Abstract
Mycoplasma agalactiae and M. bovis rank amongst the most serious pathogenic mycoplasmas infecting small ruminants and cattle, respectively. Despite considerable advances made in Mycoplasma molecular genetics in the past decade, there is still a complete lack of genetic tools to assess the pathogenic mechanisms of these two species. Studies were undertaken to develop a genetic system for the analysis of potential virulence factors of these pathogens. Transposon Tn4001mod was successfully introduced into various chromosomal sites of M. agalactiae and M. bovis with an optimal frequency of 10−6 per viable colony-forming unit (CFU). This is the first report that demonstrates the amenability of these agents to transformation and to genetic manipulation. Furthermore, Tn4001 is implicated as the first potential genetic tool available for these ruminant pathogens.
Keywords: Mycoplasma, Transformation, Transposon, Genetics, Mutagenesis
Introduction
The phylogenetically related species Mycoplasma agalactiae and M. bovis inflict serious and difficult-to-eradicate diseases in small ruminants and cattle, respectively. Clinical manifestations caused by these pathogens include mastitis, arthritis and respiratory diseases that lead to significant economic losses worldwide (Bergonier et al., 1997; Rosengarten and Citti, 1999; Nicholas and Ayling, 2003). Despite their severity, there has been limited success in controlling these Mycoplasma infections. Development of adequate prophylactic and preventive measures would largely rely on the thorough understanding and identification of the factors involved in virulence and disease progression. Due to the complete lack of a genetic system for manipulating the genome of these two Mycoplasma species, progress towards deciphering their pathogenic mechanisms has been hampered.
Mycoplasma genetics, in general, suffers largely due to the paucity of genetic selection markers, suitable cloning vectors and efficient DNA transfer methods (Minion and Kapke, 1998). Transposons have served as valuable genetic tools for mycoplasmas. Transformation of many Mycoplasma species has been demonstrated using the Gram-positive bacterial transposons, Tn916 or Tn4001 (Dybvig and Cassell, 1987; Dybvig and Alderete, 1988; Hedreyda et al., 1993; Cao et al., 1994; King and Dybvig, 1994). Tn916, which bears the tetracycline resistance marker tet M, was the first to be used in mycoplasmas (Dybvig and Cassell, 1987), but its large size (18 kb) often discourages its use in genetic studies despite its useful conjugative properties (Franke and Clewell, 1981). The 4.7-kb staphylococcal transposon Tn4001, conferring gentamicin (Gm), tobramicin and kanamicin resistance (Lyon et al., 1984), is especially suitable for Mycoplasma genetic studies as it is small enough to be used as a cloning vehicle and has been already modified to create a variety of useful versions including Tn4001mod with additional unique restriction sites (Knudtson and Minion, 1993).
The aim of the present study was to demonstrate the ability to transform M. agalactiae and M. bovis and to develop a genetic system for the analysis of their potential virulence factors.
Tn4001mod was successfully introduced into these ruminant pathogens by electroporation, thus, making it the first report that demonstrates their amenability to genetic transformation. The convenience of this insertional mutagenesis in M. agalactiae and M. bovis should stimulate interest in studying their molecular pathology, and thereby, help in evolving new improved diagnostic and prophylactic tools.
Materials and methods
Bacterial strains and culture conditions
M. agalactiae and M. bovis type strains, PG2 (Solsona et al., 1996) and PG45 (Rosengarten et al., 1994), respectively, were grown at 37 °C in standard SP-4 medium supplemented with 500 U/ml of penicillin (Tully, 1995) and with pyruvate (0.5%, w/v) and phenol red (0.005%, w/v) as growth indicators (Hannan et al., 1997). Mycoplasma species verification was performed by PCR as described (Chavez Gonzalez et al., 1995;Subramaniam et al., 1998). Transformants were selected on SP-4 agar plates containing 50 μg/ml of Gm (Sigma Chemical Co., St. Louis, MO, USA).
Plasmid DNA
Plasmid pISM2062 (Knudtson and Minion, 1993) carrying the transposon Tn4001mod was used for transformation and was maintained and amplified in Escherichia coli DH10B in Luria–Bertani broth supplemented with 7 μg/ml of Gm and 50 μg/ml of ampicillin.
