Abstract
Autophagosome fusion with a lysosome constitutes the last barrier for autophagic degradation. It is speculated that this fusion process is precisely and tightly regulated. Recent genetic evidence suggests that a set of SNARE proteins, including STX17, SNAP29, and VAMP8, are essential for the fusion between autophagosomes and lysosomes. However, it remains unclear whether these SNAREs are fusion competent and how their fusogenic activity is specifically regulated during autophagy. Using a combination of biochemical, cell biology, and genetic approaches, we demonstrated that fusogenic activity of the autophagic SNARE complex is temporally and spatially controlled by ATG14/Barkor/Atg14L, an essential autophagy-specific regulator of the class III phosphatidylinositol 3-kinase complex (PtdIns3K). ATG14 directly binds to the STX17-SNAP29 binary complex on autophagosomes and promotes STX17-SNAP29-VAMP8-mediated autophagosome fusion with lysosomes. ATG14 homo-oligomerization is required for SNARE binding and fusion promotion, but is dispensable for PtdIns3K stimulation and autophagosome biogenesis. Consequently, ATG14 homo-oligomerization is required for autophagosome fusion with a lysosome, but is dispensable for autophagosome biogenesis. These data support a key role of ATG14 in controlling autophagosome fusion with a lysosome.
Keywords: ATG14, autophagosome, Barkor, homo-oligomerization, lysosome, membrane fusion, membrane tethering, SNARE, syntaxin 17, snap29, vamp8
We provide 2 lines of evidence to demonstrate that the autophagic SNARE complex STX17-SNAP29-VAMP8 is a bona fide fusogen. First, our crystal structure analysis at a high resolution (1.4 Å) revealed that 4 SNARE core domains of autophagic SNAREs, STX17 (1), SNAP29 (2), and VAMP8 (1), form a parallel α helical bundle that includes the conserved ionic layer at the center of the complex, which is characteristic of all fusion-competent SNARE complexes. Second, membrane fusion was shown by content mixing of a self-quenched fluorescence dye in a proteoliposome system, in which STX17 is incorporated into t-vesicles with SNAP29 to mimic autophagosomes, and VAMP8 is incorporated into v-vesicles to mimic lysosomes.
SNARE-mediated fusion is often slow and spontaneous. We anticipate that fusogenic activity of autophagic SNAREs is robustly and precisely regulated, similar to other well-characterized SNAREs. We found that ATG14 is a critical switch to temporally and spatially control autophagic SNARE-mediated membrane fusion. This conclusion is supported by the following evidence; 1) Recombinant ATG14 directly interacts with the STX17-SNAP29 binary complex; 2) ATG14 colocalizes with STX17 on the mature autophagosome. Here, mature autophagosomes are defined as sealed complete autophagosomes and autolysosomes but not phagophores or lysosomes. 3) Recombinant ATG14 robustly promotes the function of STX17-SNAP29-VAMP8-reconstituted proteoliposomes in the lipid- and content- mixing assays. Although both assays evaluate the membrane fusion activity, the lipid mixing reflects an early stage in fusion by measuring mixing of different membrane lipids labeled with distinct dyes. Lipid mixing cannot distinguish hemifusion from complete fusion. Hemifusion and complete fusion differ in fusion pore opening and content exchange. Content mixing measures the complete fusion, since it evaluates the exchange of vesicle contents. Membrane rupture/leakage in content mixing can be distinguished from fusion by the fluorescence pattern (the latter is more sustainable). ATG14 not only promotes lipid mixing and hemifusion (visualized by electron microscopy), but also content mixing of STX17-SNAP29-VAMP8-reconstituted proteoliposomes. Therefore, we conclude that ATG14 controls autophagic SNARE-mediated membrane fusion.
ATG14, as an essential component of the PtdIns3K complex, is required for autophagosome biogenesis. The indispensible role of ATG14 makes it difficult to analyze its function in the late maturation stage of autophagy. The problem is solved by identification of an ATG14 mutant that affects its function only in autophagosome fusion with lysosomes, but not biogenesis. We found that the interaction with the autophagic SNARE STX17 requires ATG14 homo-oligomerization through cysteine repeats. Mutation of 4 cysteines at the N terminus of ATG14 (4C4A) completely abolishes its homo-oligomerization and STX17 binding, but not BECN1 interaction. Consequently, autophagosome fusion with a lysosome, rather than PtdIns3K activity and autophagosome biogenesis, is compromised in complemented ATG14 4C4A cells. We examined autophagosome fusion with a lysosome in the following assay: 1) Autophagy flux evaluated by LC3 and SQSTM1 protein levels; 2) Colocalization of LC3 (autophagosome) with LAMP2 (lysosome); 3) Acidification of mRFP-GFP-LC3; 4) Proteinase protection of GFP-LC3 in sealed complete autophagosomes (modified from yeast studies); 5) Electron microscopy analysis for the presence of double-membrane autophagosomes; 6) In vitro membrane tethering and fusion assay by the recombinant ATG14 4C4A mutant. All these assays strongly support the conclusion that ATG14, in its oligomeric form, is an indispensible part of the fusion machinery governing autophagosome fusion with lysosomes.
