Hepatitis C virus (HCV) infects approximately 130 million people worldwide and causes chronic liver diseases including fibrosis, cirrhosis, and hepatocellular carcinoma. A vaccine is not available and current interferon-based treatments are frequently ineffective. The development of novel therapies has been constrained by the lack of versatile small animal models. Since the natural species tropism of HCV is limited to humans and chimpanzees, establishing a mouse model of infection will likely require viral or host adaptation.
Murine host adaptation by xenotransplantation has already shown promise. The best-characterized model is based on a urokinase plasminogen activator (uPA) transgene (Alb-uPA mice). uPA expression leads to liver damage and, when crossed onto immunodeficient backgrounds, transplanted human hepatocytes have a growth advantage and repopulate the mouse liver. Such liver-chimeric mice are susceptible to HCV1 and have been used to study drug metabolism2, efficacy of antivirals3 and neutralizing antibodies4, as well as the role of lymphocytes in limiting viral infection5. Unfortunately, the Alb-uPA model is expensive, technically challenging, and low throughput and therefore other liver injury models are being explored. In mice with a targeted disruption in the fumaryl acetoacetate hydrolase gene, liver damage can be timed by withdrawal of a hepatocyte protective drug. When crossed to an immunodeficient background, these mice show average engraftment rates of 40%6. Although useful for studying aspects of human hepatotropic infections in vivo, liver-chimeric mice have the major disadvantage of an immunodeficient background, which drastically limits studies of HCV pathogenesis.
An alternate approach for host adaptation is the generation of transgenic mice expressing human-specific factors. Such animals would overcome the technical challenges of xenotransplantation and possibly provide an immunocompetent model. As a prerequisite, however, all human-specific factors necessary for HCV propagation must be found and possible species restrictions have to be overcome. Recently, CD81 and occludin (OCLN) have been identified as the minimal set of human factors required to bypass the HCV entry block in mice7. In contrast, little information is available on human-specific factors required for HCV replication, although it is known that mouse cells support viral RNA accumulation to very low levels8. Most cellular components implicated in HCV replication (reviewed in9) are conserved between humans and mice and are therefore unlikely to account for species-specific differences. However, differential expression of critical replication factors might contribute to the replication block in mice. The expression of antiviral factors may also impact tropism. For instance, interferon-regulated protein kinase R and signaling molecule interferon regulatory factor 3 efficiently restrict HCV replication in murine fibroblasts10,8. Lastly, due to the limited replication of HCV in mouse cells, production of infectious virus has not been studied and it is conceivable that further blocks exist for virus assembly and release.
Adaptation of the virus to rodent hosts is an alternative strategy for establishing a mouse model for hepatitis C. This approach has advantages, in that the resulting model is immunocompetent, can be bred, and does not require specific knowledge of restriction factors. Resistance of mice to HCV is multifactorial and at least determined by blocks in viral entry and replication7,8. Entry of HCV is a complex process facilitated by four essential membrane proteins: scavenger receptor class B type 1 (SCARB1, also referred to as SR-BI), CD81, claudin-1 (CLDN1) and OCLN7,11–13. Comparisons of the human and mouse orthologues reveal that, while mouse (m)SCARB1 and mCLDN1 support HCV entry similar to their human homologues, mCD81 and mOCLN do not7.
