Abstract
Alzheimer’s disease (AD), the major cause of dementia among the elderly world-wide, manifests in familial and sporadic forms, and the latter variety accounts for the majority of the patients affected by this disease. The etiopathogenesis of sporadic AD is complex and uncertain. The autopsy studies of AD brain have provided limited understanding of the antemortem pathogenesis of the disease. Experimental AD research with transgenic animal or various cell based models has so far failed to explain the complex and varied spectrum of AD dementia. The review, therefore, emphasizes the importance of AD related risk factors, especially those with metabolic implications, identified from various epidemiological studies, in providing clues to the pathogenesis of this complex disorder. Several metabolic risk factors of AD like hypercholesterolemia, hyperhomocysteinemia and type 2 diabetes have been studied extensively both in epidemiology and experimental research, while much less is known about the role of adipokines, pro-inflammatory cytokines and vitamin D in this context. Moreover, the results from many of these studies have shown a degree of variability which has hindered our understanding of the role of AD related risk factors in the disease progression. The review also encompasses the recent recommendations regarding clinical and neuropathological diagnosis of AD and brings out the inherent uncertainty and ambiguity in this area which may have a distinct impact on the outcome of various population-based studies on AD-related risk factors.
Keywords: Metabolic, risk factors, sporadic, Alzheimer’s disease, pathogenesis, treatment
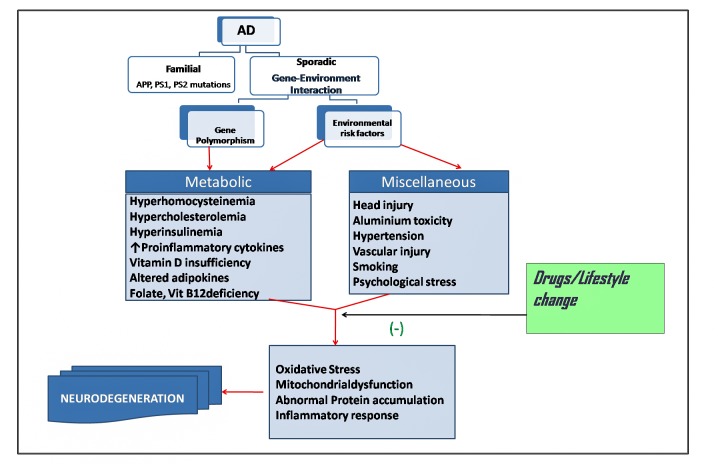
With the general increase in the life span of the population across the globe, Alzheimer’s disease (AD), a progressive neurodegenerative disorder presenting with insidious loss of memory and cognition, is fast becoming a major disease burden and socio-economic challenge for many countries. AD is the commonest form of dementia above the age of 65 years, and the estimated prevalence of this disease was 24 million in the world in 2011 which would be doubled by the year 2030 [1, 2]. The majority (more than 95 %) of the AD dementia cases belong to sporadic variety of the disease which is a multi-factorial disorder in which both genetic predisposition and environmental factors contribute to the genesis of the disease [1, 2, 3]. In contrast, the familial form of the disease (less than 5% of AD population) arises from the mutations of any of the three genes e.g. amyloid precursor protein (APP), presenilin 1 (PS 1) and presenilin 2 (PS 2) and usually appears in somewhat younger age-group than the sporadic form and follows a more aggressive downhill course [2,3,4]. The clinical features and post-mortem neuropathological hallmarks of both forms of AD are nearly identical, and thus it has been tacitly assumed that ante-mortem pathogenesis is the same in either form of the disease. The neuropathological lesions of AD are characterized by abnormal accumulation of amyloid beta protein (Aβ) derived from APP and a microtubule associated protein tau [5]. The experimental research in AD for exploring disease pathogenesis has been dominated by the attempts for identification of various neurotoxic consequences of abnormal protein accumulation on one hand, and on the other by an extensive use of transgenic AD models harboring single or multiple mutant gene(s) of human familial AD [5, 6, 7, 8]. However, this approach so far has neither explained the complexity and heterogeneity of sporadic human AD, nor produced any reasonably efficacious drug for this condition. It is now increasingly understood that in sporadic AD multiple risk factors from gene- environment interactions working in the backdrop of the aging brain activate and reinforce the disease pathogenesis [1, 2, 8]. The molecular nature of gene-environment interaction or its temporal relationship with the pathogenesis of sporadic AD is largely unknown. However, one interesting theory, the Latent Early- life Associated Regulation (LEARn) model, has envisaged that exposure to environmental risk factors (e.g. heavy metal exposure, nutritional deficiency etc.) in early developmental life brings about epigenetic modifications of AD related genes (e.g. Amyloid Precursor Protein or APP gene) that remain latent for many years till a second ‘hit’ (e.g. aging associated elevated pro-inflammatory cytokines, unhealthy mid-life diet etc.) results in sustained alterations in the expressions of these early-affected genes manifesting the full-blown disease [9, 10]. The epigenetic modifications of AD related genes affected by the early life exposure to environmental risk factors may include DNA methylation or oxidation or chromatin re-organization [9, 10]. The novel and attractive LEARn model which advocates the role of environmental risk factors and traces the origin of AD to early developmental stage, however, needs further support from experimental and epidemiological studies. On the other hand, many AD researchers tend to suggest that the environmental risk factors operate during the pre-clinical phase of the disease decades before the appearance of clinical dementia. In either way, there is overwhelming support for environmental or extra-genetic risk factors as inducers of AD pathogenesis. These environmental or extra-genetic risk factors are varied in nature and origin, but for the sake of the present review we may club a few of them as metabolic risk factors and attempt to understand their biological implications in the context of disease pathogenesis and therapeutic strategies (Fig.1). The growing literature of metabolic risk factors of sporadic Alzheimer’s disease (AD) extending from epidemiology to molecular pathogenesis and therapeutic management, however, is replete with controversies and contradictions. Nevertheless, this is an active area of research and one that holds enormous promise in the future development of treatment strategies to combat AD primarily because much of the data are obtained directly from clinical cases. Secondly, the identification of clear metabolic risk factors for AD will give us an opportunity to halt the progress of AD by dietary manipulation or life-style management. The idea of blocking or halting AD pathogenesis by dietary manipulations (e.g. supplementation with folate and vitamin B12) or life-style changes during the early developmental phase or latent phase of the disease has also been espoused by those proposing the LEARn model of sporadic AD [10, 11]. This review will try to present an overview of AD bringing out the dilemmas and uncertainties of clinical diagnosis, neuropathology and molecular pathogenesis before providing an account of predisposing metabolic risk factors of this disease.
Figure 1.
Risk factors of Alzheimer’s disease. Gene -environment interactions may be the underlying mechanism of sporadic AD. Environmental risk factors include those present in external environment or extra-genetic internal milieu of the body. The risk factors may affect the functions of AD-related genes or their regulatory regions by methylation, oxidation or other mechanisms. Gene polymorphisms, on the other hand, may aggravate the effects of the risk factors on AD pathogenesis. Drugs, diet and life-style may prevent the interaction of risk factors with AD pathogenic mechanisms.
Alzheimer’s disease: clinical diagnosis, pathology and molecular pathogenesis
Clinical Diagnosis
The clinical diagnosis of AD requires thorough history and neuropsychological evaluation following the criteria set by NINCDS/ADRDA (Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association) in 1984, which are comparable to those used by other expert bodies e.g. DSM IV (Diagnostic and Statistical Manual of Mental Disorders) [12]. Fundamentally, an insidious onset of progressive dementia with impairment of multiple cognitive domains documented by Mini Mental State Examination or Blessed Dementia Scoring or similar tests usually after 65 years of age is considered as diagnostic of probable AD in the absence of any other dementia producing systemic illness or neurological disease [12, 13]. Various mood or behavioral alterations like depression, agitation, apathy, social withdrawal, insomnia, delusion, emotional or physical outburst are also typical characteristics of AD [12, 13]. The diagnosis can be supported by MRI (magnetic resonance imaging) evidence of diffuse atrophy involving hippocampus and neocortex of the brain. However, the clinical diagnosis is often confused with other forms of dementia like Lewy body dementia, HIV dementia, Huntington’s disease, frontotemporal dementia, dementia due to Parkinson’s disease and hydrocephalus, Cruetzfeldt-Jacob disease and other conditions [12, 14]. Vascular dementia poses a special problem because of overlapping clinical features and etiopathogenesis [15, 16]. Thus, in a significant number of cases of clinically diagnosed probable AD, the final autopsy report does not confirm the presence of AD pathology. Over the last two decades, the understanding of the pathogenesis of AD and other types of dementias at the cellular and molecular level has enormously increased, and coupled with this the PET imaging methods of reduced glucose uptake or increased amyloid beta deposition in AD brain and the identifications of CSF biomarkers of this disease condition have been developed. This has necessitated some modifications of the original diagnostic criteria and introduction of new terminologies and classifications, which could help in identifying the AD patients with greater degrees of certainty. Thus, as recommended by National Institute of Aging - Alzheimer’s Association (NIA - AA) workgroups, AD is a continuum of disease process which includes pre-clinical AD, mild cognitive impairment (MCI) and AD dementia [17, 18, 19]. AD dementia patients would be further categorized as ‘ probable AD’ and ‘possible AD (i.e. AD dementia with an atypical course or with another co-morbidity such as cerebro-vascular disease, Lewy body dementia or any other disease affecting cognition)’ on the basis of history and neuropsychological examination, while the diagnosis of ‘probable AD’ or ‘possible AD’ with AD pathophysiologic process will require CSF biomarker examination (Aβ42, total tau or phosphorylated tau) or PET evidence of decreased 18F-2 deoxyglucose uptake or increased accumulation of amyloid beta peptide in brain [19]. The present recommendations have emphasized that ‘ probable AD’ or ‘ possible AD’ diagnosis is sufficient in clinical settings, while for research purposes a more definitive diagnosis of ‘probable’ or ‘possible’ AD with AD pathophysiologic process is necessary [19]. Similarly, pre-clinical AD is to be used only for research purposes e.g. for selection of cohorts for longitudinal clinical studies [17]. The inherent uncertainty and ambiguity of AD diagnosis as outlined here is to be kept in mind in the context of our current discussion on AD and its multiple risk factors, since the former can confound the results of epidemiological studies significantly.
