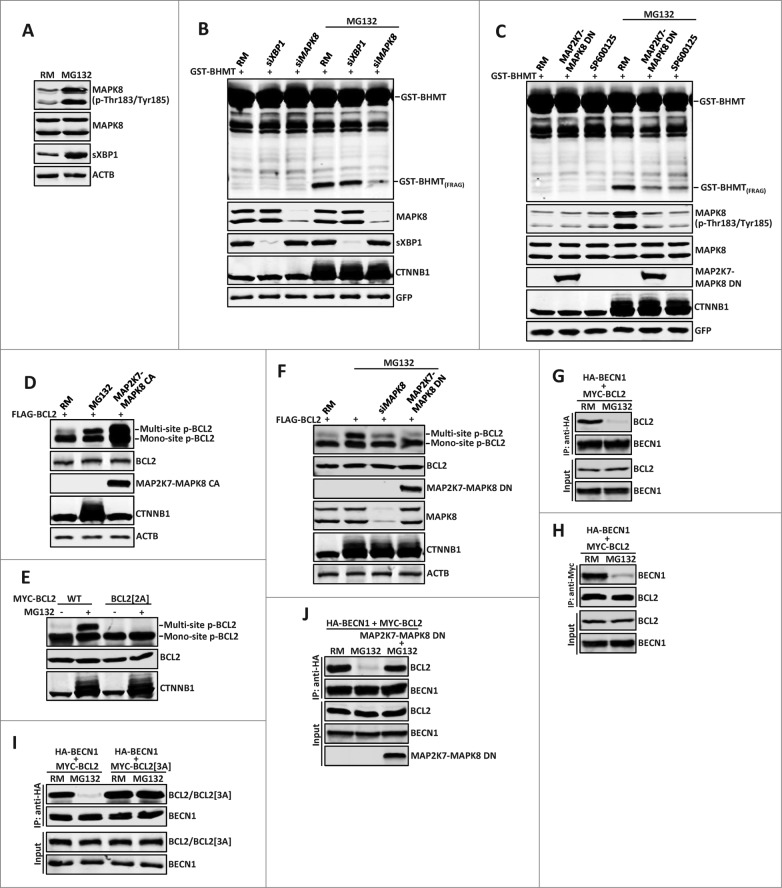
Figure 5 (See previous page).
The MAPK8-BCL2-BECN1 pathway is the main mediator downstream of ERN1 in proteasome inhibition-induced autophagy. (A) MG132 activates main downstream effectors of ERN1 signaling: MAPK8 and XBP1. 10 µM MG132 treatment for 2 h induced strong increase of dual-phosphorylated MAPK8 and spliced XBP1 (sXBP1). (B and C) MAPK8 but not XBP1 is required for MG132-induced autophagy. GST-BHMT assays. HEK293T cells were cotransfected with GST-BHMT reporter (2 µg) and (B) siRNAs against XBP1 (30 nM) or MAPK8 (20 nM), or (C) a dominant-negative MAPK8 (MAP2K7-MAPK8 DN, 2 µg) or were treated with SP600125 for 1 h before MG132 treatment. (D and E) MG132 induces multisite phosphorylation of BCL2. (D) Similar to that catalyzed by the constitutively active MAPK8 (MAP2K7-MAPK8 CA, 2 µg), MG132 significantly induced multisite phosphorylation of wild-type BCL2, (E) but not a mutant BCL2 with Thr69Ala and Ser87Ala substitutions. (F) MPAK8 is responsible for the MG132-induced multisite phosphorylation of BCL2. HEK293T cells were treated with 10 µM MG132 for 2 h. Both siRNA-mediated depletion of MAPK8 and expression of MAP2K7-MAPK8 DN significantly reduced the level of multisite phosphorylation in BCL2. (G and H) MG132 treatment induced dissociation between BCL2 and BECN1. HEK293T cells were cotransfected with 2 µg each of MYC-BCL2 and HA-BECN1. Reciprocal coimmunoprecipitation (coIP) assays were performed with (G) anti-HA or (H) anti-MYC antibodies in the absence or the presence of 10 µM MG132 (2 h incubation), as indicated. Note the dramatically reduced interaction between BCL2 and BECN1 in MG132-treated samples. (I and J) MAPK8-mediated multisite phosphorylation of BCL2 is required for the MG132-induced dissociation of BCL2 and BECN1. HA-BECN1 was cotransfected with wild-type MYC-BCL2 or in parallel with (I) mutant MYC-BCL2[3A] (T69A, S70A, S87A), or (J) a dominant-negative MAPK8 (MAP2K7-MAPK8 DN) into HEK293T cells. (I) Contrary to wild-type BCL2, mutant BCL2[3A] remained associated with BECN1 after MG132 treatment. (J) MAP2K7-MAPK8 DN blocked MG132-induced disassociation of BCL2 from BECN1. The knockdown efficiency against target proteins by different siRNAs were verified by western blotting assays. Proteasome inhibition by MG132 was confirmed by the increased accumulation of CTNNB1.