Abstract
OBJECTIVE
To identify possible mechanisms linking obesity in pregnancy to increased fetal adiposity and growth, we developed a unique mouse model of maternal obesity associated with fetal overgrowth and tested the hypothesis that maternal obesity causes up-regulation of placental nutrient transporter expression and activity.
METHODS
C57BL/6J female mice were fed a control (C) or a high fat/high sugar (HF/HS) pelleted diet supplemented by ad libitum access to sucrose (20%) solution, mated and studied at embryonic day 18.5.
RESULTS
HF/HS diet increased maternal fat mass by 2.2-fold (p<0.01) and resulted in glucose intolerance with normal fasting glucose. Maternal circulating insulin, leptin, and cholesterol were increased (p<0.05) whereas total and high molecular weight (HMW) adiponectin were decreased (p<0.05). HF/HS diet increased fetal weight (+18%, p=0.0005). In trophoblast plasma membrane (TPM) isolated from placentas of HF/HS fed animals, protein expression of glucose transporter (GLUT) 1 and 3, sodium-coupled neutral amino acid transporter (SNAT) 2 and large neutral amino acid transporters 1 (LAT1) was increased. TPM System A and L amino acid transporter activity was increased in the HF/HS group.
CONCLUSION
Up-regulation of specific placental nutrient transporter isoforms may constitute a mechanism underlying fetal overgrowth in maternal obesity.
Keywords: Fetal growth, maternal-fetal exchange, amino acids, glucose, pregnancy, mice, obese
INTRODUCTION
Babies born to obese mothers often have increased fat mass and/or birth weight and may be insulin resistant at birth (1). These metabolic disturbances increase the risk for obesity, high triglycerides, low HDL cholesterol, high blood pressure and elevated fasting glucose in childhood and development of T2DM and CVD in adult age (2). Thus, mothers with a high body mass index that give birth to heavier daughters, who are at increased risk to be obese themselves during their reproductive years, propagate a vicious, detrimental cycle of intrauterine transmission of obesity from the mother to her children (3). The strong association between maternal obesity and metabolic syndrome in childhood is of particular concern because almost 2/3 of American women of reproductive age are either overweight or obese (4) with similar trends around the world including the United Kingdom where one in five pregnant woman is obese (5).
The mechanisms underlying increased fetal adiposity and/or growth in maternal obesity are poorly understood, however fetal growth and development is critically dependent on nutrient supply, which is intimately related to placental transport of nutrients. Placental transport of glucose occurs via facilitated diffusion mediated by Glucose Transporters (GLUTs), which transport glucose down its concentration gradient. System A is a Na+-dependent transporter mediating the cellular uptake of non-essential neutral amino acids. System L is a Na+-independent exchanger mediating transport of essential amino acids. The System L amino acid transporter is a heterodimer, consisting of a light chain, typically LAT 1 (SLC7A5) or LAT2 (SLC7A8), and a heavy chain, 4F2hc/CD98 (SLC3A2). Fetal overgrowth has been reported to be associated with up-regulation of placental nutrient transporters in some studies but not all (6, 7). Specifically, placental glucose transporter activity and protein expression were found to be increased in women with type-1 diabetes and giving birth to large babies (8). Furthermore, the activity of placental System A and L amino acid transporters is up-regulated in diabetes associated with fetal overgrowth (7). We recently reported that System A activity and SNAT 2 protein expression were increased in syncytiotrophoblast microvillous plasma membranes isolated from obese women giving birth to large babies (9).
Maternal obesity is associated with increased serum levels of lipids (10) and growth factors such as insulin (1, 11), leptin (1, 11) and pro-inflammatory cytokines (1, 10) and lower serum adiponectin (11) as compared to pregnant women with a healthy weight as classified by WHO (BMI 18.5-25). Maternal metabolic hormones are key regulators of placental nutrient transport (12). For example, IGF-I, insulin (13), leptin (13) and pro-inflammatory cytokines (14) stimulate whereas adiponectin inhibits placental amino acid transporter activity by inhibiting insulin/IGF-1 signaling (15). Changes in maternal metabolism and hormone levels in obesity may therefore have profound effects on placental function, resulting in altered nutrient delivery to the fetus.