Neuropathology of AD
The histopathology of AD brain has two hallmarks of extracellular amyloid or senile plaques and neurofibrillary tangles in neuronal perikarya [20, 21, 22]. The amyloid plaques appear in several varieties e.g. neuritic plaques, diffuse amyloid plaques, amyloid lakes, cotton-wool plaques etc. [20]. The neuritic plaques are composed of a core deposit of amyloid beta peptide (predominantly Aβ42 and Aβ40, but also several other amyloid peptides of different chain lengths) in different degrees of oligomerization state surrounded by dystrophic neurites [20, 22]. The neurofibrillary tangles, on the other hand, are composed of paired helical filaments of hyperphosphorylated tau proteins detected by histochemical stains or immuno-histochemistry [20, 22]. The distributions of these typical AD lesions are approximately in the same regions and involve enterorhinal cortex, hippocampus, amygdala and different areas of neocortex [20, 21, 22]. There are other accompaying features of AD pathology like neuronal loss, degeneration of synapses, dendrites and axons, gliosis, white matter rarefaction, ganulovacuolar degeneration and cerebral amyloid angiopathy [20, 22]. Further, apart from amyloid beta peptides or abnormal tau protein, several other proteins also accumulate in various degrees in AD brain like α- synuclein, TDP-43 (TAR DNA binding protein), actin giving rise to various forms of inclusion bodies like Lewy bodies, Hirano bodies etc. [20, 22]. Since the post-mortem confirmation of AD diagnosis rests on autopsy findings, attempts have been made over the years for systematic and well standardized criteria, qualitative and quantitative, for identifying the neuropathology of AD brain [23, 24, 25]. The NIA-REGAN (National Institute of Aging / Regan Institute of the Alzheimer Association) criteria set in 1997 have been revised recently by NIA - AA workgroups and provided guidelines for autopsy reporting of AD neuropathology irrespective of clinical presentations of the subjects [20, 25]. The present recommendations have outlined the ABC scoring for describing AD neuropathology: A for amyloid deposit, B for Braak staging of neurofibrillary tangles and C for neuritic plaques as per CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) criteria. By this ABC scoring, AD neuropathlogy can be graded as ‘No’, ‘Low’, ‘Intermediate’ and ‘High’, and further NIA - AA suggestions include pathological description of all comorbidities e.g. vascular brain injury, hippocampal sclerosis etc. [20, 22].
Clinical AD vs. AD Neuropathology: Ambiguity and Controversy
It is to be emphasized that the impairment of cognitive domains on which the clinical diagnosis of AD is based is in most cases but not always correlate well with the extent of post-mortem AD neuropathology [26, 27]. In some cases significant AD neuropathology has been observed after autopsy in aged individuals who were non-demented before death which has been explained by the assumption that neuropathology of AD predates the clinical cognitive deficits by many years [17, 20]. In other cases cognitive decline is much higher in comparison to existing AD neuropathology, which implies that other co-morbidities like vascular brain injury or Lewy body disease etc. may have contributed to the dementia in such instances [20, 26]. Alternatively, such discrepancies may mean different degrees of ‘brain reserve’ or ‘cognitive reserve’ among the affected individuals [17]. Moreover, this discrepancy between the extent of neuropathology and clinical severity of the disease in many cases may also mean that AD pathology which causes dementia and cognitive impairment is not driven solely by the toxic effects of amyloid plaques or neurofibrillary tangles, but other cellular and molecular damage mechanisms such as oxidative damage or mitochondrial dysfunction or other metabolic alterations may be playing more crucial roles in the genesis of this disease [5, 20]. It remains uncertain at this moment whether histopathologic diagnosis of AD should take in to account the features of molecular damage e.g. accumulation of oxidative damage markers, structural and functional alterations in mitochondria, presence of proinflammatory markers etc. in post-mortem AD brain to obtain a better correlation of neuropathology and cognitive decline. The incongruity between neuropathology and clinical severity of AD may also explain, at least partially, the discrepancy often observed between epidemiological studies and autopsy findings with regard to the association of a particular risk factor with AD.
Pathogenesis of Alzheimer’s Disease
The central mechanisms in the pathogenesis of AD have generally revolved round two themes clearly linked to two hallmark lesions found in brain after autopsy in this disease condition. The first and foremost is the abnormal accumulation of oligomeric Aβ42 or related peptides and their multiple toxic effects on neurons and glia leading to oxidative damage, mitochondrial dysfunction, calcium dysregulation, inflammation and ER stress, which together may set in programmed death of neurons [5, 28]. This is the so called ‘Amyloid Cascade Hypothesis’. The formation of amyloid beta peptide (predominantly Aβ42) from APP through sequential actions of β and γ secretases in the amyloidogenic pathway, the intracellular trafficking of APP-Aβ from endoplasmic reticulum through protein secretory pathway to plasma membrane and outside, the degradation of Aβ42 by neprilysin, insulin degrading enzyme (IDE), endothelin converting enzyme, plasmin etc. and the clearance of Aβ42 from the brain have been the subject of several excellent reviews in the context of AD pathogenesis [28, 29, 30]. It is conceivable that the increased accumulation of amyloid beta peptides in sporadic AD results from increased expression of APP gene and / or increased processing of the APP gene product in the amyloidogenic pathway by means of β and γ secretases and / or decreased catabolism and clearance of the peptides from the brain. In case of familial AD, the mutations in APP, PS1 or PS2 gene may lead to similar effects with accumulation of amyloid beta peptides in the brain, and the ‘Amyloid Cascade Hypothesis’ as the pathogenic mechanism of AD has gained huge support from identification of these familial AD mutation [29, 31]. The other suggested mechanism in AD pathogenesis emphasizes the neuronal and synaptic dysfunctions caused by the abnormal accumulation of phosphorylated tau protein (tauopathy) [32, 33]. Tau protein acts as a substrate for phosphorylation at multiple sites by several different kinases e.g.GSK-3β, Cdk5, PKA, MARK etc. in vitro, but it is not clear which of these kinases are responsible for in vivo phosphorylation of tau in physiological or pathological conditions [33]. Under normal physiological conditions the reversible and transient phosphorylation of tau is involved in many neuronal functions such as axonal transport and neurite outgrowth, but pathological hyperphosphorylation of tau leads to oligomerization and fibrillization and decreased binding to microtubules [32, 33]. Despite a plethora of evidence from animal models (transgenic and chemical induced), cell based models and post-mortem brain of AD subjects supporting the ‘Amyloid Cascade Hypothesis’ or ‘tauopathy’, it is not fundamentally clear how these alterations of abnormal protein accumulation and aggregation are triggered in the aging brain especially in the case of sporadic AD. The LEARn model mentioned earlier posits that environmental risk factor like heavy metal exposure or nutritional deficiency can bring about epigenetic modifications in the regulatory regions of APP or β-secretase gene to cause increased production of amyloid beta peptide [9, 10]. In general, it may be surmised that the risk factors tend to affect the expression, processing and trafficking of APP and amyloid beta peptide or change the phosphorylation state of tau proteins through multiple kinases and phosphatases. Furthermore, extensive post-mortem evidence of accumulation of oxidative damage markers of phospholipids (malondialdehyde, 4-hydroxynonenal (4-HNE), F2 isoprostane etc.), protein (protein carbonyls, HNE-protein adducts) and nucleic acids in AD brain is available [34, 35, 36]. Likewise evidence of mitochondrial dysfunction and intramitochondrial accumulation of amyloid beta peptide in AD brain has been clearly documented in postmortem AD brain [37, 38]. The inflammatory response by activated microglia and also astrocytes leading to production of cytokines, chemokines and reactive oxygen species (ROS) with associated neuronal damage is another important feature of AD pathogenesis [39, 40]. A growing body of recent evidence has indicated the activation of several types of inflammosomes in microglia, and also possibly in neurons, that drives the inflammatory response and neurodegeneration in AD brain [41, 42]. It is a moot point whether all these alterations are simply the secondary consequences of abnormal accumulation of amyloid beta peptide or hyperphosphorylated tau in AD brain or they represent independent damage mechanisms working in concert in the backdrop of an aged brain to bring about neurodegeneration as well as characteristic AD proteinopathy.
