Abstract
Patient: Male, 6
Final Diagnosis: Cerebral palsy secundary perinatal hypoxia
Symptoms: Cognitive impairment • epilectic seizure
Medication: Platelet rich plasma
Clinical Procedure: Cognitive improvement with neuroestimulator and neuroregenerator power of platelet rich plasma injection
Specialty: Hematology
Objective:
Unusual clinical course
Background:
The use of platelet-rich plasma is a now a common medical technique known as regenerative medicine, through power cell activation and differentiation, which produces growth factors called platelets derived both locally and systematically. Here, we report the case of a cerebral palsy patient who received intravenous platelet-rich plasma.
Case Report:
We administered an intravenous injection of concentrated platelet-rich plasma (25 cc) in a 6-year-old boy with perinatal cerebral palsy, cognitive impairment, and marked and severe generalized spasticity. We performed follow-up at 3 and 6 months after the injection. All serum samples for determination were obtained by ELISA technique. Cognitive scales (Bayley, Battelle, M.S.C.A, Kaufman ABC, and Stanford-Binet Intelligence scale) were used before and after treatment. The determination protocol that was applied before the analysis was performed manually and the autotransfusion was considered suitable for treatment. We determined the plasma levels of factor similar to insulin-1 (IGF-1), platelet-derived growth factor (PDGF), vasculo-endothelial growth factor (VEGF), and transforming growth factor B (TGF-B) before and during treatment monitoring.
Conclusions:
No adverse effects were observed in the patient except for a small hematoma in the area channeling venous access. We observed a clear improvement in the cognitive sphere (memory, ability to perform more complex tasks, and acquisition of new skills) and in language, maintaining stable levels of growth factor in plasma 3–5 times higher than average for his age group at both 3- and 6-month follow-up. Positron emission tomography (PET) images showed an evident increased demarcation in the cerebral cortex. We propose that this therapy is useful in these patients to harness the neurostimulative and neuroregenerative power of endogenous growth factors derived from platelets.
MeSH Keywords: Cerebral Palsy, Insulin-Like Growth Factor I, Neuronal Plasticity, Platelet-Derived Growth Factor, Platelet-Rich Plasma, Transforming Growth Factors
Background
The use of platelet-rich plasma in various fields of medicine and traumatology, dentistry, and general surgery is experiencing an extraordinary development given the larger generation capacity, chemotaxis, modulating angiogenesis, and cellular plasticity of injured tissues that produce growth factors such as insulin growth factor type I (IGF-1), transforming growth factor B (TGF-B), vasculo-endothelial-derived growth factor (VEGF), and platelet-derived growth factor (PDGF), which are the plasma growth and platelet factors that are most reported in the literature due to their power of neurostimulation and neuroregeneration. Regenerative medicine relies on the power of the cell to perform activation and differentiation, producing growth factors derived from platelets both locally and systematically.
However, there are other fields of application in medicine that are opening new roads, such as neuroendocrinology and neurorehabilitation, which are now used either locally or systemically in autologous transplantation, given the intrinsic activating antiapoptotic pathways controlling the biochemical level of neuronal cells, which have specific receptors on their membranes for these growth factors derived from platelets. In addition, it has been demonstrated that in patients with neuronal degenerative diseases (e.g., Alzheimer disease, vascular encephalopathy, multiple sclerosis, ALS, and hypoxic or anoxic encephalopathy), proteins were detected at low levels in serum, as well as being modulated by mechanisms of cerebral hypoxia. Furthermore, a neuroprotective function has also been shown in neuronal tissue.
Case Report
We report the case of a 6-year-old boy diagnosed with cerebral palsy at age 2 months, secondary to perinatal hypoxia, with spastic tetraparesis and moderate cognitive impairment, who received a 25-cc intravenous infusion of PRP with clinical, laboratory, and radiological monitoring prior to infusion and at 3 and 6 months later, without a clinical trial. Authorization for the treatment was provided by the patient’s legal representatives. We obtained 60 cc of the patient’s whole blood. After titration, we performed analytical biochemistry, determination of plasma growth factors, and complete blood count serology to ensure the suitability of the treatment. The technique for obtaining PRP used only 1 centrifugation at 3500 rpm for 30 min at 18°C.