To explore the mechanisms by which exposure to the abnormal metabolic environment of obese mothers impact fetal development and leads to metabolic syndrome in the adult offspring, a large number of animal models have been developed in rodents (16), sheep (17), and non-human primates (18) by feeding a diet rich in fat and/or sugar prior to and/or during pregnancy. Many of these models, however, fail to reproduce key aspects of the human condition, raising questions as to their relevance for obese pregnant women. In particular, very few of these models have been able to replicate fetal overgrowth (19, 20), which is common in human pregnancy. In addition, very little is known about the impact of maternal obesity on placental function in these models. We have previously established a model where female mice were fed a high fat diet before and during pregnancy, resulting in fetal overgrowth (21), however dams were not obese. Our current approach was to use a highly palatable western diet in the form of pellets complemented with a sucrose solution to induce maternal obesity. Thus, mice were fed a diet high in saturated fat, cholesterol and simple sugars, resembling a diet common in Western societies.
The aim of this study was to develop a mouse model of maternal obesity, resulting in fetal overgrowth and associated with maternal metabolic alterations similar to that observed in the pregnant woman with increased BMI. Once established, we used the model to test the hypothesis that maternal obesity causes up-regulation of placental nutrient transporter expression and activity.
MATERIALS AND METHODS
Animals and diets
The Institutional Animal Care and Use Committee at the University of Texas Health Science Center San Antonio approved all protocols. Female C57BL/6J mice (n=80), proven breeders (one previous litter) and approximately 12 weeks old (The Jackson Laboratory, Bar Harbor, ME, USA) were housed 5 per cage under controlled conditions (25°C, 12-hour light/dark cycle). Starting at 13 weeks of age, animals were fed ad libitum with a control (D12489B, 10.6 kcal% fat) or high fat pellet diet (Western Diet D12079B, 41 kcal% fat) supplemented with ad libitum access to sucrose (20 %) solution (High Fat/High Sugar, HF/HS). The sucrose solution was supplemented with vitamins (Vitamin Mix V10001, 10 gm/ 4000 kcal) and minerals (Mineral Mix S10001, 35 gm/ 4000 kcal). Diets were purchased from Research Diets (New Brunswick, NJ, USA). All animals had free access to water. Daily food intake was determined by weighing remaining food at the end of each week and used to calculate daily caloric intake. Consumption of sucrose solution (20%) by the HF/HS group was recorded every day. When females on the HF/HS diet had increased 25% in body weight they, and age-matched females on control diet, were mated with males on control diet. The presence of a plug represented embryonic day (E) 0.5 and dams were maintained on the respective diets throughout gestation. At E18.5, dams were euthanized for collection of blood and tissue samples or animals were anesthetized and a glucose tolerance test was performed.
Measurement of body composition
When females on the HF/HS diet had increased 25% in body weight dual-energy X-ray absorptiometry (DEXA) and whole body quantitative magnetic resonance imaging (qMRI) were carried out in a subgroup of animals to determine pre-pregnancy body composition in the non-pregnant state. Estimates of precision for these and other key analyses used in this study are provided in Supplemental materials. For quantitative magnetic resonance measurements, live mice were placed into a thin-walled plastic cylinder (4.7 cm ID, 0.15 cm thick), where they were free to turn around but were limited to 4-cm vertical movements by a plastic insert. The plastic cylinder containing the live mice was then placed into the qMRI machine (EchoMRI, Echo Medical Systems, Houston, TX) for measurement of lean and fat mass. DEXA measurements were performed using Lunar PIXImus mouse bone densitometer (GE, Madison, WI) and data were analyzed with PIXImus software. Animals subjected to qMRI or DEXA were euthanized following the procedure.
Collection of tissue and blood samples
Dams were fasted (4 hr) and then euthanized at E18.5 by carbon dioxide inhalation. Maternal blood was collected by cardiac puncture, allowed to clot, and spun at 4000 rpm to collect serum and snap-frozen for subsequent analysis. After laparotomy, fetuses and placentas were collected and quickly dried on blotting paper, any remaining fetal membranes were removed, and fetuses and placentas were weighed. All placentas in each litter (approximate weight 0.5 g) were pooled and washed in phosphate buffer saline and transferred to 3 ml of buffer D [250 mM sucrose, 10 mM Hepes-Tris, and 1 mM EDTA (pH 7.4) at 4°C], protease and phosphatase inhibitor cocktail (Sigma-Aldrich Corp., St. Louis, MO, USA) was added at a dilution of 1:1000, and the mixture was homogenized using a Polytron (Kinematica, Bohemia, NY, USA), frozen in liquid nitrogen, and stored at –80° C until analysis.