Metabolic and Endocrine Components in AD pathogenesis:
One of the earliest manifestations of AD is the decreased cerebral utilization of glucose as evidenced by PET imaging of 18F-2 deoxyglucose uptake in the AD brain, and the phenomenon is progressive with the disease and well correlated with the degree of cognitive decline in AD brain [43, 44]. The multiple reasons for cerebral glucose hypometabolism in AD, its relationship with oxidative stress and the use of alternative substrates under such condition have been well reviewed [43, 44]. The metabolic implications of AD pathogenesis in the context of brain insulin deficiency and insulin resistance have been extensively discussed in several recent publications [45, 46]. Although multiple causes have been attributed to altered food intake and loss of body weight in AD, the fact also implies a role of hormones like insulin and adipokines in its pathogenesis [48, 49, 50]. The mitochondrial dysfunction and inhibition of key metabolic enzymes involved in energy production like the glycolytic enzymes as also pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, cytochrome oxidase are established features of AD pathology indicating general metabolic perturbation in brain in this condition [50, 51]. The transcriptome data from neurodegenerative and neurological diseases incorporated in brain-specific metabolic network models have indicated alterations in several key metabolites and pathways in AD brain that are related to energy, lipid and ROS metabolism [52]. The role of glucose and lactate metabolism in AD brain has been recently analyzed from the proposed alternative view of brain metabolism based on astrocyte-neuron-lactate-shuttle [53]. Metabolic syndrome, a condition characterized by multiple biochemical and physiological alterations like hyperglycemia, dyslipidemia, hypertension, obesity etc., has been linked with the genesis of sporadic AD [54]. Thus, there is substantial evidence supporting a strong metabolic component in AD pathogenesis which calls for a systematic discussion of metabolic risk factors of sporadic AD.
Metabolic Risk Factors of AD: Present Status
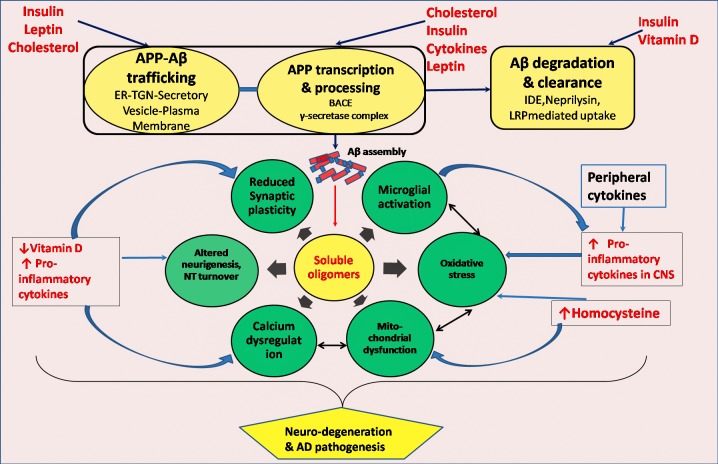
Although in the context of the present discussion, we are primarily concerned with the metabolic risk factors of sporadic AD, it is to be understood that gene polymorphisms associated with sporadic AD may modulate the role of these risk factors in multiple ways in the disease pathogenesis. The polymorphism of APOE is considered as a definite risk factor for AD with ɛ4 allele (APOE4) showing strong association with the disease, but the estimated risk attributable to APOE4 varies in different populations depending on the frequency of this allele in a particular population [55]. The protein coded by APOE is apolipoprotein E which is involved in cholesterol transport in the periphery as well as cholesterol trafficking within the brain, and APOE genotype and serum total cholesterol interaction has been suggested in AD progression [56]. Recent large genome-wide association studies have identified several other genetic susceptibility loci for AD which include CR 1, SORL 1, PICALM, CLU etc. [57]. Apart from the gene polymorphisms, population based long-term prospective as well as cross-sectional retrospective studies have identified many risk factors of sporadic AD, and some of these like hypercholesterolemia, type 2 diabetes, hyperhomocysteinemia, elevated circulating proinflammatory cytokines, vitamin D deficiency and altered blood levels of adipokines have obvious metabolic connotation. The possible links of these metabolic risk factors with disease pathogenesis are presented in Fig.2.
Figure 1.
Metabolic risk factors and molecular pathogenesis of AD. AD pathogenesis is represented by interacting damage pathways spearheaded by soluble oligomers of amyloid beta peptide. Altered levels of metabolic risk factors e.g. hormones, vitamins, cytokines, metabolites etc. can affect APP expression and processing or intracellular trafficking, catabolism and clearance of amyloid beta peptide or induce oxidative stress or inflammatory response through interactions at multiple sites leading to neurodegeneration and characteristic proteinopathy of AD. ER-TGN, Endoplasmic reticulum trans- golgi network; BACE, Beta-site APP cleaving enzyme; IDE, Insulin degrading enzyme; LRP, Low density lipoprotein receptor related protein; NT, Neurotransmitter.
Hypercholesterolemia:
Large-scale population-based prospective studies have indicated that mid-life hypercholesterolemia is an independent risk factor for AD even when corrected for age and other confounding factors [58, 59]. Several other prospective studies have also reported that hypercholestolemia is a risk factor for AD even after adjustment of APOE4 attributed risk [60]. One population based study of African Americans has shown that higher serum cholesterol is associated with increased risk of AD in APOE4 negative subjects, but not in those carrying at least one ɛ4 allele [61]. Many case - control studies have also confirmed that mild hypercholesterolemia accompanies late-onset AD [49, 62, 63]. However, a 32-year long follow-up study of 1462 women has failed to find an association of mid-life hypercholesterolemia with increasing risk of AD, and even some studies have indicated that a high blood cholesterol especially in late-life is actually protective against AD [64, 65, 66]. The statin group of cholesterol lowering agents has been shown to produce beneficial effects in AD subjects in some studies [67]. A recent careful analysis of existing literature has shown that the association of hypercholesterolemia with AD is quite compelling, but the beneficial effect of statin therapy is not firmly established [68, 69].
In experimental cell based research, cholesterol distribution within membrane is seen to have effects on APP metabolism, trafficking of APP, activities of β, γ and α secretases and Aβ synthesis [70, 71]. In transgenic AD mice high cholesterol diet promotes brain amyloidogenesis [72]. The human brain contains a high amount of cholesterol, mostly in the unesterified form, in two pools of which the major one is in myelin and the minor pool in the plasma membranes of glia and neurons [73, 74]. Since very little cholesterol is transported from the plasma via lipoproteins through the blood-brain barrier, the brain cholesterol is maintained by in situ synthesis and recycling with a small amount moving out from the CSF to the plasma [73, 74]. The recycling and trafficking of cholesterol in the brain requires apoE, low-density lipoprotein receptor related protein (LRP) and VLDL receptor, and the details of this complex pathway have been worked out [71, 75]. In the cell membrane cholesterol is associated with special lipid microdomains known as lipid rafts, and because amyloidogenic processing of APP and oligomerization of Aβ peptide take place within lipid rafts, cholesterol could modulate the latter phenomena [75]. What is not clearly understandable is how hypercholesterolemia can be an important risk factor for AD when circulating cholesterol containing lipoproteins do not gain entry in to brain in significant amount. However, high serum total cholesterol is an important vascular risk factor and vascular brain injury may confound epidemiological data linking hypercholesterolemia and AD. Alternatively, vascular brain injury and hypoperfusion of brain can initiate and promote brain amyloid pathology as has been suggested earlier [16]. APOE polymorphism is considered as a strong risk factor for sporadic AD in subjects carrying ɛ4 allele, and it has been shown in experiments with cultured neurons that apoE4 isoform is less efficient compared to apoE2 or apoE3 in carrying out efflux of cholesterol from the neurons [76]. The latter effect may promote neuronal production of amyloid β peptide. The cholesterol/ ApoE isoform interaction in the progression of AD pathogenesis has also been observed in population based study [77]. Another interesting connection with cholesterol and AD is through the oxidation product of cholesterol, 24-hydroxyhydroxy cholesterol, which is formed exclusively in the brain [73, 78, 79]. This oxysterol can pass through the blood brain barrier and constitutes an efflux pathway of cholesterol from the brain, and thus the plasma level of 24-hydroxycholesterol is an indicator of brain cholesterol turnover [73, 79]. In AD, the plasma level of 24 - hydroxycholesterol is increased presumably as a result of neuronal death and membrane cholesterol turnover [78, 79].