PCR-based detection of Tn4001 insertion in picked transformants
Crude DNA extracts were prepared from 1-ml cultures by essentially the same method as described below for genomic DNA isolation except that RNase treatments and phenol extractions were not performed. Instead, the crude DNA was directly precipitated using isopropanol and pellets obtained thereof were suspended in 25 μl of Tris-EDTA buffer. PCR assays were conducted using 2–4 μl of the crude DNA as template in 25-μl reaction mixtures, with 1 U of Taq DNA polymerase (Promega) in 1 × buffer supplied by the manufacturer, 200 μM dNTPs, 1.5 mM MgCl2 and 1.4 μM of each primer, Tn1 (5′-ACATGAATTACACGAGGGC-3′) and Tn2 (5′-GTTCTTCTTCTGACATAGTAG-3′). The amplification was performed in a Perkin Elmer GeneAmp thermal cycler over 30 cycles, each consisting of 1 min at 95 °C, 1 min at 54 °C, and 1 min at 72 °C besides the initial denaturation step of 5 min at 95 °C and the concluding chain termination at 72 °C for 5 min. In presence of the Tn4001mod, this PCR assay resulted in a 400-bp Tn-PCR product that was detected after agarose gel electrophoresis and ethidium bromide staining.
DNA isolation, manipulations and Southern hybridization
Plasmid DNA was isolated from E. coli cultures using the Qiagen Mega Kit or Peqlab Midi Kit. Mycoplasma genomic DNA was obtained by lysing the cells in a buffer containing 1% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl (pH 8.0), and 10 mM EDTA for 10 min at 50 °C and then for 10 min at 37 °C. The lysate was treated with RNase (37 °C/40 min) and proteinase K (37 °C/40 min) before precipitation with 10 mM potassium acetate at 12,000g for 15 min at 4 °C. Standard procedures were used for phenol/chloroform/isoamyl alcohol extractions of the supernatant, and the subsequent ethanol precipitation (Sambrook et al., 1989). Restriction endonucleases (Promega) were used according to the manufacturer’s instructions. Southern hybridizations using a digoxigenin (DIG)-labeled probe corresponding to the 400-bp Tn-PCR product were performed as previously described (Winner et al., 2003). The same procedure was used to detect plasmid-specific sequences using a DIG-labeled PCR probe produced by primers AmpF (5′-GGCATGACAGTAAGAG-3′) and AmpR (5′-GTGCTGCAATGATACC-3′) under the PCR conditions described above for detection of the transposon.
Results and discussion
Transformation
Several preliminary experiments were conducted to identify conditions under which transformation/transposition of Tn4001 could be detected in M. agalactiae/M. bovis. As many Mycoplasma species are naturally resistant to Gm (Hannan, 1995), a prerequisite step was to assess the sensitivity of M. agalactiae and M. bovis to Gm. A concentration of 50 μg/ml Gm was evaluated to be completely inhibitory for growth, with a rate of spontaneous resistance mutants calculated to be less than 3.9 × 10−9.
Transformation procedures were initially based on methods previously described for other Mycoplasma species (Hedreyda et al., 1993; Winner et al., 2003) but were later modified to best suit M. agalactiae and M. bovis after several pilot experiments. For an optimum transformation frequency (TF), Mycoplasma cells from 1 ml late log-phase culture (~108–109 CFU/ml) were harvested at 10,000g for 20 min at 4 °C and washed three times with chilled electroporation buffer (EB; 272 mM sucrose, 8 mM HEPES, pH 7.4) before final resuspension in 100 μl of chilled EB. Contrary to the general concept of associating better cell competence with early logarithmic growth phase, late log-phase cells gave more consistent and 10-fold higher TF. A DNA concentration of 1–3 μg gave the best results under these conditions, and a 10-fold increase in DNA concentration led to an about 13-fold lower TF, suggesting that an appropriate ratio of DNA to cell number might be critical, as also observed for other bacteria (Dunny et al., 1991). The DNA-cell mixture was allowed to stand on ice for 30 min, transferred to prechilled 2-mm electrocuvettes and then electroporated in a BIO-RAD Gene-PulserRII apparatus. After assessing various electrical settings, the electroporation conditions using 2.5 kV voltage, 25 μF capacitance and 100Ω resistance, were found to be most suitable. No losses in cell viability were observed after mock electroporations under these conditions. This was true for electrocuvettes of both 1- and 2-mm electrode gap, unlike the case with M. pneumoniae where the viability was 10-fold higher with 2-mm electrocuvettes (Hedreyda et al., 1993). Immediately after the electric pulse, 900 μl of chilled SP-4 broth was added and cells were kept on ice for 15 min, and then at 37 °C for 2 h to express the Gm resistance (Gmr) determinant. A 100-μl aliquot of the 106-fold diluted culture was plated on SP-4 plates for viability estimation before adding 50 μg/ml Gm, which was then plated directly on selective media. The plates were examined up to a maximum of 9 days using a stereomicroscope, and single colonies were seeded in 1 ml of SP-4 broth containing 50 μg/ml of Gm for further analysis. Surprisingly, under these conditions a large number of colonies were also observed on selective SP-4 agar plates corresponding to the “No-DNA” mock transformation. These clones failed to grow further in Gm broth due to the lack of Tn4001mod as also revealed by PCR analyses. In different transformations, the percentage of these “pseudoresistant” clones varied between 16% and 86% of the total CFU obtained after actual pISM2062 transformations. This implied that an equivalent number of colonies on selective plates corresponding to the pISM2062 transformations might represent “pseudotransformants”. Attempts to decrease the frequency of such “pseudotransformants” by increasing the Gm concentration in selective agar media failed.