In their recent work14, Bitzegeio et al make a first important step towards developing a murine tropic virus. Using an unbiased selection approach, the authors adapt a laboratory strain of HCV to use a mouse entry factor. Taking advantage of the high mutational plasticity of HCV, Bitzegeio et al. identified three adaptive mutations in the viral glycoproteins E1 and E2 that allowed the virus to enter cells expressing human (h)SCARB1, hCLDN1, hOCLN and mCD81. Interestingly, both mutations in E2 are located in the hypervariable region 1, which is thought to be dispensable for CD81 binding. These mutations strikingly increased the affinity of the virus for the large extracellular loop of hCD81, suggesting an indirect enhancement by exposing a CD81 binding site. Moreover, the mCD81-adapted virus permitted entry via rat and hamster orthologues. In addition to modifying CD81 tropism, the adaptive mutations altered usage of hSCARB1 and hOCLN. Blocking antibodies against hSCARB1 and silencing of hOCLN had a less pronounced effect on the entry of the mutant virus as compared to the parental strain, suggesting that the mCD81-adapted virus was less dependent on SCARB1 and OCLN. In addition, mouse fibroblasts expressing all four murine entry factors supported the uptake of adapted virus and this entry could be blocked with anti-mCD81 antibodies, indicating that the species-restriction to human OCLN was altered while CD81-dependence was maintained.
Structural changes in the murine adapted E1/E2 complex were evident, since affinities for neutralizing antibodies targeting conformational epitopes were drastically altered. Increased fusogenic activity of the mutant E1/E2 complex indicated that the adapted proteins might adopt a structure resembling that acquired during receptor interactions. It has previously been demonstrated that HCV requires not only a low pH shift, but also additional primers for efficient membrane fusion15. The latter requirement appears to be less stringent in the adapted glycoprotein complex, as measured by temperature shift assays. The mCD81-adaptive mutations therefore seem to affect the overall E1/E2 structure, leading to a conformation that mimics a late stage of HCV entry, thus reducing the dependence on early binding events and receptor-induced fusion. Such observations raise the question of the similarity between entry processes of the adapted virus versus naturally occurring HCV particles.
The data presented by Bitzegeio et al forms an exciting precedent, suggesting that species barriers may be overcome with a few adaptive mutations. Although this study did not demonstrate HCV entry into primary murine hepatocytes, this does not preclude the hope that the adapted virus can enter hepatocytes in vivo. It is known that culture of primary hepatocytes is technically challenging and that their phenotype can be quickly lost ex vivo. Clearly, overcoming the species block at the level of entry is a major step towards the development of a murine-tropic HCV strain. Together with increasing knowledge of the determinants of virus replication, this study provides the basis for generating an adapted virus that can infect mice without the need for human hepatocyte xenotransplantation. However, testing of neutralizing antibodies might be misleading in such a system due to conformational differences in the adapted E1/E2 complex. In addition, therapeutics generated to block HCV entry may be human specific and thus difficult to test. Nonetheless, an immunocompetent small animal model based on a murine-tropic virus strain would be a milestone in HCV research.