Maternal serum biochemical analysis
Maternal serum analyses of total adiponectin, leptin, (Millipore, St Charles, Missouri), insulin and multimeric high-molecular weight (HMW) adiponectin (Alpco Diagnostics, Salem, NH, USA) were performed using commercially available ELISA kits. Maternal serum cholesterol, phospholipids, non-esterified fatty acids and triglycerides were analyzed by the Mouse Metabolic Phenotyping Center at the University of Cincinnati.
Glucose tolerance test
Glucose tolerance tests were performed in a subset of mice on E18.5 after a 4-h fast and under isoflurane anesthesia. Mice were injected i.p. with glucose (2 g/kg body weight), and blood (5 μl) was sampled in triplicate from the tail vein at 0, 15, 30, 60, and 90 min after the glucose load. Blood glucose was measured in triplicate immediately using a One Touch Ultra-2 (Life Scan Inc, Milpitas, CA, USA).
Isolation of trophoblast plasma membranes (TPM)
TPM were isolated from frozen placental homogenates using differential centrifugation and Mg2+ precipitation as described (21, 22). This protocol results in the isolation of the maternal-facing plasma membrane of trophoblast layer II of mouse placenta, which is believed to be functionally analogous to the syncytiotrophoblast microvillous plasma membrane of the human placenta (22). Protein concentration was determined using the Lowry assay (Bio Rad, CA) and TPM purity was assessed by TPM/homogenate enrichments of alkaline phosphatase activity. The average alkaline phosphatase enrichment for TPM vesicles isolated from control placentas (25.5 ± 5.2, n=5) was not significantly different from the enrichment in TPM vesicles obtained from placentas of HF/HS animals (22.3± 7.2, n=5).
TPM amino acid transporter activity measurements
The activity of System A and L amino acid transporters in TPM was determined using radiolabelled amino acids and rapid filtration techniques as previously described (22). Na+-dependent uptake of MeAIB (corresponding to system A activity) was calculated by subtracting Na+-independent uptakes from total uptakes. For leucine, mediated uptake was calculated by subtracting non-mediated transport, as determined in the presence of 20 mM unlabelled leucine, from total uptake. The intra-assay coefficient of variation for System A and L was 10 and 9.5%, respectively.
Western blot analysis
We determined TPM protein expression of the System A amino acid transporter isoforms (SNAT) 2 and 4, the System L amino acid transporter isoforms LAT1 and LAT2 and CD98, the heavy chain associated with LAT1 and 2. A polyclonal SNAT2 antibody generated in rabbits was received as a generous gift from Dr V. Ganapathy and Dr P. Prasad at the University of Georgia, Augusta. GLUT1 and GLUT3 antibody was purchased from Millipore (Billerica, MA). Western blotting was carried out as previously described (23). Analysis of the blots was performed by densitometry.
Data presentation and statistics
Data are presented as means + SEM or ± SEM. For fetal and placental data, means of each litter were calculated and used in statistical analysis. Thus, n represents the number of litters. Statistical significance of differences between control and HF/HS groups was assessed using Student's unpaired t test. A P value <0.05 was considered significant.
RESULTS
Body composition in female mice prior to mating
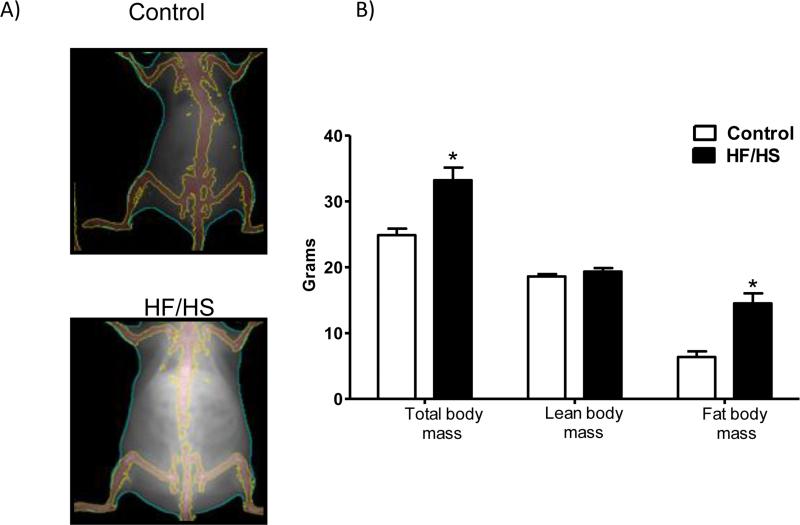
Feeding mice HF/HS diet for 4-6 weeks resulted in obesity as illustrated by measurement of body composition prior to mating using DEXA and qMRI (Fig 1). As shown in Fig 1B, maternal fat tissue mass was increased by 2.2-fold (n=5, p<0.01) in animals fed HF/HS diet as compared to control. However, lean tissue mass was comparable between HF/HS and control group.