With the goal of overcoming the problem of ‘pseudotransformants’ observed with Gm selection, we decided to test the alternate tetM marker which provides resistance against tetracycline (Dybvig and Cassell, 1987). For this purpose the mini-Tn4001tet seemed to be the most appropriate choice as it is expected to have an additional advantage of stabilizing the Tn4001 insertions by decreasing secondary multiple insertions (Pour-El et al., 2002). However, several attempts to generate M. agalactiae transformants using mini-Tn4001tet were unsuccessful.
Finally, to surmount the problem related to pseudoresistance, the transformation mix was incubated for an additional 10–14 h in Gm broth, following the usual 2-h growth in non-selective media. Results showed that the number of pseudoresistant clones decreased significantly after a 10-h outgrowth in selective medium and represented, on average, a mere 5% of the total transformants. Attempts to shorten this outgrowth time to limit the bias introduced by the multiplication of original transformants for calculating the TF were unsuccessful. Using this procedure, Gmr colonies of M. agalactiae and M. bovis were estimated to occur at frequencies ranging from 1.6 × 10−7 to 3.5 × 10−6 per-viable CFU. Though these TFs may be slightly overestimated due to the possible multiplication of the original clones during the 10 h outgrowth period, data presented here demonstrate for the first time a procedure for introducing foreign DNA into the M. agalactiae and M. bovis genomes. This was confirmed by analyzing individual colonies randomly selected from solid selective media following transformation.
Analyses of transformed clones
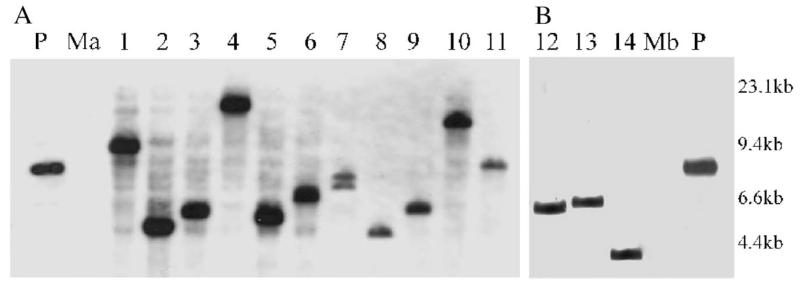
Detailed analyses showed that when replated on increasing Gm concentrations most of the transformants could tolerate at least 100 μg/ml Gm. This resistance correlated with the presence of Tn4001mod as these clones yielded the expected 400-bp Tn-PCR fragment (Fig. 1) using Tn4001-specific primers Tn1 and Tn2 (Winner et al., 2003), while the pseudoresistant clones picked from the “No-DNA” transformation plates were negative in a similar assay (data not shown). Insertion of Tn4001mod in the M. agalactiae and M. bovis genomes was further demonstrated by Southern blot analyses using BamHI/EcoRI-restricted chromosomal DNA and a DIG-labeled probe corresponding to the 400-bp region of the Gmr determinant (Fig. 2). As there is no EcoRI site within Tn4001mod and as BamH1 has a single unique site in one of the IS256 elements of Tn4001mod (Knudtson and Minion, 1993), each hybridization band would correspond to a single Tn4001 insertion. All the analyzed transformants displayed a single Tn4001mod insertion at different chromosomal loci except one that depicted two hybridization signals (Fig. 2A, lane 7), suggesting that either this clone was simultaneously transformed with two copies of pISM2062 or that Tn4001mod was transposed to secondary sites in the chromosome after the initial insertion event. Such multiple insertions and/or replicative transposition was previously observed with Tn4001 (Cao et al., 1994; Pour-El et al., 2002) and Tn916 (Dybvig and Alderete, 1988) in other mycoplasmas, and thought to be due to uncontrolled transposase expression in the heterologous Mycoplasma genetic system. Integration of the entire plasmid in the M. agalactiae/M. bovis genome as described for pAM120 in M. hyorhinis and M. pulmonis (Dybvig and Alderete, 1988) was ruled out by Southern blot analyses using a plasmid-specific probe. This indicates that transposition is the major event responsible for the chromosomal insertion of the Tn4001mod. The pISM2062 plasmid contains an E. coli replicon and is not expected to replicate in M. agalactiae/M. bovis. This was further confirmed by PCR using primer IS256rev (Winner et al., 2003) and by Southern hybridization using the plasmid-specific probe. In none of these experiments the extrachromosomal plasmid was detected, when total genomic DNA of selected transformants was used as target DNA.