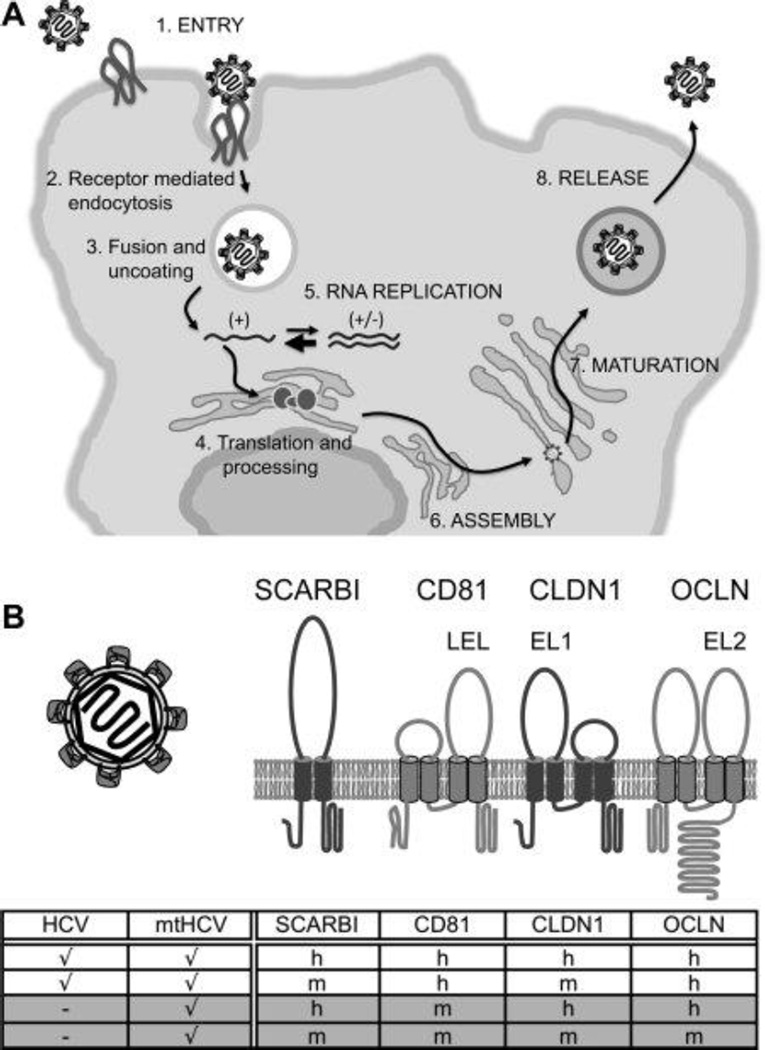
Figure 1.
Species blocks in HCV infection and phenotype of mtHCV. (A) HCV life cycle and blocks in murine cells. HCV entry and replication are blocked in murine cells, whereas endocytosis, membrane fusion, uncoating, translation, and polyprotein processing are supported. There are no data available on the assembly, maturation, and release of infectious HCV particles in murine cells. Capital letters indicate steps in the HCV life cycle that presumably require adaptation to the murine host. (B) Membrane topology model of HCV entry factors and entry factor usage by HCV and mtHCV. HCV entry requires four factors: SCARBI, CD81, CLDN1, and OCLN. The large extracellular loop (LEL) of CD81 and extracellular loops 1 and 2 (EL1 and EL2) of CLDN1 and OCLN, which are critical for HCV entry, are labeled. Entry factors restricting entry into mouse hepatocytes are depicted in light gray. The table shows human and mouse entry factor usage of parental HCV and adapted mtHCV (a check mark means that entry is supported, and a dash means that entry is blocked; h and m indicate human and mouse, respectively). Newly acquired tropisms of mtHCV are shaded in gray.
References
- 1.Dandri M, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- 2.Tateno C, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol. 2004;165:901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meuleman P, Leroux-Roels G. The human liver-uPA-SCID mouse: a model for the evaluation of antiviral compounds against HBV and HCV. Antiviral Res. 2008;80:231–238. doi: 10.1016/j.antiviral.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Law M, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 5.Ohira M, et al. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J Clin Invest. 2009;119:3226–3235. doi: 10.1172/JCI38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissig KD, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ploss A, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin LT, et al. Replication of Subgenomic HCV Replicons in Mouse Fibroblasts is Facilitated by Deletion of Interferon Regulatory Factor-3 and Expression of Liver-Specific MicroRNA-122. J Virol. 2010 doi: 10.1128/JVI.00559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ploss A, Rice CM. Towards a small animal model for hepatitis C. EMBO Rep. 2009;10:1220–1227. doi: 10.1038/embor.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang KS, et al. Replication of hepatitis C virus (HCV) RNA in mouse embryonic fibroblasts: protein kinase R (PKR)-dependent and PKR-independent mechanisms for controlling HCV RNA replication and mediating interferon activities. J Virol. 2006;80:7364–7374. doi: 10.1128/JVI.00586-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarselli E, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pileri P, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 13.Evans MJ, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 14.Bitzegeio J, et al. Adaptation of hepatitis C virus to mouse CD81 permits infection of mouse cells in the absence of human entry factors. PLoS Pathog. 2010;6:e1000978. doi: 10.1371/journal.ppat.1000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tscherne DM, et al. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J Virol. 2006;80:1734–1741. doi: 10.1128/JVI.80.4.1734-1741.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]