Figure 1.
Body composition of mice fed a control or high fat/high sugar diet (HF/HS). When females on the HF/HS diet had increased 25% in body weight dual-energy X-ray absorptiometry (DEXA, A) and whole body quantitative magnetic resonance imaging (qMRI, B) was performed (n=5/group). Values are given as means + SEM; *P < 0.05 vs. control; unpaired Student's t-test.
Food and calorie intake in pregnant mice
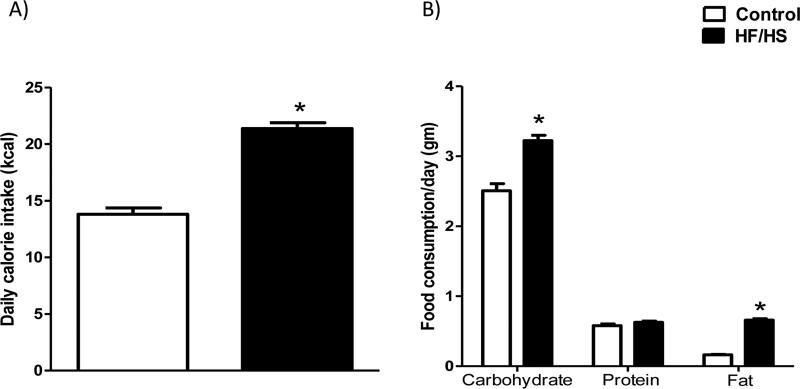
Pregnant mice fed a HF/HS diet had a higher daily calorie intake (+55 %, n=15, p<0.0001) than those fed a control diet (Fig 2A). As shown in Fig 2B, HF/HS fed mice had an increased intake of carbohydrates (+1.3-fold, p<0.001, n=15) and fat (+4-fold, p<0.0001, n=15). Notably, protein intake was not significantly altered in HF/HS fed animals.
Figure 2.
Daily calorie (A) and food intake (B) in pregnant mice fed control (n=15) or HF/HS (n=15) diet. Data was collected by weighing the remaining pelleted food at the end of each of the following gestational periods: E 0.5 to 6.5, E 7.5 to 13.5 and E 14.5 to 17.5. In addition, the amount of consumed sugar was determined every day at the time of refreshing the sucrose water. This information was used to calculate the average daily food and calorie intake for the entire gestation period. Values are given as means + SEM; *P < 0.05 vs. control; unpaired Student's t-test.
Maternal endocrine and metabolic profile at E18.5
At E18.5, maternal fasting serum leptin and insulin levels were significantly increased by 44% (p=0.01) and 3.6-fold respectively (p=0.0001) , in animals fed the HF/HS diet (Table 1). Total maternal serum adiponectin levels were reduced by 11.5 % (p=0.05, Table 1) and multimeric HMW adiponectin levels were decreased by 58 % (p=0.0001, Table 1) in HF/HS group as compared to control. Furthermore, maternal serum levels of cholesterol (p<0.0002) and phospholipids (p<0.0002) were elevated at E18.5 in animals fed a HF/HS group as compared to controls (Table 1). However, maternal fasting triglyceride, non-esterified fatty acids and glucose levels were comparable between the HF/HS and control groups.
Table 1.
The maternal endocrine and metabolic profile at E18.5.
| Analysis | Control Diet | HF/HS Diet | p-value |
|---|---|---|---|
| Leptin (ng/ml) | 22.7 ± 2.6 | 32.6 ± 2.8 | 0.01 |
| Insulin (ng/ml) | 0.21 ± 0.06 | 0.76 ± 0.12 | 0.0001 |
| Adiponectin (μg/ml) | 21.8 ± 0.97 | 19.2 ± 0.78 | 0.05 |
| HMW adiponectin (μg/ml) | 16.6 ± 1.4 | 6.9 ± 0.5 | 0.0001 |
| Glucose (mg/dl) | 146 ± 7.8 | 143± 7.6 | 0.8 |
| Cholesterol (mg/dl) | 44.3 ± 2.8 | 65.6 ± 4.5 | 0.0002 |
| NEFA (mEq/l) | 0.84 ± 0.06 | 1.0 ± 0.06 | 0.08 |
| Phospholipids (mg/dl) | 72.5 ± 5.9 | 117.3 ± 9.5 | 0.0002 |
| Triglycerides (mg/dl) | 46 ± 3.2 | 52 ± 3.9 | 0.40 |
Fasting (4 hours) serum samples at E18.5, n = 18-20 in each group; Values are means ± SEM, unpaired Student's t-test. NEFA; Non-esterified fatty acids. Data were tested for normal distribution (Shapiro Wilk test) and insulin, NEFA and TGs were found to deviate from normal distribution. Therefore, this data was log transformed, which made the data normally distributed, and Students t-test was performed on the log transformed data.