There are many unsolved questions to be addressed in future studies. For instance, how does ATG14 promote membrane fusion mediated by autophagic SNAREs? ATG14 might form a clasp to bind to the binary (or the partially folded trans-SNARE complex) on autophagosomes and prime it for binding to VAMP8 on lysosomes. We found that the ATG14 coiled-coil domain (CCD) interacts with the α helix of the STX17 SNARE motif, and this interaction is important for its fusion-promoting activity. This interaction may allow ATG14 to function as a clasp to stabilize the α helical structure formed by STX17 and SNAP29 (Fig. 1). Alternatively, the ATG14 CCD might bundle with the α helices within the SNARE motifs of STX17 and SNAP29 to form an intermediate helical complex, which is ready to bundle with VAMP8 via replacement. The potential benefit of this intermediate helical structure is to avoid loosened or misaligned STX17-SNAP29 helical structures. The detailed mechanism can be revealed by future structural analyses. ATG14 might also facilitate membrane fusion through membrane tethering. We found that recombinant ATG14 functions as a robust member tether of “naked” liposomes, and this membrane tethering activity requires its membrane binding BATS domain and cysteine repeats for homo-oligomerization. However, ATG14 cannot induce membrane fusion of “naked” liposomes by itself. The contribution of membrane tethering and SNARE binding by ATG14 relative to its fusion-promoting activity remains to be determined.
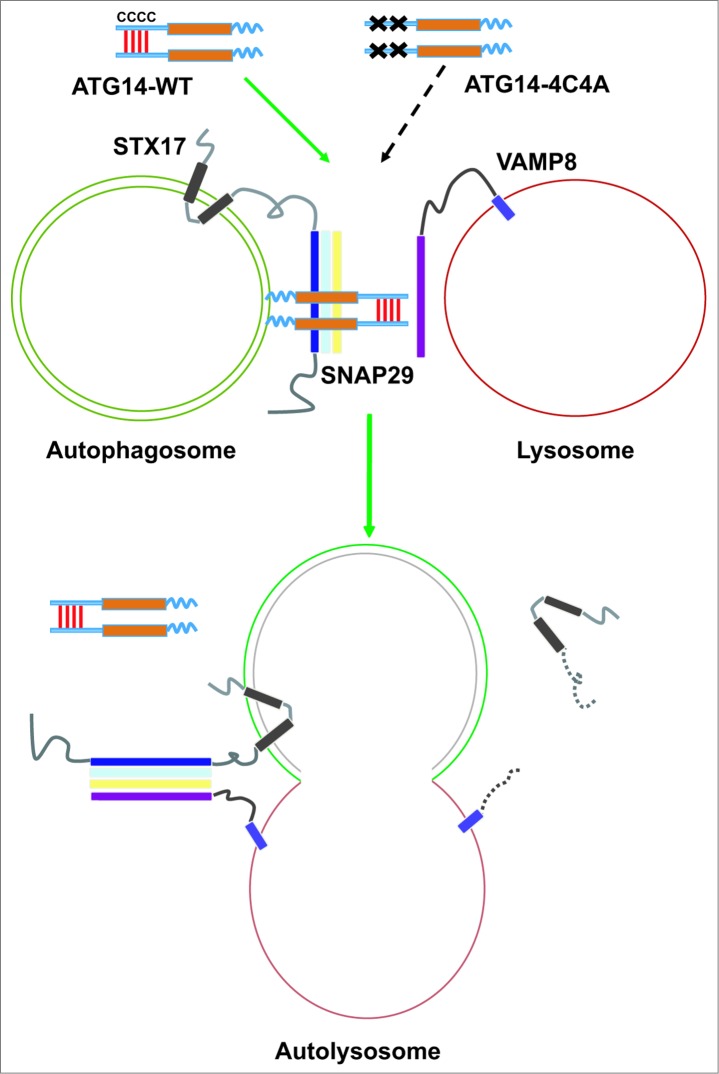
Figure 1.
ATG14 promotes STX17-SNAP29-VAMP8-mediated autophagosome fusion with a lysosome. ATG14 is homo-oligomerized through cysteine repeats. Oligomeric ATG14 localizes to complete autophagosomes and clasps the STX17-SNAP29 binary complex, primes it for binding to VAMP8 on lysosomes, and promotes the fusion between autophagosomes and lysosomes. After fusion, ATG14 dissociates from the cis-SNARE complex and is retrieved from autolysosomes.
ATG14 associates with PtdIns3K through direct interaction with BECN1. STX17 does not co-immunoprecipitate with BECN1 and vice versa, suggesting that STX17 is not in the PtdIns3K complex. ATG14-STX17-SNAP29 might represent a distinct complex separated from the ATG14-BECN1-PIK3C3/VPS34-PIK3R4/VPS15 PtdIns3K complex. ATG14 cysteine repeats have been previously shown to be important for its targeting to the ER membrane. Interestingly, we found that ATG14 4C4A still localizes to ER or ERGIC membrane, while the localization signal is reduced probably due to lack of homo-oligomerization.
It remains unclear how ATG14 is recruited to complete autophagosomes and when it dissociates from autolysosomes. It is suggested that ATG14, along with the other PtdIns3K components, begins to dissociate from autophagosomes upon membrane sealing. A portion of ATG14 might continue to stay on the membrane and function in the fusion process. Alternatively, ATG14 might be recruited anew to complete autophagosomes when fusion starts. The retrieval of ATG14 (and STX17) from fused autolysosomes represents another crucial question, since the recycling of this component could be vital for fusion efficiency.
Funding
The work was supported by grants to QZ from the Welch Foundation (I-1864), CPRIT (RP140320), the American Cancer Society Research Scholar Grant (RSG-11–274–01-CCG) and NIH R01 (CA133228). The work was partially supported by China Scholarship Council to RL.