Fig. 1.
PCR analysis of M. agalactiae and M. bovis Tn4001mod transformants. Tn4001-specific primers were used to detect the presence of Tn4001 in the gentamicin-resistant clones of M. agalactiae (lanes 1–4) and M. bovis (lanes 5–8) obtained after transformation. Non-transformed M. agalactiae (Ma) and M. bovis (Mb) are shown as negative controls and pISM2062 (P) as positive control. M, DNA size marker.
Fig. 2.
Southern hybridization analysis of transformant DNA of M. agalactiae (A, lanes 1–11) and M. bovis (B, lanes 12–14) double-digested with BamHI/EcoRI and probed with a DIG-labeled fragment corresponding to the 400-bp region within the HindIII fragment of Tn4001. Digested DNA corresponding to non-transformed M. agalactiae (Ma) and non-transformed M. bovis (Mb) served as negative controls and pISM2062 (P) as positive control. DNA size standards are indicated in the right margin.
Stability of the clones
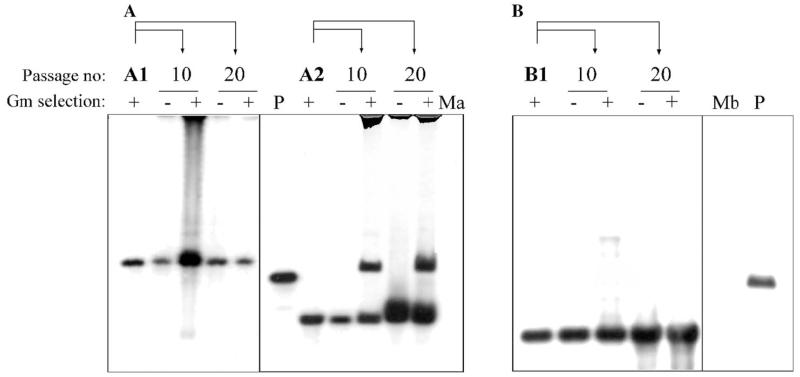
To test the stability of Tn4001 insertions in M. agalactiae/M. bovis, independent transformants were subjected to 20 serial passages in both non-selective and selective broth. Cultures of the 16th and 20th passage showed no significant difference in the total number of CFU, when plated on selective and non-selective plates. As illustrated for three selected clones (Fig. 3), Southern blot analyses of genomic DNA obtained from cultures of the 10th and the 20th passage using the Tn4001-specific probe revealed that the transposon is stable in non-selective broth. Interestingly, before the 10th passage in selective medium one of the M. agalactiae clones (clone A2) underwent a second transposition event resulting in the occurrence of an additional hybridizing fragment as shown in Fig. 3A (lanes corresponding to passage 10 and 20). Individual colonies derived from these two passages were found to harbor the same two insertions, negating the occurrence of a mix of two different mutants. Transposition of the IS element alone – if at all occurring in these mutants – was not assessed.
Fig. 3.
Stability of Tn4001mod insertions in M. agalactiae and M. bovis as revealed by Southern hybridization using a Tn4001-specific probe. Genomic DNA of independent pISM2062 transformants was harvested after 10 or 20 additional passages in SP-4 broth, in presence (+) or absence (−) of Gm. (A) BamHI/EcoRI-digested DNA of non-transformed M. agalactiae (Ma) and its two different pISM2062 transformants, A1 and A2 (the same as depicted in lanes 1 and 5 of Fig. 2A, respectively). (B) BamHI/EcoRI-digested DNA of non-transformed M. bovis (Mb) and its pISM2062 transformant B1 (the same as depicted in lane 14 of Fig. 2B). Digested pISM2062 (P) containing the Tn4001mod served as a positive control in both cases.
