone biopsy further supported primary lung cancer (positive for CK 7, TTF-1 and negative for ER, CD 10, and CA 125).
A follow-up CT scan in October 2014 showed stable lung nodules and enlarging renal masses when compared to the prior CT scan. The CT findings and unchanged clinical performance status of the patient was now suggestive of bilateral renal cancer. The case was presented to the tumor board. The diagnosis was felt to be either lung carcinoma metastasizing to the kidney or a renal carcinoma metastasizing to the lung. Renal biopsy was recommended. The bone biopsy specimen, taken earlier, was also sent to Rosetta Genomics to assess tumor origin.
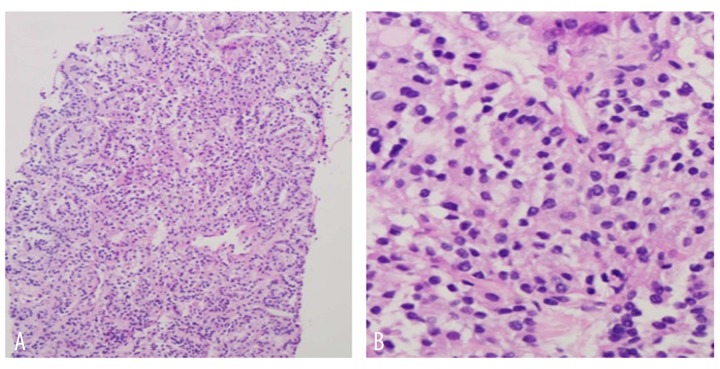
The renal biopsy revealed papillary carcinoma with clear cell features, with morphology similar to that of the lung biopsy (Figure 8). IHC staining was positive for CK 7, TTF-1 and negative for CK 20, CD10, CA 125, ER, and progesterone receptor (PR). The tumor showed diffuse (100%) nuclear staining for Paired box 8 (PAX-8) and TTF-1 (Figure 9). While the histological features were not typical, the IHC profile was a better fit for thyroid carcinoma (as PAX-8 is extremely rare in lung carcinomas and renal cell carcinoma may show only focal staining for TTF-1). Additional immunostaining for thyroglobulin, napsin A (the lung marker), and carbonic anhydrase IX (the renal marker) to confirm the origin were recommended. The results from Rosetta Genomics came back revealing follicular thyroid cancer as the tumor of origin. The immunostaining was strongly positive for thyroglobulin (Figure 10) and negative for napsin A and carbonic anhydrase IX. This confirmed the thyroid as the primary site.
Figure 8.
(A, B) Kidney biopsy showing papillary carcinoma with clear cell features.
Figure 9.
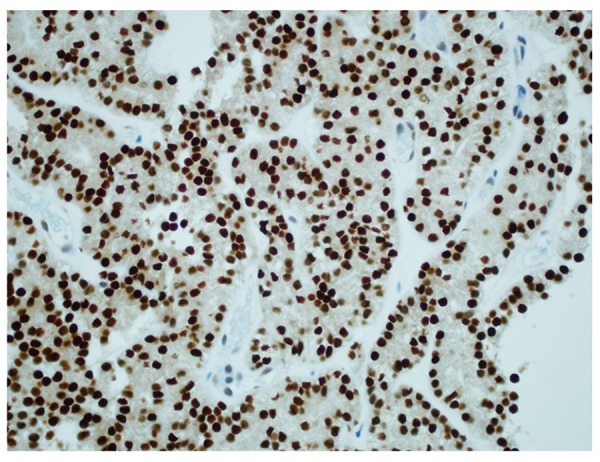
Immunohistochemical staining of kidney biopsy revealing tumor cells positive for thyroid transcription factor-1.
Figure 10.