Table 1 shows the cellular and protein values in peripheral blood of the patient and the final product obtained.
Table 1.
Growth factor values and cell count in peripheral blood and final product obtained.
| Peripheral blood | PRP | |
|---|---|---|
| PDGF-AB (10–50 pg/ml) | 45 pg/ml | 360 pg/ml |
| TGF-B1 (10–70 pg/ml) | 35 pg/ml | 320 pg/ml |
| VEGF (15–85 pg/ml) | 55 pg/ml | 560 pg/ml |
| IGF-1 (0.5–19.5 pg/ml) | 13 pg/ml | 175 pg/ml |
| Platelets (150.000–350.000/mm3) | 265.000/mm3 | 1.250.000/mm3 |
| Leucocytes (3.200–9000/mm3) | 5.600/mm3 | 20.000/mm3 |
| Granulocytes | 60% (3.330/mm3) | 24% (480/mm3) |
| Mononuclears | 35% (1.960/mm3) | 70% (14.000/mm3) |
| CD 34+ | 0.5/mm3 | 175/mm3 |
While the laboratory sample was processed, a 20-G venous access was created in the elbow flexure and we infused the RPP within 30 min of obtaining it, at the rate of 1 mL per s.
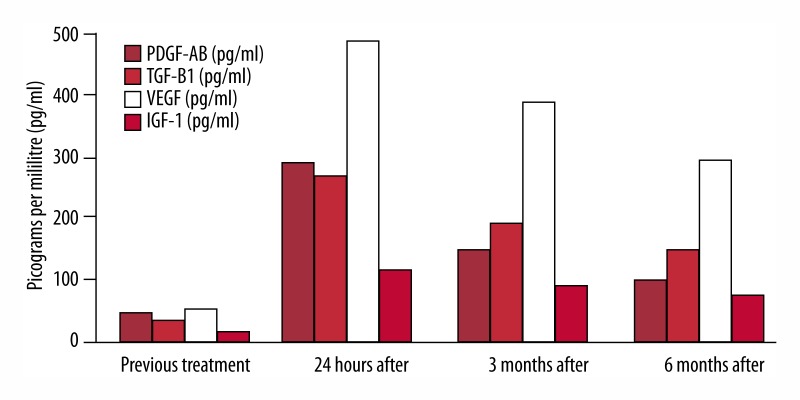
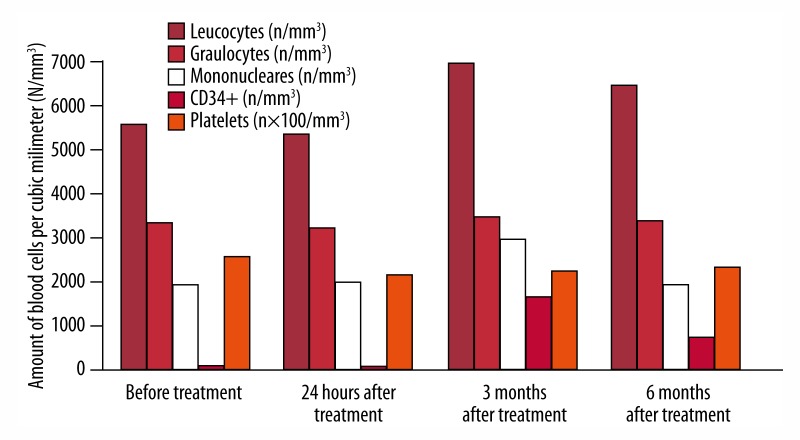
Before the injection and at 24 hours and 3 and 6 months after the injection, we determined plasma levels of serum growth factors by ELISA technique and leucocyte count. Values are shown in Figures 1 and 2.
Figure 1.
Levels of growth factors before injection and at 24 hours and 3 and 6 months after the treatment.