Type 2 Diabetes:
A population based cohort study has estimated the Relative Risk (RR) of 2.27 for men and 1.37 for women relating AD with type 2 diabetes [80]. Large- scale population based prospective studies have shown that type 2 diabetes increases the risk of late-onset AD, and different studies have calculated the RR varying from 1.44 to 1.9 [81, 82, 83, 84]. Another population based follow up study showed that hyperinsulinemia or type 2 diabetes increases the risk of of AD with a hazard ratio of 2.2 [85]. A mean follow-up study of 5.5 years for a population of 1138 persons has shown that diabetes is a strong risk factor for AD (hazard ratio 2.4) and the risk is further augmented when other risk factors like hypertension, heart disease and smoking are simultaneously present [86]. Several meta-analysis studies from published literature have estimated an aggregated relative risk of approximately 1.5 linking type 2 diabetes with AD, and the relative risk increases considerably by the presence of hypertension, smoking and APOE4 positivity [87, 88, 89, 90]. A population based cross-sectional study has also shown the significant association of hyperglycemia and hyperinsulinemia with AD independent of APOE4 status [91]. However, many epidemiological studies have linked type 2 diabetes more with vascular dementia than AD, and cognitive decline as a consequence of type 2 diabetes per se is also well documented [92, 93, 94]. On the other hand, there is a clear discrepancy between epidemiological results and neuropathological autopsy findings in linking AD with type 2 diabetes. Neuropathological autopsy studies do not support that type 2 diabetes predisposes to development of AD pathology in the brain of aged persons when compared to age-matched controls [95]. Several epidemiological studies also have examined a proportion of their study cohorts at autopsy for neuropathological changes typical of AD and concluded that type 2 diabetes does not promote AD pathology in the brain and instead vascular pathologies in brain are more commonly associated with type 2 diabetes [89, 96].
While the controversy is persisting between epidemiological findings and neuropathological observations in relating type 2 with AD, in experimental AD research insulin and insulin resistance have been linked to AD pathogenesis in multiple ways. In the brain insulin and IGF signaling regulates a broad range of functions such as glucose and energy metabolism, growth, differentiation, migration, survival and plasticity of neurons [97, 98]. In particular in the context of amyloid beta metabolism, insulin stimulates APP-Aβ trafficking from trans-golgi network to plasma membrane and the extra-cellular release of Aβ, and insulin resistance in mice induced by high fat diet causes increased activities of β and γ secretases with accumulation of amyloid beta peptide [45, 99, 100]. Likewise in db/db mice brain or in insulin resistant cultured cells of neural origin, an accumulation of autophagosomes and amyloid beta peptide has been noticed [100]. In addition, insulin competes with Aβ for degradation by IDE (insulin-degrading enzyme) and thus may promote Aβ accumulation in the brain in hyperinsulinemic condition, although the evidence suggests that the peripheral hyperinsulinemia of type 2 diabetes actually causes a decreased entry of insulin into brain [101]. The role of insulin in tau phosphorylation has also been explored in different experimental models, and it is suggested that insulin resistance through impaired PI3/Akt signaling may lead to activation of GSK-3β and increased phosphorylation of tau [101, 102].
It has been envisaged that Alzheimer’s disease may be a type of ‘brain diabetes’ or ‘type 3 diabetes’ resulting from both insulin deficiency and insulin resistance in the brain [46, 84, 101]. This is supported by a body of evidence such as the decreased level of insulin, insulin mRNA and insulin receptor protein in brain, de-sensitization of insulin receptor and decreased levels and impaired functioning of downstream components of insulin signaling pathway in AD brain [46, 103, 104, 105, 106, 107]. Although the reasons for brain insulin deficiency or resistance in AD are not clear, it is worthwhile to state in this context that Aβ oligomers which accumulate in AD brain as a primary pathologic process can bind to brain insulin receptors and induce the re-distribution of the receptors from the membrane to the cytosol leading to impaired insulin signaling [98]. Likewise, Aβ oligomers can abnormally activate TNF-α/JNK pathway causing phosphorylation of serine residues of IRS-1 and blocking insulin signaling downstream [98]. Whatever may be the mechanism, insulin deficiency and resistance of ‘type 3’ diabetes can directly contribute to the genesis of AD pathology in the brain as a primary process by enhancing Aβ accumulation and tau phosphorylation. In conformity with this idea of brain insulin deficiency and resistance in AD, intracerebro-ventricular administration of streptozotocin in mice causes AD like neuropathology and cognitive deficits [46, 108]. However, this proposition of AD per se as a case of ‘brain diabetes’, though attractive, needs further scrutiny, and rather it may be more logical to think that insulin deficiency and resistance is conferred on AD brain secondarily by coexisting peripheral insulin resistance diseases like type 2 diabetes. In turn, the brain insulin resistance aggravates the AD pathologic process presumably by altering APP expression, processing, trafficking and degradation and / or phosphorylation of tau or by causing metabolic perturbations, oxidative stress and mitochondrial dysfunctions through inter-dependent pathways. The possibility that co-existing type 2 diabetes accelerates AD pathogenesis is not only supported by epidemiological evidence, but also by studies in transgenic Tg2576 AD mice in which dietary high fat induced insulin resistance promotes Aβ peptide generation and amyloid plaque formation in the brain [109]. Another recent study has shown that cross-mating of ob/ob or NSY diabetic mice models with APP transgenic mice results in double transgenic animals in which both AD and type 2 diabetes pathologies are aggravated [110]. Although acute infusion of insulin in peripheral circulation tends to raise CSF insulin, in type 2 diabetes, chronic hyperglycemia and hyperinsulinemia lead to decreased entry of insulin in to brain resulting in lower CSF and brain insulin levels and a state of brain insulin deficiency [101, 111, 112]. Thus both insulin deficiency and insulin resistance would be present in AD brain as a result of co-existing type 2 diabetes. However, the molecular defect that generates insulin resistance in type 2 diabetes is neither precisely known, nor it is known whether the mechanism of insulin resistance is the same for all tissues like skeletal muscles, adipose tissue or liver. Thus, it will be an interesting area of exploration to elucidate the mechanism of brain insulin resistance in type 2 diabetes and its consequence on the co-existing AD pathology. A corollary of this complex interaction of type 2 diabetes and AD is the possibility of a beneficial effect of insulin or insulin-sensitizers in blocking the genesis and progression of sporadic AD, but the results are still not unequivocal in this respect [103, 113, 114].
Hyperhomocysteinemia
Hyperhomocysteinemia has been well recognized as a cardiovascular risk factor from epidemiological and experimental studies [115]. The causes of hyperhomocysteinemia in general population are multiple and include both genetic and non-genetic mechanisms. The mutations of genes coding for major enzymes involved in homocysteine remethylation or transulfuration pathways e.g. cystathionine β synthase, methionine synthase, methylene tetrahydrofolate reductase etc. can lead to hyperhomocysteinemia [116]. The deficiency of vitamin cofactors of homocysteine metabolism like vitamin B12, folate and pyridoxine may also be responsible for hyperhomocysteinemia in general population [116].
Many case-control as well as prospective studies have associated high plasma homocysteine with AD which has been well reviewed in a recent publication [117]. Several large -scale long follow-up studies have demonstrated an increased risk of AD with high plasma total homocysteine after adjustment of other variables [118, 119]. In the Framingham Offspring Study, 2096 participants have been examined seven times for multiple parameters for 30 years followed by a battery of neuropsychological tests, and an inverse association of plasma total homocysteine with multiple cognitive domains has been noted in subjects above 60 years or above [120]. A recent meta-analysis of eight cohort studies has also confirmed the association of hyperhomocysteinemia with increased risk of AD with an odds ratio of 1.35 for every 5 µmol/L increase in plasma homocysteine [121]. Many epidemiological studies have simultaneously measured plasma total homocysteine, folate, vitamin B12, vitamin B6 in cohorts in relation to AD risk and established that hyperhomocysteinemia is associated with deficiency of these vitamins [118, 122, 123, 124, 125]. There has been a general optimism that corrections of hyperhomocysteinemia by supplementation with folate, vitaminB12 and vitamin B6 would be beneficial in preventing or halting AD progression [126]. However, several interventional epidemiological studies have shown that while hyperhomocysteinemia can be reversed by supplementation with vitamin B12, folate, pyridoxine etc., the dementia is not improved [127, 128]. Since homocysteine is a known vascular risk factor, it is likely that hyperhomocysteinemia probably contributes to vascular component of AD pathology. Several case-control studies have shown that the degree of hyperhomocysteinemia is more in typical vascular dementia than in AD [129, 130]. Nevertheless, many experimental studies have shown that homocysteine in higher than physiological concentrations can induce a wide range of neurotoxic effects involving oxidative stress, excitotoxicity, mitochondrial dysfunction, DNA damage and apoptosis implying that hyper-homocysteinemia may directly contribute to AD neurodegeneration [117, 131, 132, 133].