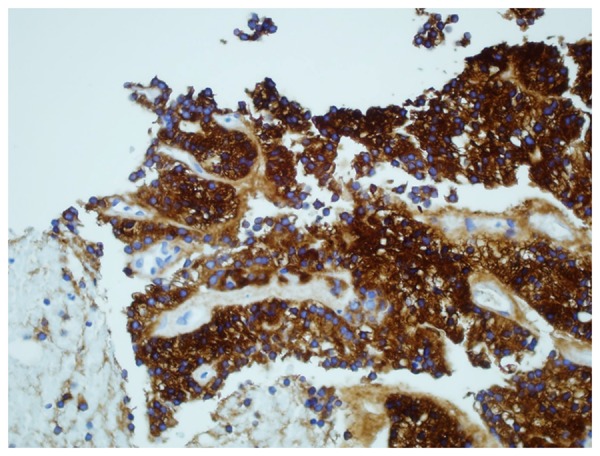
Immunohistochemical staining of kidney biopsy revealing tumor cells positive for thyroglobulin.
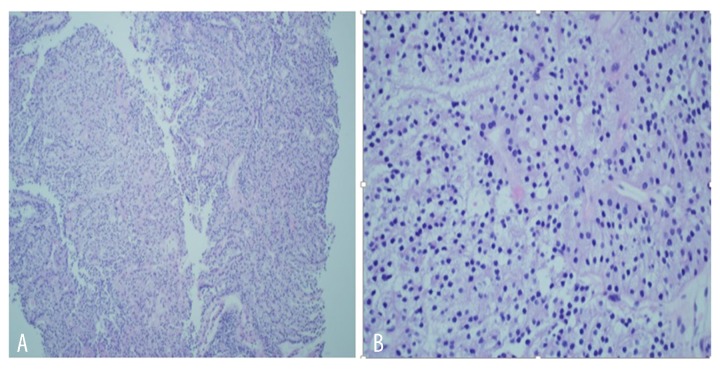
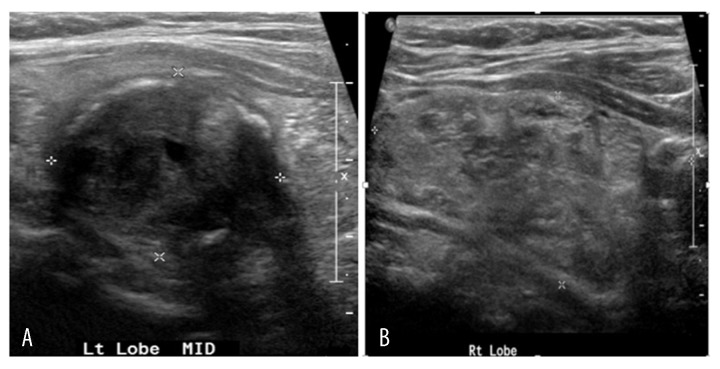
Thyroid ultrasound (US) showed a 5.0×2.4 cm nodule almost entirely occupying the right lobe and 2 nodules in the left lobe measuring 2.9×2.3 cm and 1.1×1.0 cm (Figure 11). Fine-needle aspiration biopsy of the thyroid revealed follicular neoplasm. The patient underwent total thyroidectomy in December of 2014. Histopathology of the surgical specimen revealed follicular variant of papillary thyroid cancer with clear cell differentiation and lymphovascular invasion (Figure 12). The regional lymph nodes were negative. Postoperatively, the patient was started on levothyroxine suppression therapy and received radioactive iodine ablation (200 mCi) in April 2015. An iodine-131 whole-body scan was performed, showing severe widespread disease in the lungs, liver, bilateral kidney (right more than left), and the right greater trochanter (Figure 13).
Figure 11.
(A, B) Ultrasonography of thyroid gland showing abnormal texture with focal nodules in both the thyroid lobes. A solid nodule occupies the entire right lobe. Solid nodule with calcified borders noted in the middle part of the left lobe.
Figure 12.
(A, B) Histology of the thyroidectomy specimen showing papillary carcinoma, follicular variant, with focal clear cell differentiation.
Figure 13.