Figure 2.
Count of blood cells before and at 24 hours and 3 and after 6 months after treatment.
Similarly, rigorous monitoring was performed for neurorehabilitative skills acquired by the patient by 3 and 6 months after treatment by applying cognitive scales (Bayley, Battelle, M.S.C.A, Kaufman ABC, and Stanford-Binet intelligence scale) and comparing the results with those obtained before treatment. We did PET brain imaging immediately before and at 6 months after the injection. Images and report results are shown in Figure 3.
Figure 3.
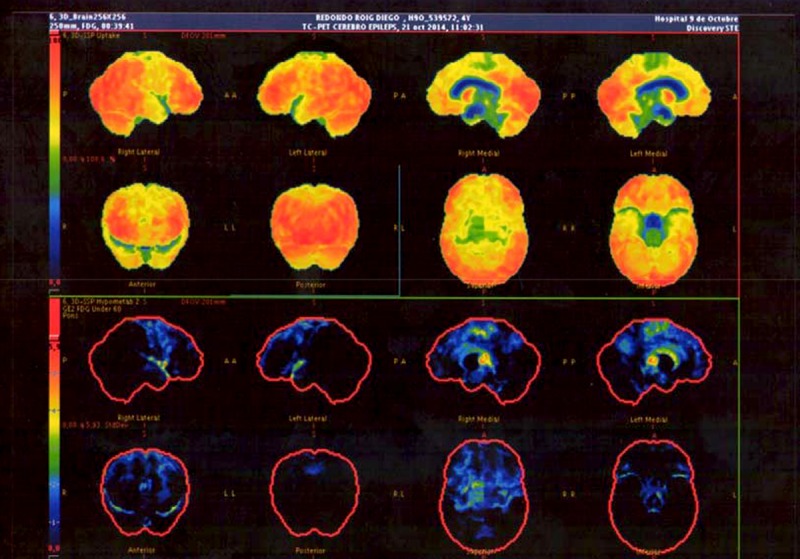
Cerebral PET images taken immediately before treatment and at 6-month follow-up.
Clinical monitoring and analytical values of the obtained platelet growth factors and imaging were satisfactory, obtaining a high peak in levels of plasmatic growth factors at 24 hours after the treatment, which remained stable at above-normal levels at 3 and 6 months after the injection. This resulted in a clearly progressive improvement of the patient’s symptoms, especially in the cognitive sphere (e.g., capacity to eat solid food, remarkable improvement in coordinated movements, speaking 2- and 3-syllable words, and fixing his gaze on moving objects, evident at 3- month follow-up and maintaining progress at 6-month follow-up. Neuroradiological evaluation revealed a clear increase in glucose metabolism level in the entire cortex, suggestive of neuronal plasticity phenomena, and we did not observe any changes in deep brain structures (hippocampus and amygdala).
Discussion
Progress in regenerative medicine in several clinical areas is revolutionizing the field of tissue repair, providing a treatment tool that is economical, easy to use, without adverse effects, and less invasive [1,2]. However, scientific and social requirements make it necessary to design appropriate clinical trials to establish treatment protocols in each particular medical application [1,3].
Today, the medical areas with the most scientific evidence about the use of RPP are dentistry (for repairing dental alveolar bed) and traumatology (arthropathy, tendinopathy, ligament injuries, and meniscopathy), with the design of randomized trials in phase I–II [1,4]. But the empirical use in many diseases and medical specialties is based on weak scientific evidence [5].