Adipokines
The fact that AD is accompanied by significant metabolic changes in the brain and altered food intake and body weight, some significant studies, epidemiological and experimental, have been made on the role of adipokines like leptin and adiponectin in AD. Adiponectin regulates glucose, lipid and energy metabolism and insulin sensitivity in many tissues through AdipoR1 and AdipoR2 receptors using complex signaling mechanisms involving AMPK, p3-MAPK, PPAR-α and NF-kβ [134, 135, 136]. The role of adiponectin in regulating brain metabolism and function is less established, but the globular, trimeric and hexameric forms of the hormone are believed to cross the blood-brain barrier and act on receptors present in different brain areas [137]. Under physiological conditions, adiponectin may be responsible for regulation of energy metabolism of the brain and control of food intake [137]. The other important adipokine, leptin, acts through Ob-Rb (the long form of the receptor) utilizing an array of downstream signaling mechanisms e.g. Jak-Stat, Erk, PI3K/Akt and AMPK [138, 139] Leptin is known to affect satiety, food intake and body weight through its action in hypothalamus, but the hormone has more diverse neurotrophic and neuroprotective actions in the brain and also affects hippocampal neurigenesis and long-term potentiation [138, 139, 140]. Several case-control and large population based prospective studies have shown an inverse association of circulating leptin levels with AD or general cognitive decline [141, 142, 143]. In case of adiponectin the epidemiological link with AD risk is less clear. Some case-control and large follow-up studies have shown an association of higher circulating level of adiponectin with increased risk of AD, but others have not replicated these findings [144, 145, 146]. A recent case-control study has measured the serum levels of adiponectin and leptin in the same cohorts of AD and confirmed a raised level of adiponectin and a lower leptin level in serum of AD subjects which are in good correlation with the severity of the disease as measured by MMSE scores [49]. Extensive experimental studies in cell lines and AD transgenic mice have indicated that leptin can affect APP-Aβ trafficking, Aβ accumulation and clearance, βsecretase expression as also phosphorylation of tau protein [139, 140, 147]. Adiponectin on the other hand may have a proinflammatory role in AD brain [137].
Proinflammatory cytokines
The AD brain elicits a strong inflammatory response primarily by activated microglia and to a lesser extent by astrocytes. The microglia during inflammatory response show typical morphological changes with expression of specific surface markers and release of proinflammatory interleukins, interferons, chemokines and reactive oxygen species [39, 40, 148, 149]. On the other hand, as part of this inflammatory response, an alternative activation of microglia and acquired deactivation take place which helps in repair, resolution and neuroprotection [149]. In AD brain the activation of the microglia is triggered by oligomeric amyloid beta peptide and amyloid fibrils and the resulting pro- inflammatory response mediated by IL-1, IL-6 and TNF-α, complement system, chemokines etc. plays an important role in neurodegeneration of AD, while anti-inflammatory cytokines IL-4, IL-10 and TGF-β also liberated from the microglia may assist in neuroprotection [40, 148, 150, 151, 152]. The role of pro-inflammatory cytokines in AD pathogenesis has recently gained some intense attention because of the evidence suggesting the activation of the inflammosome, NLRP3, in the brain of transgenic AD mice and patients of mild cognitive impairment and AD dementia [153, 154]. The inflammosomes, the intracellular multi-protein complexes consisting of nucleotide binding oligomerization domain (NOD)- like receptor proteins (NLRP), adaptor proteins and downstream effector proteins, become activated by a wide array of pathogen and danger related molecular patterns through NLRP [42, 154]. There are several types of inflammosomes, but the microglial NLRP3 which is coupled to ASC (apoptosis associated spec-like protein containing caspase recruitment domain) and caspase 1 probably plays a key role in AD pathogenesis [42, 154]. The phagocytosed fibrillar amyloid beta peptide within microglia activates NLRP3 through lysosomal degradation leading to activation of caspase 1 that induces maturation and secretion of pro-inflammatory cytokines IL-1β and IL-18 [41, 154]. However, independent of the brain immune response, raised levels of circulating proinflammatory cytokines have been generally reported in AD subjects in both longitudinal as well as case-control studies, though significant variability has also been noted [155, 156, 157, 158]. A systematic meta-analysis of published reports has clearly shown that raised serum levels of IL-6, TNF-α and IL-1 are associated with increased risk of AD [159]. Some studies have measured serum and CSF levels of cytokines simultaneously in AD subjects, but generally serum level is not reflective of that of CSF [159]. A recent case-control study of a relatively small sample size has suggested that raised levels of proinflammatory cytokines like IL-6, IL-1β and TNF-α are present only in AD patients with associated depression [160]. The communication pathways between the peripheral immune system and the CNS have been identified in details which include both humoral and neural mechanisms, and through this the raised levels of peripheral proinflammatory cytokines can increase the CNS production of cytokines which have varied actions in CNS through widely distributed receptors [161, 162]. The cytokines have neuromodulatory and neurotrophic actions and can also affect neurigenesis, synaptic plasticity, synaptic scaling, neurotrasmitter turnover and the activity of hypothalamus-pituitary axis [162, 163]. Thus, the activation of peripheral immune system during systemic infection or after systemic injection of lipopolysaccharides or endotoxins or interleukins can cause behavioral and cognitive alterations in animals and human beings [164, 165, 166]. Additionally, this mechanism may be causal to many psychiatric and neurological diseases [148, 167, 168]. In the context of AD pathogenesis, the central pro-inflammatory cytokines have been shown to enhance the phosphorylation of tau or production of amyloid beta peptide and potentiate the neurodegeneration process [148, 169, 170].
Vitamin D
Vitamin D3 synthesized from 7-dehydrocholesterol in the epidermis by photochemical reaction is sequentially hydroxylated in liver and kidney by cytochrome P450 dependent enzymes to produce 1,25 dihydroxy vitamin D3, the active form of vitamin D3, which acts as a hormone producing diverse effects in tissues apart from its classical role in Ca2+ and phosphate metabolism [171]. The serum level of vitamin D3 is typically monitored by measuring 25-hydroxy vitamin D3. Vitamin D has both genomic and non-genomic actions mediated by nuclear and membrane receptors respectively, and the receptor VDR is identical in both the locations [171, 172]. The nuclear VDR heterodimerizes with retinoid X receptor and in concert with several coactivators regulates the transcription of a wide variety of genes in different tissues [171, 172]. In CNS development, vitamin D helps in neuronal differentiation and axonal growth, and in the adult brain it regulates the synthesis of neurotrophic factors and neurotransmitters and also exhibits neuroprotective and antioxidative functions [173, 174, 175].
The association of VDR polymorphism with increased risk of AD has been shown in several studies. The polymorphism in intron 8 recognized by ApaI, but not one recognized by TaqI in exon 9, is associated with increased risk of AD [176]. Another promoter SNP (single-nucleotide polymorphism) corresponding to the transcription factor Cdx-2 binding region of VDR gene has also been linked to increased risk of AD [177]. Haplotype analysis of 5 polymorphic sites has shown that the frequency of TaubF haplotype (alleles of TaqI, ApaI, Tru9I, BsmI, FokI) is significantly higher in AD patients [178]. Several observational case-control studies have indicated that the deficiency of plasma 25-hydroxy vitamin D3 is associated with cognitive decline in elderly or with AD [179, 180]. A large population based prospective studies of Danish subjects over a follow up period of 30 years has concluded that 25-hydroxy vitamin D3 deficiency increases the risk of AD [181]. Other studies have shown that plasma vitamin D is an important determinant of cognitive status, CSF Aβ42 level and brain volume in patients of AD, MCI and subjective cognitive impairment [182].
In cell based experiments, vitamin D has been shown to inhibit Aβ induced cytotoxicity and apoptosis in cultured cortical neurons [183]. In post-mortem AD brain, a decreased level of VDR mRNA has been reported in hippocampal region [184]. 1, 25 dihydroxy vitamin D has been shown to increase the clearance of Aβ peptide from mouse brain [185]. In neuroblastoma cells, VDR overexpression or vitamin D treatment increases APP transcription [177]. The macrophages from AD subjects show decreased phagocytosis of soluble Aβ, and thus delay the clearance of amyloid beta load of the brain [186]. 1, 25, dihydroxy vitamin D3 alone or in combination with curcuminoids can strongly activate the macrophages from AD subjects to remove Aβ by phagocytosis [186]. A vitamin D3-enriched diet has been shown to decrease the amyloid plaque load and inflammatory reaction and increase NGF production in the brain of AD transgenic mice [187]. It appears plausible that vitamin D, because of its diverse genomic and non-genomic effects in CNS, will impact amyloid pathogenesis at multiple levels.
Metabolic Risk Factors of AD: Future Possibilities
Epidemiological studies as mentioned here have identified several important metabolic risk factors having moderate to strong association with AD, but these reports also exhibit extreme variability in many cases. Moreover, neuropathological autopsy studies in large cohorts have often not supported the epidemiological data. The experimental studies have also not convincingly elucidated the pathways that could link these risk factors to the disease pathogenesis. Much of this confusion has arisen from the inherent uncertainty of AD diagnosis, absence of simple and specific biomarkers for disease identification, ambiguity in neuropathology, incomplete understanding of pathogenesis and frequent absence of correlation between clinical severity and the extent of neuropathology. Thus, the effectiveness of risk factor modification by drugs (e.g. statins, B vitamins, vitamin D, insulin or other anti-diabetic agents and anti-depressants) or life-style management to halt the progression of AD has not been firmly established. However, this is a possibility with significant optimism because of the scarcity of effective drugs for the treatment of AD, the recent major failure of anti-amyloid therapeutics in clinical trials and uncertain effectiveness of potential disease-modifying agents [188, 189]. Another important issue should also be addressed at this point with regard to experimental models used for AD research. There is no proper animal model for sporadic AD, but an overwhelming emphasis has been given on transgenic AD models to understand the disease pathogenesis. It is intuitively illogical to assume that the pathogenesis of sporadic AD which occurs without any definite mutation would be explained by a transgenic model harboring single or multiple mutations. The clear identification of metabolic risk factors may help us to develop a disease model that would truly simulate sporadic AD pathogenesis.