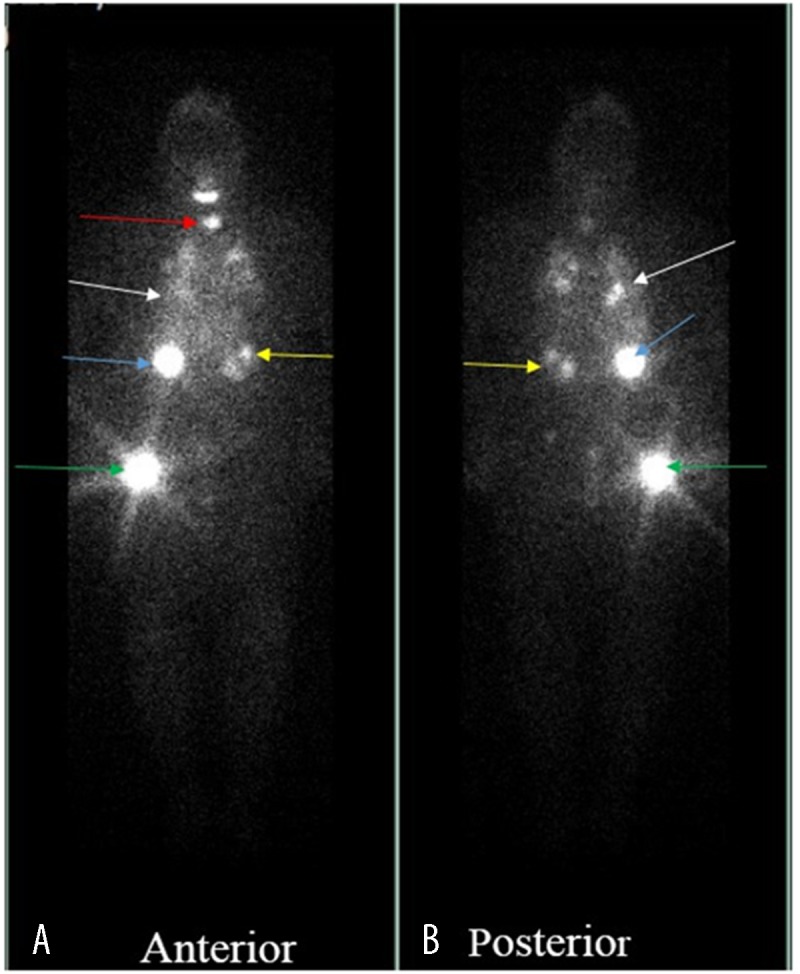
A total body iodine-131 scan demonstrates severe, widespread iodine avid metastatic disease seen in the thyroid lungs (red arrow), right (blue arrow) and left (yellow arrow) kidney, lungs (white arrow) and greater trochanter (green arrow).
The plan was to perform left radical nephrectomy (as it was not iodine-avid, possibly due to necrosis) and hysterectomy. However, left radical nephrectomy was not performed due to the possible risk of deterioration of renal function, which had remained at baseline over the period of one and a half years. Hysterectomy was deferred since the patient had low-grade endometrial cancer with no further uterine bleeding. The revised treatment plan is to repeat the whole-body iodine scan in 3–6 months to assess the response to iodine ablation. The patient is being followed with thyroglobulin and thyroid-stimulating hormone (TSH) levels. The patient will also receive prophylactic radiation therapy for the right trochanteric lytic lesion.
Discussion
For certain malignancies, such as lung cancer, the kidney is not an uncommon site for metastases but for thyroid cancer it is extremely rare. Bracken et al., in an autopsy study of 11 328 patients who died of malignant disease, reported that 816 (7.2%) had renal metastases. Out of all the cases of renal metastases, melanoma was the most common primary (35.5%) site followed by lung (17.8%), gastrointestinal tract (11.5%), genitourinary tract (10.5%), breast (8.3%), and gynecologic tract (4.3%) [3]. Thyroid cancer metastasizing to the kidney is rare and only 24 cases have been reported so far in the English literature. Out of these, 6 were FV-PTC, and this is the 7th case of FV-PTC (Table 1). The clinical manifestations of these tumors were palpable abdominal mass, abdominal/flank pain, or hematuria, with only 2 cases detected incidentally, including the present case. This is the first reported case of FV-PTC with bilateral renal metastases. In the present case there was no history of thyroid cancer, thyroid surgery, or radiation exposure, in contrast to other reported cases of FV-PTC.
Table 1.