Conclusion
The findings of our study have opened the field of molecular genetic studies in these, so far, untransformed and poorly understood pathogens. Tn4001 and/or its Gmr determinant can serve as valuable tool to genetically manipulate M. agalactiae/M. bovis to identify and study the factors involved in their virulence and pathogenicity. For instance, the Vpma family of variable surface lipoproteins identified by our group in M. agalactiae (Glew et al., 2000) is believed to play an important role in immune evasion and rapid adaptation in the host. The presence of a putative xer1 recombinase, which is suspected of controlling the phenotypic switches between different vpma members, and a tRNA-lys (CTT) gene in the vpma locus is typical of a pathogenicity island (Glew et al., 2002). This poses the question of whether Vpmas per se, or switches in Vpma phenotype, contribute to virulence in M. agalactiae. To answer this question, disruption of the recombinase would be critical to generate phase-locked single Vpma expressors whose virulence potential and role in disease prognosis could then be suitably evaluated by in vivo or in vitro studies. These genetic disruptions are now technically possible, as our study provides not only the first reliable transformation protocol but also suggests the use of Tn4001 as a powerful insertional mutagenesis tool.
In conclusion, the current study provides the first report of transformation and transpositional insertion of Tn4001 in M. agalactiae and M. bovis. Overall, data present evidence that these pathogens are amenable to transformation by electroporation and that the Gmr gene and the transposase genes required for Tn4001 transposition are functional in these two Mycoplasma species. This finding makes Tn4001 the first genetic tool available for genetic studies in these two ruminant pathogens, paving the way towards deciphering their mechanisms of pathogenicity.
Acknowledgments
This work was supported in part by grant P14725-GEN (C. Citti and R. Rosengarten) from the Austrian Science Fund (FWF). The authors thank W. Jechlinger for helpful discussion and K. Siebert-Gulle for technical assistance.
References
- Bergonier D, Berthelot X, Poumarat F. Contagious agalactia of small ruminants: current knowledge concerning epidemiology, diagnosis and control. Rev. Sci. Tech. 1997;16:848–873. doi: 10.20506/rst.16.3.1062. [DOI] [PubMed] [Google Scholar]
- Cao J, Kapke PA, Minion FC. Transformation of Mycoplasma gallisepticum with Tn916, Tn4001, and integrative plasmid vectors. J. Bacteriol. 1994;176:4459–4462. doi: 10.1128/jb.176.14.4459-4462.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez Gonzalez YR, Ros Bascunana C, Bolske G, Mattsson JG, Fernandez Molina C, Johansson KE. In vitro amplification of the 16S rRNA genes from Mycoplasma bovis and Mycoplasma agalactiae by PCR. Vet. Microbiol. 1995;47:183–190. doi: 10.1016/0378-1135(95)00058-i. [DOI] [PubMed] [Google Scholar]
- Dunny GM, Lee LN, LeBlanc DJ. Improved electroporation and cloning vector system for gram-positive bacteria. Appl. Environ. Microbiol. 1991;57:1194–1201. doi: 10.1128/aem.57.4.1194-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K, Alderete J. Transformation of Mycoplasma pulmonis and Mycoplasma hyorhinis: transposition of Tn916 and formation of cointegrate structures. Plasmid. 1988;20:33–41. doi: 10.1016/0147-619x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Dybvig K, Cassell GH. Transposition of gram-positive transposon Tn916 in Acholeplasma laidlawii and Mycoplasma pulmonis. Science. 1987;235:1392–1394. doi: 10.1126/science.3029869. [DOI] [PubMed] [Google Scholar]
- Franke AE, Clewell DB. Evidence for conjugal transfer of a Streptococcus faecalis transposon (Tn916) from a chromosomal site in the absence of plasmid DNA. Cold Spring Harb. Symp. Quant. Biol. 1981;45:77–80. doi: 10.1101/sqb.1981.045.01.014. [DOI] [PubMed] [Google Scholar]