Acknowledgments
We thank the Department of Biotechnology, Govt. of India, New Delhi for the financial support to our research on Alzheimer’s disease. We also thank ‘The West Bengal University of Health Sciences’ for their help and encouragement.
Footnotes
Conflicts of Interest:
The authors declare no conflict of interest.
References:
- [1].Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Duthey B. Background Paper 6.11. Alzheimer Disease and other Dementias. 2013. pp. 4–74. 6.11.
- [3].van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76:v2–7. doi: 10.1136/jnnp.2005.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bird TD. Early-onset familial Alzheimer disease. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews. Seattle: NCBI; 2012. pp. 1–17. [Google Scholar]
- [5].Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clin Interv Aging. 2007;2:347–359. [PMC free article] [PubMed] [Google Scholar]
- [6].Armstrong RA. The pathogenesis of Alzheimer’s disease: a reevaluation of the “amyloid cascade hypothesis”. Int J Alzheimers Dis. 2011;2011:630865. doi: 10.4061/2011/630865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elder GA, Gama Sosa MA, De Gasperi R. Transgenic mouse models of Alzheimer’s disease. Mt Sinai J Med. 2010;77:69–81. doi: 10.1002/msj.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mattson MP. Pathways towards and away from Alzheimer’s Disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry. 2009;14:992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lahiri DK, Maloney B, Basha MR, Ge YW, Zawia NH. How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr Alzheimer Res. 2007;4:219–228. doi: 10.2174/156720507780362164. [DOI] [PubMed] [Google Scholar]
- [11].Lahiri DK, Maloney B. The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp Gerontol. 2010;45:291–296. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [13].Forstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249:288–290. doi: 10.1007/s004060050101. [DOI] [PubMed] [Google Scholar]
- [14].Geldmacher DS, Whitehouse PJ., Jr Differential diagnosis of Alzheimers disease. Neurology. 1997;48(Suppl 6):2S–9S. doi: 10.1212/wnl.48.5_suppl_6.2s. [DOI] [PubMed] [Google Scholar]
- [15].Pimmentel EML. Role of neuropsychological assessment in the differential diagnosis of Alzheimer’s disease and vascular dementia. Dement Neuropsychol. 2009;3:214–221. doi: 10.1590/S1980-57642009DN30300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- [17].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khachaturian ZS. Diagnosis of Alzheimer’s Disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- [24].Joachim CL, Morris JH, Selkoe DJ. Clinically diagnosed Alzheimer’s disease: autopsy results in 150 cases. Ann Neurol. 1988;24:50–56. doi: 10.1002/ana.410240110. [DOI] [PubMed] [Google Scholar]
- [25].Hyman BT, Trojanowski JQ. Editorial on Consensus Recommendations for the post-mortem Diagnosis of Alzheimer Disease from the National Institute on Aging and the Reagan Institute Working Group on Diagnostic Criteria for the neuropathological assessment of Alzheimer Disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- [26].Serrano-Pozo A, Qian J, Monsell SE, Frosch MP, Betensky RA, Hyman BT. Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center. J Neuropathol Exp Neurol. 2013;72:1182–1192. doi: 10.1097/NEN.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dong S, Duan Y, Hu Y, Zhao Z. Advances in the pathogenesis of Alzheimer’s disease: a re-evaluation of amyloid cascade hypothesis. Transl Neurodegener. 2012;1:1–18. doi: 10.1186/2047-9158-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol Rev. 2002;54:469–525. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- [31].Bali J, Gheinani AH, Zurbriggen S, Rajendran L. Role of genes linked to sporadic Alzheimer’s disease risk in the production of β-amyloid peptides. Proc Natl Acad Sci USA. 2012;109:15307–15311. doi: 10.1073/pnas.1201632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- [34].Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- [35].Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chakrabarti S, Sinha M, Thakurta IG, Banerjee P, Chattopadhyay M. Oxidative stress and amyloid beta toxicity in Alzheimer’s disease: intervention in a complex relationship by antioxidants. Curr Med Chem. 2013;20:4648–4664. doi: 10.2174/09298673113209990152. [DOI] [PubMed] [Google Scholar]
- [37].Santos RX, Correia SC, Wang X, Perry G, Smith MA, Moreira PI, et al. A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer’s disease. J Alzheimers Dis. 2010;20:S401–S412. doi: 10.3233/JAD-2010-100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2010;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zotova E, Nicoll JA, Kalaria R, Holmes C, Boche D. Inflammation in Alzheimer’s disease: relevance to pathogenesis and therapy. Alzheimers Res Ther. 2010;2:1. doi: 10.1186/alzrt24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer’s pathology. J Cell Mol Med. 2008;12:2255–2262. doi: 10.1111/j.1582-4934.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu L, Chan C. The role of inflammasome in Alzheimer’s disease. Ageing Res Rev. 2014;15:6–15. doi: 10.1016/j.arr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mamelak M. Sporadic Alzheimer’s disease: the starving brain. J Alzheimers Dis. 2012;31:459–474. doi: 10.3233/JAD-2012-120370. [DOI] [PubMed] [Google Scholar]
- [45].Erol A. An integrated and unifying hypothesis for the metabolic basis of sporadic Alzheimer’s disease. J Alzheimers Dis. 2008;13:241–253. doi: 10.3233/jad-2008-13302. [DOI] [PubMed] [Google Scholar]
- [46].de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol. 2014;88:548–559. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Besser LM, Gill DP, Monsell SE, Brenowitz W, Meranus DH, Kukull W, et al. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gillette-Guyonnet S, Nourhashemi F, Andrieu S, de Glisezinski I, Ousset PJ, Riviere D, et al. Am J Clin Nutr. 2000;71:637S–642S. doi: 10.1093/ajcn/71.2.637s. [DOI] [PubMed] [Google Scholar]
- [49].Khemka VK, Bagchi D, Bandyopadhyay K, Bir A, Chattopadhyay M, Biswas A, et al. Altered serum levels of adipokines and insulin in probable Alzheimer’s disease. J Alzheimers Dis. 2014;41:525–533. doi: 10.3233/JAD-140006. [DOI] [PubMed] [Google Scholar]
- [50].Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gibson GE, 1, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- [52].Mustafa S, Kutlu U, Tunahan Ç. Systematic analysis of transcription-level effects of neurodegenerative diseases on human brain metabolism by a newly reconstructed brain-specific metabolic network. FEBS Open Bio. 2014. In press. [DOI] [PMC free article] [PubMed]
- [53].Newington JT, Harris RA, Cumming RC. Reevaluating metabolism in Alzheimer’s disease from the perspective of the astrocyte-neuron lactate shuttle model. J Neurodeg Dis. 2013;2013:234572. doi: 10.1155/2013/234572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, Sancarlo D. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev. 2010;9:399–417. doi: 10.1016/j.arr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- [55].Crean S, Ward A, Mercaldi CJ, Collins JM, Cook MN, Baker NL, et al. Apolipoprotein E ε4 prevalence in Alzheimer’s disease patients varies across global populations: a systematic literature review and meta-analysis. Dement Geriatr Cogn Disord. 2011;31:20–30. doi: 10.1159/000321984. [DOI] [PubMed] [Google Scholar]
- [56].Evans RM, Hui S, Perkins A, Lahiri DK, Poirier J, Farlow MR. Cholesterol and APOE genotype interact to influence Alzheimer disease progression. Neurology. 2004;62:1869–1871. doi: 10.1212/01.wnl.0000125323.15458.3f. [DOI] [PubMed] [Google Scholar]
- [57].Kamboh MI, Demirci FY, Wang X, Minster RL, Carrasquillo MM, Pankratz VS, et al. Genome-wide association study of Alzheimer’s disease. Transl Psychiatry. 2012;2:e117. doi: 10.1038/tp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Evans RM, Emsley CL, Gao S, Sahota A, Hall KS, Farlow MR, et al. Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: a population-based study of African Americans. Neurology. 2000;54:240–242. doi: 10.1212/wnl.54.1.240. [DOI] [PubMed] [Google Scholar]
- [61].Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 2011;68:1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ramdane S, Daoudi-Gueddah D. Mild hypercholesterolemia, normal plasma triglycerides, and normal glucose levels across dementia staging in Alzheimer’s disease: a clinical setting-based retrospective study. Am J Alzheimers Dis Other Demen. 2011;26:399–405. doi: 10.1177/1533317511414552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Paragh G, Balla P, Katona E, Seres I, Egerházi A, Degrell I. Serum paraoxonase activity changes in patients with Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2002;252:63–67. doi: 10.1007/s004060200013. [DOI] [PubMed] [Google Scholar]
- [64].Mielke MM, Zandi PP, Shao H, Waern M, Östling S, Guo X, et al. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology. 2010;75:1888–1895. doi: 10.1212/WNL.0b013e3181feb2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cedazo-Mínguez A, Ismail MA, Mateos L. Plasma cholesterol and risk for late-onset Alzheimer’s disease. Expert Rev Neurother. 2011;11:495–498. doi: 10.1586/ern.11.36. [DOI] [PubMed] [Google Scholar]