FV-PTC with renal metastases.
| Reference | Age/gender | Clinical presentation | Location/side & size | Interval from Initial diagnosis of thyroid cancer/surgery (years) | Other sites of metastases | Follow up | |
|---|---|---|---|---|---|---|---|
| 1. | Tur et al. (1994) [4] | 72/F | Incidental | Right 3 kidney, 3 cm | Liver – caudate lobe | No recurrence or new complaints 7 months s/p surgery | |
| 2. | Graham et al. (1995) [5] | 75/M | Hematuria | Left No Interval kidney, 8.5 cm | None | No recurrence or new complaints 36 months s/p surgery | |
| 3. | Gamboa-Dominguez et al. (1999) [6] | 50/F | Hematuria, Left flank pain | Left Kidney, 8 cm | No interval | Two cerebral metastases | Died in 11 months due to brain metastasis |
| 4. | Smallridge et al. (2001) [7] | 61/F | Upper back mass | Left Kidney, 3 cm | 1/4 | Left shoulder/scapula | No recurrence 2 years post treatment |
| 5. | Liou et al. (2005) [8] | 50/F | Low back pain and palpable thyroid nodule | Right Kidney, 1.9 cm | No interval | Lung, bones | No follow up available |
| 6. | Varinot et al. (2014) [9] | 77/M | Hematuria | Right kidney, 4.5 cm | 6 | Bones, brain | No follow up available |
| 7. | Present case (2015) | 70/F | Incidental | Bilateral (left 6.4 cm right 3.6 cm) | Thyroid cancer was found later than its metastasis | Bones, lungs and liver | Patient is under treatment |
All the patients were ≥50 years of age, out of total FVPTC cases 5 were female (71.4%), out of 7 FVPTC cases 3 metastasized to right and 3 to the left kidneys. Only the present case metastasized to both kidneys.
The most frequent distant sites of thyroid metastases are the lungs and bones, but metastases to the kidney are very rare and most often are detected during autopsy. In a study of 161 autopsy cases of metastatic thyroid cancer, 6.1% of well-differentiated thyroid cancer metastasized to the kidney [10].
There are many possible reasons for poor detection of renal metastasis. It may be due to the inability of the metastatic lesions to trap iodide on iodine-131 scan, secondary to mis-directed, altered, or intracellularly entrapped sodium iodide symporter (NIS) protein. NIS, a transmembrane glycoprotein, mediates uptake of iodide into the follicular cells of the thyroid gland in the synthesis of thyroid hormone [7]. Other possible reasons are: inability of the magnetic resonance imaging (MRI) to differentiate between the renal metastases and solid kidney tumors (since metastatic lesions usually appear isointense with surrounding tissue on T1-weighted images of MRI) [11]; misinterpretation of iodine-131 scan in the abdomen as physiological uptake [7]; and low diagnostic yield of routine imaging modalities such as CT or US [12]. Moreover, metastases to the kidney occur very late due to the indolent nature of thyroid cancer. Often these metastases do not produce any signs or symptoms. One case had metastases 37 years after the diagnosis of thyroid cancer [13].
The diagnosis of FV-PTC depends on characteristic nuclear features of papillary carcinoma, even when there is no extension beyond the thyroid gland [2]. FV-PTC is difficult to diagnosis clinically and pathologically because most it usually occurs in the background of nodular goiter, giving the false impression of follicular adenoma or adenomatoid nodule. Furthermore, FVPTC sometimes does not possess all of the characteristic nuclear features, or these may be present only focally, while the rest of the tumor exhibits benign features [1]. Additionally, there is an overlap of histologic features between FV-PTC and other follicular neoplasms. It has been reported that FNAB has a low sensitivity for diagnosing FVPTC, therefore requiring thyroidectomy for accurate diagnosis [14].
To clearly identify the primary neoplasm, immunohistochemistry plays an important role. One marker may be positive for more than 1 tumor, and thus a combination of markers is needed to confirm the diagnosis. The CK7, CK20, and TTF-1 are important IHC markers for the diagnosis of lung tumors and are useful to differentiate primary pulmonary adenocarcinomas from metastatic adenocarcinomas to the lung [15]. TTF-1 is found in the lung and the thyroid gland but is negative in renal tumors [16]. PAX8 is a highly sensitive marker for thyroid cancer and renal tumors, but it is not found in lung adenocarcinoma [17]. Thyroglobulin is a sensitive and specific histogenetic marker for follicular cell-derived thyroid carcinomas [18].