Some of the most promising medical fields for application are neurology, neuroendocrinology, and neurorehabilitation. Neuroregenerative, antiapoptotic, immunomodulatory, and neurotropic effects differentiating platelet-derived growth factors in neuronal tissue makes this a totally feasible therapy treatment from the medical point of view, to be applied in neurodegenerative or anoxic and hypoxic pathologies like Alzheimer disease, cerebral vascular accidents, spinal cord injury, and cerebral palsy of hypoxic or anoxic origin [5,6]. Spontaneous remission of cerebral palsy signs is rare due to degenerative neuronal and glial mass secondary to hypoxic effects in the evolution of the disease [7]. Neuroprotective and neuroregeneration stimulator effects were observed in these patients managed with synthetic growth hormone (HGF), which results in functional improvement, especially in the cognitive field (e.g., memory, language, ability to perform complex tasks, and acquisition of new skills). In these patients, the neuronal degenerative effect has been accompanied by a marked qualitative and quantitative decrease in plasmatic growth factors like HGFIGF-1-VEGD, PDGF, and TGF-B [8,9], regulated by the hypothalamic-pituitary axis that produces a neuroprotective effect, and by the neurotropic effect of chemotaxis, cell differentiation, and neuroplasticity on neuronal tissue. However, treatment with synthetic growth hormone is costly, not only from the economic standpoint, but also from the clinician’s viewpoint. The possibility of RPP as a final product, rich in these growth factors, for local or systemic administration in a single dose, and maintenance of plasma levels stable enough for achieving a long-term therapeutic effect similar to clinical hormone synthetic growth, without the oncological secondary effects [5,8], make it attractive for these patients by significantly reducing the economic and clinical cost of treatment.
Conclusions
We report the case of a patient with cerebral palsy, in whom the application of systemic PPR in a single dose increased the level of platelet-derived growth factors to above-normal levels and that remained stable over time (at 6-month clinical follow-up), resulting in clinical improvement, especially in the cognitive sphere, coinciding with the results obtained by other authors who used isolated growth factors in patients with perinatal hypoxia. This is the first case report published in the literature of use of autologous growth factors in this clinical application with successful results. Well-designed, randomized, clinical trials are needed to further develop the utility of this treatment in these patients.
Acknowledgments
We thank the patient, his family, and the professionals who trusted in us.
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
References:
- 1.Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 2.Weibrich G, Kleis W, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17(2):184–90. [PubMed] [Google Scholar]
- 3.Lorente A, Ortega R, Martín M, et al. Quantification of growth factors by using a new system for obtaining platelet-rich plasma. Med Oral Patol Oral Cir Bucal. 2011;16(4):e614–18. doi: 10.4317/medoral.16.e614. [DOI] [PubMed] [Google Scholar]
- 4.Weibrich G, Kleis W, Hafner G. Growth factor levels in platelet-rich plasma and correlations with donor age, sex and platelet count. J Craniomaxillofac Surg. 2002;30(2):97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 5.Harguindey S. [Apoptosis and anti-apoptosis in cancer, Alzheimer’s disease and neurodegenerative processes: a dialectic of opposites? New range of therapeutic possibilities and potential hazards] Oncologia. 2004;27(10):579–89. [in Spanish] [Google Scholar]
- 6.Brea D, Sobrino T, Ramos P, Castillo J. Reorganización de la vascularización cerebral tras la isquemia. [Reorganization of brain vascularization after ischemia.] Rev Neurol. 2009;49(12):645–54. [in Spanish] [PubMed] [Google Scholar]
- 7.Taudorf K, Hansen FJ, Mechior JC, Pedersen H. Spontaneous remission of cerebral palsy. Neuropediatrics. 1986;17(1):19–22. doi: 10.1055/s-2008-1052493. [DOI] [PubMed] [Google Scholar]
- 8.Legido A, Valencia I, Katsetos C, Papadopoulos M. [Neuroprotection in ischemic perinatal hypoxic encephalopathy. Treatments with demonstrated clinical efficacy and future prospects] Medicina (Buenos Aires) 2007;67(6/1):543–55. [PubMed] [Google Scholar]
- 9.Devesa J, Alonso B, Casteleiro N, et al. Effects of recombinant growth hormone (GH) replacement and psycomotor and cognitive stimulation in the neurodevelopment of GH-deficient (GHD) children with cerebral palsy: a pilot study. Ther Clin Risk Manag. 2011;7:199–206. doi: 10.2147/TCRM.S21403. [DOI] [PMC free article] [PubMed] [Google Scholar]