- [66].Mielke MM, Zandi PP, Sjögren M, Gustafson D, Ostling S, Steen B, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- [67].Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- [68].Scott HD, Laake K. Statins for the prevention of Alzheimer’s disease. Cochrane Database Syst Rev. 2001;4:CD003160. doi: 10.1002/14651858.CD003160. [DOI] [PubMed] [Google Scholar]
- [69].Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol. 2011;68:1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wolozin B. A fluid connection: cholesterol and Abeta. Proc Natl Acad Sci USA. 2001;98:5371–5373. doi: 10.1073/pnas.101123198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- [72].Umeda T, Tomiyama T, Kitajima E, Idomoto T, Nomura S, Lambert MP, et al. Hypercholesterolemia accelerates intraneuronal accumulation of Aβ oligomers resulting in memory impairment in Alzheimer’s disease model mice. Life Sci. 2012;91:1169–1176. doi: 10.1016/j.lfs.2011.12.022. [DOI] [PubMed] [Google Scholar]
- [73].Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- [74].Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- [75].Simons M, Keller P, Dichgans J, Schulz JB. Cholesterol and Alzheimer’s disease: is there a link? Neurology. 2001;57:1089–1093. doi: 10.1212/wnl.57.6.1089. [DOI] [PubMed] [Google Scholar]
- [76].Michikawa M, Fan QW, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74:1008–1016. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- [77].Evans RM, Hui S, Perkins A, Lahiri DK, Poirier J, Farlow MR. Cholesterol and APOE genotype interact to influence Alzheimer disease progression. Neurology. 2004;62:1869–1871. doi: 10.1212/01.wnl.0000125323.15458.3f. [DOI] [PubMed] [Google Scholar]
- [78].Zuliani G, Donnorso MP, Bosi C, Passaro A, Dalla Nora E, Zurlo A, et al. Plasma 24S-hydroxycholesterol levels in elderly subjects with late onset Alzheimer’s disease or vascular dementia: a case-control study. BMC Neurol. 2011;11:121. doi: 10.1186/1471-2377-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Vega GL, Weiner MF, Lipton AM, Von Bergmann K, Lutjohann D, Moore C, et al. Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Arch Neurol. 2003;60:510–515. doi: 10.1001/archneur.60.4.510. [DOI] [PubMed] [Google Scholar]
- [80].Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- [81].Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- [82].Brayne C, Gill C, Huppert FA, Barkley C, Gehlhaar E, Girling DM, et al. Vascular risks and incident dementia: results from a cohort study of the very old. Dement Geriatr Cogn Disord. 1998;9:175–180. doi: 10.1159/000017043. [DOI] [PubMed] [Google Scholar]
- [83].Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- [84].Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;56:384–402. doi: 10.1016/j.brainresrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [85].Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–92. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- [86].Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- [88].Exalto LG, Whitmer RA, Kappele LJ, Biessels GJ. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp Gerontol. 2012;47:858–864. doi: 10.1016/j.exger.2012.07.014. [DOI] [PubMed] [Google Scholar]
- [89].Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer’s disease: The confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152–160. doi: 10.1093/epirev/mxs012. [DOI] [PubMed] [Google Scholar]
- [90].Luchsinger JA. Diabetes, related conditions, and dementia. J Neurol Sci. 2010;299:35–38. doi: 10.1016/j.jns.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- [93].Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26:507–519. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- [94].McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- [95].Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective post-mortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- [96].Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- [97].Neumann KF, Rojo L, Navarrete LP, Farias G, Reyes P, Maccioni RB. Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Curr Alzheimer Res. 2008;5:438–447. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- [98].De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123:531–539. doi: 10.1172/JCI64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, et al. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Son SM, Song H, Byun J, Park KS, Jang HC, Park YJ, Mook-Jung I. Altered APP processing in insulin-resistant conditions is mediated by autophagosome accumulation via the inhibition of mammalian target of rapamycin pathway. Diabetes. 2012;61:3126–3138. doi: 10.2337/db11-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Correia SC, Santos RX, Carvalho C, Cardoso S, Candeias E, Santos MS, et al. Insulin signaling, glucose metabolism and mitochondria: major players in Alzheimer’s disease and diabetes interrelation. Brain Res. 2012;1441:64–78. doi: 10.1016/j.brainres.2011.12.063. [DOI] [PubMed] [Google Scholar]
- [102].Yang Y, Song W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience. 2013;250:140–150. doi: 10.1016/j.neuroscience.2013.07.009. [DOI] [PubMed] [Google Scholar]
- [103].Tang J, Pei Y, Zhou G. When aging-onset diabetes is coming across with Alzheimer disease: comparable pathogenesis and therapy. Exp Gerontol. 2013;48:744–750. doi: 10.1016/j.exger.2013.04.013. [DOI] [PubMed] [Google Scholar]
- [104].Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225:54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- [106].Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- [107].Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- [108].Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- [109].Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- [110].Park SA. A common pathogenic mechanism linking type-2 diabetes and Alzheimer’s disease: evidence from animal models. J Clin Neurol. 2011;7:10–18. doi: 10.3988/jcn.2011.7.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, Figlewicz DP, Jacobson L, Beard JC, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- [112].Bosco D, Fava A, Plastino M, Montalcini T, Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J Cell Mol Med. 2011;15:1807–1821. doi: 10.1111/j.1582-4934.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Freiherr J, Hallschmid M, Frey WH, 2nd, Brünner YF, Chapman CD, Holscher C, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27:505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Williamson R, McNeilly A, Sutherland C. Insulin resistance in the brain: an old-age or new-age problem? Biochem Pharmacol. 2012;84:737–745. doi: 10.1016/j.bcp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- [115].Ciaccio M, Bellia C. Hyperhomocysteinemia and cardiovascular risk: effect of vitamin supplementation in risk reduction. Curr Clin Pharmacol. 2010;5:30–36. doi: 10.2174/157488410790410551. [DOI] [PubMed] [Google Scholar]
- [116].Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39–54. doi: 10.1146/annurev.med.60.041807.123308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhuo JM, Wang H, Pratico D. Is hyperhomocysteinemia an Alzheimer’s disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol Sci. 2011;32:562–571. doi: 10.1016/j.tips.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- [119].Zylberstein DE, Lissner L, Björkelund C, Mehlig K, Thelle DS, Gustafson D, et al. Midlife homocysteine and late-life dementia in women. A prospective population study. Neurobiol Aging. 2009;32:380–386. doi: 10.1016/j.neurobiolaging.2009.02.024. [DOI] [PubMed] [Google Scholar]
- [120].Elias MF, Sullivan LM, D’Agostino RB, Elias PK, Jacques PF, Selhub J, et al. Homocysteine and cognitive performance in the Framingham offspring study: age is important. Am J Epidemiol. 2005;162:644–653. doi: 10.1093/aje/kwi259. [DOI] [PubMed] [Google Scholar]
- [121].Wald DS, Kasturiratne A, Simmonds M. Serum homocysteine and dementia: meta-analysis of eight cohort studies including 8669 participants. Alzheimers Dement. 2011;7:412–417. doi: 10.1016/j.jalz.2010.08.234. [DOI] [PubMed] [Google Scholar]
- [122].Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- [123].Mizrahi EH, Bowirrat A, Jacobsen DW, Korczyn AD, Traore F, Petot GJ, et al. Plasma homocysteine, vitamin B12 and folate in Alzheimer’s patients and healthy Arabs in Israel. J Neurol Sci. 2004;227:109–113. doi: 10.1016/j.jns.2004.08.011. [DOI] [PubMed] [Google Scholar]
- [124].Postiglione A, Milan G, Ruocco A, Gallotta G, Guiotto G, Di Minno G. Plasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer’s dementia. A case-control study Gerontology. 2001;47:324–329. doi: 10.1159/000052822. [DOI] [PubMed] [Google Scholar]
- [125].Nilsson K, Gustafson L, Hultberg B. Elevated plasma homocysteine level is not primarily related to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;34:121–127. doi: 10.1159/000342612. [DOI] [PubMed] [Google Scholar]
- [126].Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer’s disease? J Alzheimers Dis. 2006;9:393–398. doi: 10.3233/jad-2006-9404. [DOI] [PubMed] [Google Scholar]
- [127].Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sun Y, Lu CJ, Chien KL, Chen ST, Chen RC. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin Ther. 2007;29:2204–2214. doi: 10.1016/j.clinthera.2007.10.012. [DOI] [PubMed] [Google Scholar]
- [129].Miller JW, Green R, Mungas DM, Reed BR, Jagust WJ. Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology. 2006;58:1471–1475. doi: 10.1212/wnl.58.10.1471. [DOI] [PubMed] [Google Scholar]