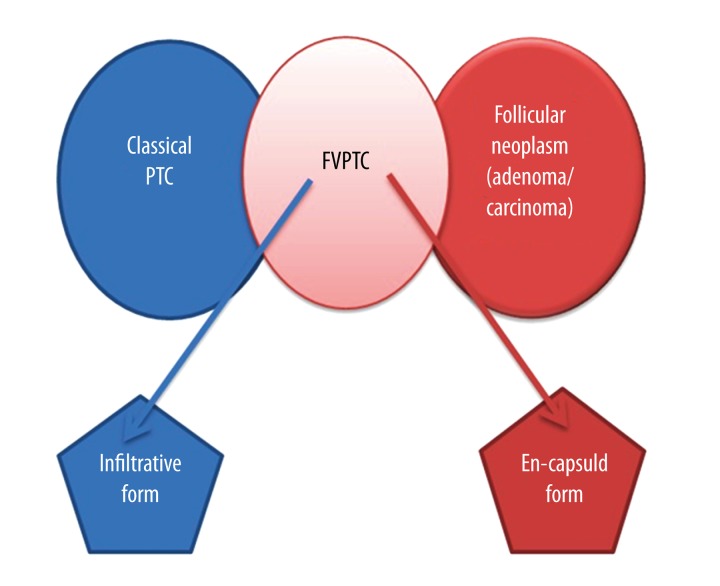
Surgery offers a favorable prognosis for FV-PTC. It has been reported recently that encapsulated FV-PTC and infiltrative FV-PTC demonstrate clinical behavior similar to follicular adenoma and classic PTC, respectively (Figure 14), and should be treated similarly [19]. The best treatment approach to metastatic differentiated thyroid cancer is the surgical removal of the thyroid gland and metastatic lesions followed by iodine-131 ablation therapy. Whole-body scintigraphy should be performed in high-risk cases, especially in patients with elevated thyroglobulin levels. Patients should be given thyroxin suppression therapy following surgical treatment [1]. Mortality in FV-PTC related to distant metastases was 67% in a clinicopathological study done by Mizukami et al. [20].
Figure 14.
Encapsulated FV-PTC and infiltrative FV-PTC have clinical behavior similar to follicular adenoma and classic PTC, respectively.
Conclusions
This case adds to the rare reports in the literature of FV-PTC metastasizing to kidneys. The presence of pulmonary nodules and kidney masses do not always suggest the lung or the kidney as primary tumor sites. The clinician should be aware of the possibility of metastases and look for the primary source, which in the present case was the thyroid. The possibility of thyroid cancer gets overlooked since it rarely metastasizes to the kidneys, so clinicians should have a high index of suspicion even if there is no history of thyroid cancer or thyroid surgery. In patients with metastatic disease, immunohistochemistry plays an important role in determining the primary site of origin since it may have a major impact on therapy and prognosis. It is important to note that in case of multiple organ metastases, each metastatic lesion should be biopsied for definitive diagnosis and appropriate treatment. Since FNAB has a low sensitivity for diagnosing FVPTC, accurate diagnosis requires thyroidectomy.
Footnotes
Conflict of interest
There is no conflict of interest for the authors.