- Glew MD, Papazisi L, Poumarat F, Bergonier D, Rosengarten R, Citti C. Characterization of a multigene family undergoing high-frequency DNA rearrangements and coding for abundant variable surface proteins in Mycoplasma agalactiae. Infect. Immun. 2000;68:4539–4548. doi: 10.1128/iai.68.8.4539-4548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew MD, Marenda M, Rosengarten R, Citti C. Surface diversity in Mycoplasma agalactiae is driven by site-specific DNA inversions within the vpma multigene locus. J. Bacteriol. 2002;184:5987–5998. doi: 10.1128/JB.184.21.5987-5998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan PC. Antibiotic susceptibility of Mycoplasma fermentans strains from various sources and the development of resistance to aminoglycosides in vitro. J. Med. Microbiol. 1995;42:421–428. doi: 10.1099/00222615-42-6-421. [DOI] [PubMed] [Google Scholar]
- Hannan PC, Windsor GD, de Jong A, Schmeer N, Stegemann M. Comparative susceptibilities of various animal-pathogenic mycoplasmas to fluoroquinolones. Antimicrob. Agents Chemother. 1997;41:2037–2040. doi: 10.1128/aac.41.9.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreyda CT, Lee KK, Krause DC. Transformation of Mycoplasma pneumoniae with Tn4001 by electroporation. Plasmid. 1993;30:170–175. doi: 10.1006/plas.1993.1047. [DOI] [PubMed] [Google Scholar]
- King KW, Dybvig K. Transformation of Mycoplasma capricolum and examination of DNA restriction modification in M. capricolum and Mycoplasma mycoides subsp. mycoides. Plasmid. 1994;31:308–311. doi: 10.1006/plas.1994.1033. [DOI] [PubMed] [Google Scholar]
- Knudtson KL, Minion FC. Construction of Tn4001lac derivatives to be used as promoter probe vectors in mycoplasmas. Gene. 1993;137:217–222. doi: 10.1016/0378-1119(93)90009-r. [DOI] [PubMed] [Google Scholar]
- Lyon BR, May JW, Skurray RA. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol. Gen. Genet. 1984;193:554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Minion FC, Kapke PA. Transformation of mycoplasmas. Methods Mol. Biol. 1998;104:227–234. doi: 10.1385/0-89603-525-5:227. [DOI] [PubMed] [Google Scholar]
- Nicholas RA, Ayling RD. Mycoplasma bovis: disease, diagnosis, and control. Res. Vet. Sci. 2003;74:105–112. doi: 10.1016/s0034-5288(02)00155-8. [DOI] [PubMed] [Google Scholar]
- Pour-El I, Adams C, Minion FC. Construction of mini-Tn4001tet and its use in Mycoplasma gallisepticum. Plasmid. 2002;47:129–137. doi: 10.1006/plas.2001.1558. [DOI] [PubMed] [Google Scholar]
- Rosengarten R, Citti C. The role of ruminant mycoplasmas in systemic infection. In: Stipkovits L, Rosengarten R, Frey J, editors. Mycoplasmas of Ruminants, Pathogenicity, Diagnostics, Epidemiology and Molecular Genetics. Vol. 3. European Commission; Brussels: 1999. pp. 14–17. [Google Scholar]
- Rosengarten R, Behrens A, Stetefeld A, Heller M, Ahrens M, Sachse K, Yogev D, Kirchhoff H. Antigen heterogeneity among isolates of Mycoplasma bovis is generated by high-frequency variation of diverse membrane surface proteins. Infect. Immun. 1994;62:5066–5074. doi: 10.1128/iai.62.11.5066-5074.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A laboratory manual. second ed Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Solsona M, Lambert M, Poumarat F. Genomic, protein homogeneity and antigenic variability of Mycoplasma agalactiae. Vet. Microbiol. 1996;50:45–58. doi: 10.1016/0378-1135(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Bergonier D, Poumarat F, Capaul S, Schlatter Y, Nicolet J, Frey J. Species identification of Mycoplasma bovis and Mycoplasma agalactiae based on the uvrC genes by PCR. Mol. Cell. Probes. 1998;12:161–169. doi: 10.1006/mcpr.1998.0160. [DOI] [PubMed] [Google Scholar]
- Tully JG. Culture medium formulation for primary isolation and maintenance of mollicutes. In: Razin S, Tully JG, editors. Molecular and Diagnostic Procedures in Mycoplasmology. Vol. 1. Academic Press; San Diego: 1995. pp. 33–39. [Google Scholar]
- Winner F, Markova I, Much P, Lugmair A, Siebert-Gulle K, Vogl G, Rosengarten R, Citti C. Phenotypic switching in Mycoplasma gallisepticum hemadsorption is governed by a high-frequency, reversible point mutation. Infect. Immun. 2003;71:1265–1273. doi: 10.1128/IAI.71.3.1265-1273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]