Abstract
The Atg8 family protein LC3 is indispensible for autophagy and plays critical roles in multiple steps of the process. Despite this functional significance, the regulation of LC3 activity at the posttranslational level remains poorly understood. In a recent study, we report that the conserved Ste20 kinases STK3 and STK4, the mammalian orthologs of Hippo kinase, are essential for autophagy in diverse organisms, and both can phosphorylate LC3 on amino acid Thr50. STK3/STK4-mediated phosphorylation is critical for fusion of autophagosomes with lysosomes, as well as the ability of cells to clear intracellular bacteria, an established cargo for autophagy. Our discovery of a novel mode of autophagy regulation involving direct phosphorylation of LC3 by STK3/STK4 significantly enhances our molecular understanding of the autophagy process. Moreover, our findings raise the exciting possibility that STK3/STK4's known roles in immunity are exerted through their ability to regulate autophagy via LC3 phosphorylation.
Keywords: autophagy flux, Hippo kinase, LC3, MST1/MST2, Ste20 kinases
Regulation of autophagy proteins by posttranslational modifications, elicited by signaling kinases, has emerged as a powerful means for controlling their response to various cellular inputs. For example, under nutrient-rich conditions, the kinase TOR inhibits autophagy by phosphorylating Atg13, which prevents formation of the autophagy initiating Atg1-Atg13-(Atg17-Atg31-Atg29) complex. Examples such as this one, highlight the fact that most of our understanding of phosphorylation events in autophagy regulation has been restricted to factors that act upstream to trigger the process. However, once autophagy is activated, a complex choreography involving several autophagy proteins is mobilized to ensure formation and completion of the phagophores, recruitment of cargo, as well as fusion of the autophagosome with lysosomes for cargo turnover and recycling. Unlike upstream regulation of the pathway, the roles played by posttranslational modifications in coordinating these downstream core steps remain underexplored.
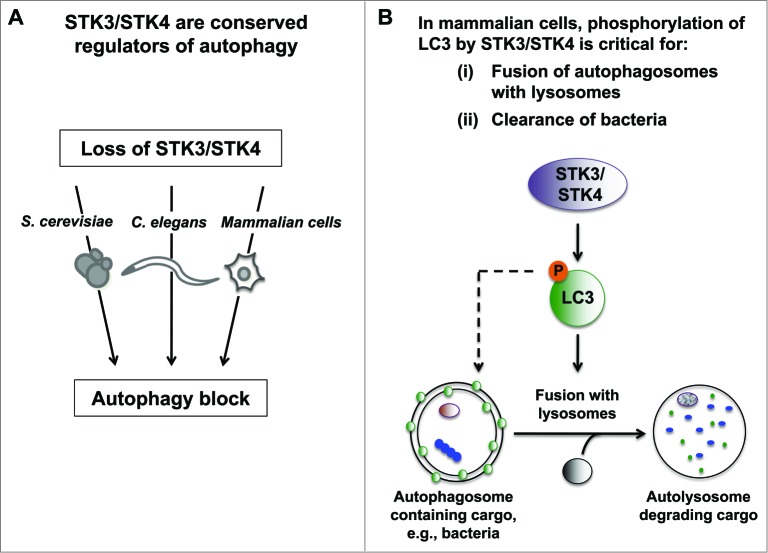
In our recent study, we made inroads toward bridging this gap by showing that the conserved Ste20 kinases STK3/MST2 and STK4/MST1 directly phosphorylate the essential autophagy protein LC3 to mediate fusion of autophagosomes with lysosomes (Fig. 1). Our investigations revealed that 2 mammalian cells types (i.e., mouse embryonic fibroblasts [MEFs] and myoblasts), as well as the model organisms C. elegans and S. cerevisiae lacking the STK3 ortholog or its functionally redundant paralog STK4, display several phenotypes consistent with an autophagy block. Specifically, loss of STK3/STK4 results in a dramatic accumulation of autophagic structures, high levels of the processed LC3-II form as well as the autophagy substrate SQSTM1/p62, and impaired autophagic flux. Intriguingly, these data are consistent with published loss-of-function studies in flies that have suggested a putative role for Hippo (the fly ortholog of Stk3/Stk4) in promoting autophagy, although no mechanism has been ascribed to it.
Figure 1.