- [130].Ray L, Khemka VK, Behera P, Bandyopadhyay K, Pal S, Pal K, et al. Serum homocysteine, dehydroepiandrosterone sulphate and lipoprotein (a) in Alzheimer’s disease and vascular dementia. Aging Dis. 2013;4:57–64. [PMC free article] [PubMed] [Google Scholar]
- [131].Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- [132].Lin N, Qin S, Luo S, Cui S, Huang G, Zhang X. Homocysteine induces cytotoxicity and proliferation inhibition in neural stem cells via DNA methylation in vitro. FEBS J. 2014;281:2088–2096. doi: 10.1111/febs.12764. [DOI] [PubMed] [Google Scholar]
- [133].Kim WK, Pae YS. Involvement of N-methyl-d-aspartate receptor and free radical in homocysteine-mediated toxicity on rat cerebellar granule cells in culture. Neurosci Lett. 1996;216:117–120. doi: 10.1016/0304-3940(96)13011-1. [DOI] [PubMed] [Google Scholar]
- [134].Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- [135].Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- [136].Soodini GR. Adiponectin and leptin in relation to insulin sensitivity. Metab Syndr Relat Disord. 2004;2:114–123. doi: 10.1089/met.2004.2.114. [DOI] [PubMed] [Google Scholar]
- [137].Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165:313–327. doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Morton GJ. Hypothalamic leptin regulation of energy homeostasis and glucose metabolism. J Physiol. 2007;583:437–443. doi: 10.1113/jphysiol.2007.135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Marwarha G, Ghribi O. Leptin signaling and Alzheimer’s disease. Am J Neurodegener Dis. 2012;1:245–265. [PMC free article] [PubMed] [Google Scholar]
- [140].Lee EB. Obesity, leptin, and Alzheimer’s disease. Ann N Y Acad Sci. 2011;1243:15–29. doi: 10.1111/j.1749-6632.2011.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rizoulis AA, Karaoulanis SE, Rizouli KA, Papadimitriou A. Serum leptin levels in patients with Alzheimer’s disease. Int J Caring Sci. 2005;5:43–49. [Google Scholar]
- [144].Une K, Takei YA, Tomita N, Asamura T, Ohrui T, Furukawa K, et al. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur J Neurol. 2011;18:1006–1009. doi: 10.1111/j.1468-1331.2010.03194.x. [DOI] [PubMed] [Google Scholar]
- [145].van Himbergen TM, Beiser AS, Ai M, Seshadri S, Otokozawa S, Au R, et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Arch Neurol. 2012;69:594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Warren MW, Hynan LS, Weiner MF. Lipids and adipokines as risk factors for Alzheimer’s disease. J Alzheimers Dis. 2012;29:151–157. doi: 10.3233/JAD-2012-111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Tezapsidis N, Johnston JM, Smith MA, Ashford JW, Casadesus G, Robakis NK, et al. Leptin: A novel therapeutic strategy for Alzheimer’s disease. J Alzheimers Dis. 2009;16:731–740. doi: 10.3233/JAD-2009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain res bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Enciu AM, Popescu BO. Is there a causal link between Inflammation and dementia? Biomed Res Int. 2013;2013:316495. doi: 10.1155/2013/316495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Weisman D, Hakimian E, Ho GJ. Interleukins, inflammation, and mechanisms of Alzheimer’s disease. Vitam Horm. 2006;74:505–530. doi: 10.1016/S0083-6729(06)74020-1. [DOI] [PubMed] [Google Scholar]
- [152].Rubio-Perez JM, Morillas-Ruiz JM. A review: Inflammatory process in Alzheimer’s disease, Role of cytokines. Scientific World Journal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Tan MS, Yu JT, Jiang T, Zhu XC, Tan L. The NLRP3 inflammasome in Alzheimer’s disease. Mol Neurobiol. 2013;48:875–882. doi: 10.1007/s12035-013-8475-x. [DOI] [PubMed] [Google Scholar]
- [155].Licastro F, Pedrini S, Caputo L, Annoni G, Grimaldi LME. Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- [156].Mraka RE, Griffin WST. Potential Inflammatory biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2005;8:369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- [157].Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;28:281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- [158].Lanzrein A, Johnston CM, Perry VH, Jobst KA, King EM, Smith AD. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1β, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-α, the soluble tumor necrosis factor receptors I and II, and α1 antichymotrypsin. Alzheimer Dis Assoc Disord. 1998;12:215–227. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- [159].Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- [160].Khemka VK, Ganguly A, Bagchi D, Ghosh A, Bir A, Biswas A, et al. Raised serum proinflammatory cytokines in Alzheimer’s disease with depression. Aging Dis. 2014;5:170–176. doi: 10.14336/AD.2014.0500170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Banks WA, Farr SA, Morley JE. Entry of Blood-Borne Cytokines into the Central Nervous System: Effects on Cognitive Processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- [162].Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- [163].McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [164].Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- [165].Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- [166].Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- [167].Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Shaftel SS, Griffin WS, O’Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNF alpha plus IFN gamma induce the production of Alzheimer beta-amyloid peptides, and decrease the secretion of APPs. FASEB J. 1999;13:63–68. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- [171].Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- [172].Revelli A, Massobrio M, Tesarik J. Nongenomic effects of 1 alpha, 25-dihydroxyvitamin D3. Trends Endocrinol Metab. 1998;9:419–427. doi: 10.1016/s1043-2760(98)00100-3. [DOI] [PubMed] [Google Scholar]
- [173].Wrzosek M, Łukaszkiewicz J, Wrzosek M, Jakubczyk A, Matsumoto H, Piątkiewicz P, et al. Vitamin D and the central nervous system. Pharmacol Rep. 2013;65:271–278. doi: 10.1016/s1734-1140(13)71003-x. [DOI] [PubMed] [Google Scholar]
- [174].Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- [175].Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- [176].Gezen-Ak D, Dursun E, Ertan T, Hanağasi H, Gürvit H, Emre M, et al. Association between vitamin D receptor gene polymorphism and Alzheimer’s disease. Tohoku J Exp Med. 2007;12:275–282. doi: 10.1620/tjem.212.275. [DOI] [PubMed] [Google Scholar]
- [177].Wang L, Hara K, Van Baaren JM, Price JC, Beecham GW, Gallins PJ, et al. Vitamin D receptor and Alzheimer’s disease: a genetic and functional study. Neurobiol Aging. 2012;33:1844.e1–9. doi: 10.1016/j.neurobiolaging.2011.12.038. [DOI] [PubMed] [Google Scholar]
- [178].Gezen-Ak D, Dursun E, Bilgiç B, Hanağasi H, Ertan T, Gürvit H, et al. Vitamin D receptor gene haplotype is associated with late-onset Alzheimer’s disease. Tohoku J Exp Med. 2012;228:189–196. doi: 10.1620/tjem.228.189. [DOI] [PubMed] [Google Scholar]
- [179].Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2013;33:659–674. doi: 10.3233/JAD-2012-121432. [DOI] [PubMed] [Google Scholar]
- [180].Zhao Y, Sun Y, Ji HF, Shen L. Vitamin D levels in Alzheimer’s and Parkinson’s diseases: a meta-analysis. Nutrition. 2013;29:828–832. doi: 10.1016/j.nut.2012.11.018. [DOI] [PubMed] [Google Scholar]
- [181].Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. 2014;10:296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- [182].Hooshmand B, Lokk J, Solomon A, Mangialasche F, Miralbell J, Spulber G, et al. Vitamin D in relation to cognitive impairment, cerebrospinal fluid biomarkers, and brain volumes. J Gerontol A Biol Sci Med Sci. 2014. [Epub ahead of print] [DOI] [PubMed]
- [183].Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer’s disease: vitamin D receptor suppression by amyloid-β and preventing the amyloid-β induced alterations by vitamin D in cortical neurons. J Alzheimers Dis. 2011;23:207–219. doi: 10.3233/JAD-2010-101377. [DOI] [PubMed] [Google Scholar]
- [184].Sutherland MK, Somerville MJ, Yoong LK, Bergeron C, Haussler MR, McLachlan DR. Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: correlation with calbindin-28k mRNA levels. Brain Res Mol Brain Res. 1992;13:239–250. doi: 10.1016/0169-328x(92)90032-7. [DOI] [PubMed] [Google Scholar]
- [185].Ito S, Ohtsuki S, Nezu Y, Koitabashi Y, Murata S, Terasaki T. 1 alpha, 25-dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-β peptide (1–40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS. 2011;8:20. doi: 10.1186/2045-8118-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, et al. 1 alpha, 25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17:703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- [187].Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S, et al. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J Alzheimers Dis. 2011;25:295–307. doi: 10.3233/JAD-2011-101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Jiang T, Yu JT, Tan L. Novel disease-modifying therapies for Alzheimer’s disease. J Alzheimers Dis. 2012;31:475–492. doi: 10.3233/JAD-2012-120640. [DOI] [PubMed] [Google Scholar]
- [189].Townsend M. When will Alzheimer’s disease be cured? A pharmaceutical perspective. J Alzheimers Dis. 2011;24(Suppl 2):43–52. doi: 10.3233/JAD-2011-110020. [DOI] [PubMed] [Google Scholar]