References:
- 1.Salajegheh A, Petcu EB, Smith RA, Lam AK. Follicular variant of papillary thyroid carcinoma: a diagnostic challenge for clinicians and pathologists. Postgrad Med J. 2008;84(988):78–82. doi: 10.1136/pgmj.2007.064881. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Z, Gandhi M, Nikiforova MN, et al. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120(1):71–77. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 3.Bracken RB, Chica G, Johnson DE, Luna M. Secondary renal neoplasms: an autopsy study. South Med J. 1979;72(7):806–7. doi: 10.1097/00007611-197907000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Tur GE, Asanuma Y, Sato T, et al. Resection of metastatic thyroid carcinomas to the liver and the kidney: report of a case. Surg Today. 1994;24(9):844–48. doi: 10.1007/BF01636320. [DOI] [PubMed] [Google Scholar]
- 5.Graham LD, Roe SM. Metastatic papillary thyroid carcinoma presenting as a primary renal neoplasm. Am Surg. 1995;61(8):732–34. [PubMed] [Google Scholar]
- 6.Gamboa-Dominguez A, Tenorio-Villalvazo A. Metastatic Follicular Variant of Papillary Thyroid Carcinoma Manifested as a Primary Renal Neoplasm. Endocr Pathol. 1999;10(3):256–68. [PubMed] [Google Scholar]
- 7.Smallridge RC, Castro MR, Morris JC, et al. Renal metastases from thyroid papillary carcinoma: study of sodium iodide symporter expression. Thyroid. 2001;11(8):795–804. doi: 10.1089/10507250152484664. [DOI] [PubMed] [Google Scholar]
- 8.Liou MJ, Lin JD, Chung MH, et al. Renal metastasis from papillary thyroid microcarcinoma. Acta Otolaryngol. 2005;125(4):438–42. doi: 10.1080/00016480410022822. [DOI] [PubMed] [Google Scholar]
- 9.Varinot J, Ménégaux F, Bitker MO, Compérat E. Renal metastasis from thyroid carcinoma: a case report. Anal Quant Cytopathol Histpathol. 2014;36(1):46–50. [PubMed] [Google Scholar]
- 10.Heitz P, Moser H, Staub JJ. Thyroid cancer: a study of 573 thyroid tumors and 161 autopsy cases observed over a thirty-year period. Cancer. 1976;37(5):2329–37. doi: 10.1002/1097-0142(197605)37:5<2329::aid-cncr2820370523>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Cheon M, Choi JY, Kim HK, et al. Renal Metastasis from Follicular Thyroid Carcinoma Diagnosed by I-131 Whole-body Scan Mimicking Renal Cell Carcinoma on Contrast-Enhanced Computed Tomography. Nucl Med Mol Imaging. 2011;45(1):72–75. doi: 10.1007/s13139-010-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen C, Iuanow E, Oates E, et al. Post-therapy iodine-131 localization in un-suspected large renal cyst: possible mechanisms. J Nucl Med. 1998;39:2158–61. [PubMed] [Google Scholar]
- 13.Johnson MW, Morettin LB, Sarles HE, Zaharopoulos P. Follicular carcinoma of the thyroid metastatic to the kidney 37 years after resection of the primary tumor. J Urol. 1982;127(1):114–16. doi: 10.1016/s0022-5347(17)53634-4. [DOI] [PubMed] [Google Scholar]
- 14.Finnerty BM, Kleiman DA, Scognamiglio T, et al. Navigating the management of follicular variant papillary thyroid carcinoma subtypes: a classic PTC comparison. Ann Surg Oncol. 2015;22(4):1200–6. doi: 10.1245/s10434-014-4126-3. [DOI] [PubMed] [Google Scholar]
- 15.Su YC, Hsu YC, Chai CY. Role of TTF-1, CK20, and CK7 immunohistochemistry for diagnosis of primary and secondary lung adenocarcinoma. Kaohsiung J Med Sci. 2006;22(1):14–19. doi: 10.1016/S1607-551X(09)70214-1. [DOI] [PubMed] [Google Scholar]
- 16.Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol. 2010;41(1):20–25. doi: 10.1016/j.humpath.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35(6):816–26. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 18.Kavishwar VS, Phatak AM, Rege JD. Immunohistochemistry of thyroid carcinoma – an experience with thyroglobulin and calcitonin. Indian J Pathol Microbiol. 1998;41(2):163–67. [PubMed] [Google Scholar]
- 19.Walts AE, Mirocha JM, Bose S. Follicular variant of papillary thyroid carcinoma (FVPTC): histological features, BRAF V600E mutation, and lymph node status. J Cancer Res Clin Oncol. 2015 doi: 10.1007/s00432-015-1939-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Mizukami Y, Michigishi T, Nonomura A, et al. Distant metastases in differentiated thyroid carcinomas: a clinical and pathologic study. Hum Pathol. 1990;21(3):283–90. doi: 10.1016/0046-8177(90)90228-w. [DOI] [PubMed] [Google Scholar]