Novel role for Hippo kinases STK3/STK4 in regulation of autophagy. (A) Loss of STK3/STK4 kinase orthologs results in an autophagy block in S. cerevisiae, C. elegans and in mammalian cells (i.e., mouse embryo fibroblasts and myoblasts). (B) STK3/STK4 directly phosphorylate LC3B at amino acid Thr50 in mammalian cells. This phosphorylation event plays an important role in mediating fusion of autophagosomes with lysosomes, and is important for mouse embryo fibroblasts to clear intracellular bacteria. Since Thr50 is not conserved in C. elegans and S. cerevisiae, we propose that alternate sites and/or alternate substrates are utilized by STK3/STK4 to regulate autophagy in these organisms.
We hypothesized that the underlying mechanism of STK3/STK4-mediated autophagy regulation likely involved phosphorylation of key autophagy proteins since a recent genome-wide analysis of the human autophagy proteome showed that STK3/STK4 kinases interact strongly with the Atg8 family of autophagy proteins in vitro. In line with this observation, we demonstrated that STK3 and STK4 robustly phosphorylate the Atg8 family member LC3B at residue Thr50. Remarkably, reintroducing a phosphomimetic version of LC3B (hereafter referred to as LC3) into STK3/STK4-deficient MEFs restores autophagic flux, indicating that LC3 Thr50 phosphorylation is functionally relevant in vivo. Furthermore, we ascertained that STK3/STK4-mediated autophagy regulation is physiologically relevant because loss of LC3 Thr50 phosphorylation impairs the ability of MEFs to clear intracellular bacteria via the autophagy pathway. This discovery is especially timely since recent reports have indicated a putative role for STK3/STK4 kinases in immunity, although no mechanism has been established for this link. Our experiments are the first to suggest that STK3/STK4's regulation of the autophagy process via LC3 phosphorylation might be integral to the ability of cells to fight cellular infections.
How does loss of LC3 Thr50 phosphorylation compromise its function? Since LC3 has known roles in autophagosome formation and cargo sequestration, we first tested whether these processes were impaired in Stk3/Stk4-deficient MEFs and found no apparent defects. In contrast, under fully fed conditions there was a large increase in autophagosome numbers in Stk3/Stk4-deficient MEFs, and this number failed to change when exposed to autophagy stimuli, indicating a block in fusion of autophagosomes with lysosomes. In further support of this hypothesis, we directly measured colocalization between LC3 and LAMP1 (a lysosomal marker) and found a significantly impairment of autophagosome-lysosome fusion in Stk3/Stk4-deficient MEFs. Intriguingly, lysosomes appeared markedly clustered around the perinuclear region in Stk3/Stk4-deficient MEFs, a phenotype that has previously been associated with defects in components of the fusion machinery. These data suggest that in addition to cargo sequestration and autophagosome formation, LC3 may play essential roles in mediating fusion of autophagosomes with lysosomes.
Several exciting questions emerge from our studies. First, the exact mechanism by which loss of LC3 phosphorylation results in impaired fusion of autophagosomes with lysosomes is unknown. We hypothesize that critical proteins involved in fusion and/or loading of autophagosomes along microtubules may fail to mobilize in Stk3/Stk4-deficient MEFs. Second, the identity of the upstream signals needed to trigger STK3/STK4's autophagy function needs investigation. STK3/STK4 kinases are best studied as tumor suppressors that respond to cell surface receptors to suppress tumor growth. Whether STK3/STK4 rely on their canonical upstream signaling components or whether noncanonical signals are involved in regulation of autophagy, remains an outstanding question. Third, the potential role for STK3/STK4 kinases in immunity via autophagy needs detailed investigation, which could yield valuable targets for drug development. Finally, there is evidence to suggest a broad function for STK3/STK4 kinases in regulating autophagy, since a recent study demonstrated that STK4 inhibits autophagy in heart tissue in response to stress by phosphorylating BECN1. Our study, conversely, suggests that STK3/STK4 can promote autophagy by using the LC3B isoform as a substrate. Future experiments should address whether other Atg8 family members can also function as STK3/STK4 kinase substrates. In conclusion, we propose that STK3/STK4 kinases are likely to play critical yet complex roles in regulating autophagy by targeting autophagy proteins in a context-specific manner. Future studies will shed light on these exciting questions, while improving our molecular understanding of the autophagy process.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
DSW was supported by a Glenn Foundation for Aging Research fellowship, and MH by NIH/NIA grants AG038664 and AG039756.