Maternal glucose tolerance at E18.5
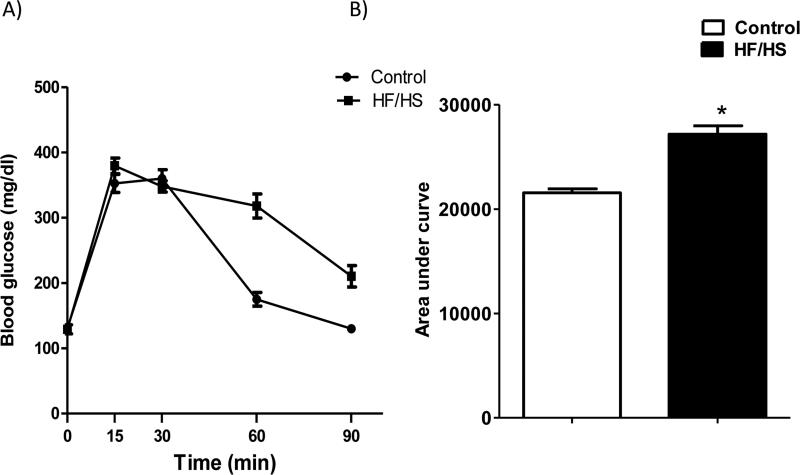
As shown in Fig 3, HF/HS diet fed pregnant mice were glucose-intolerant as determined by an increased area under the blood-glucose curve (+28%, n=4-5, p<0.01) following an intraperitoneal glucose injection at E18.5.
Figure 3.
Maternal glucose tolerance test (A) at E18.5 after a 4-hour fast in mice fed a control or HF/HS diet. An i.p. injection of 2 g/kg of a 30% glucose solution was given, and blood glucose was determined in samples obtained from the tail vein 0-90 min after glucose administration. Histogram (B) summarize the area under curve of GTT. Values are means ± SEM; n= 4 (Control) and 5 (HF/HS). *P < 0.05 vs. control; unpaired Student's t-test.
Fetal and placental weight and litter size at E18.5
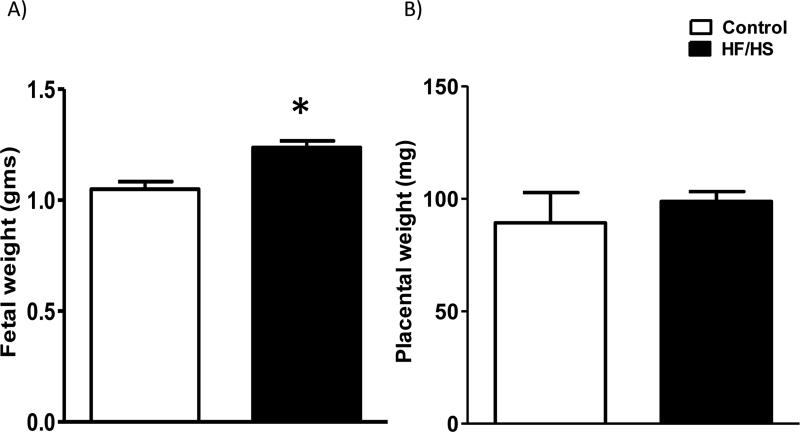
Fetal weights were increased by 18% (p<0.01; n=12 in each group) at E18.5 in the HF/HS group (Fig 4). This was not due to a difference in litter size, which was essentially the same in the control (6.9 ± 0.14, n=12) and obese groups (6.8 ± 0.32, n=12). Placental weights were not different between groups (Fig 4).
Figure 4.
Fetal (A) and placental (B) weights determined at E18.5 in mice fed a control and HF/HS diet. Values are means + SEM, n= 12 in each group, *P < 0.05 vs. control; unpaired Student's t-test.
TPM nutrient transporter isoform expression
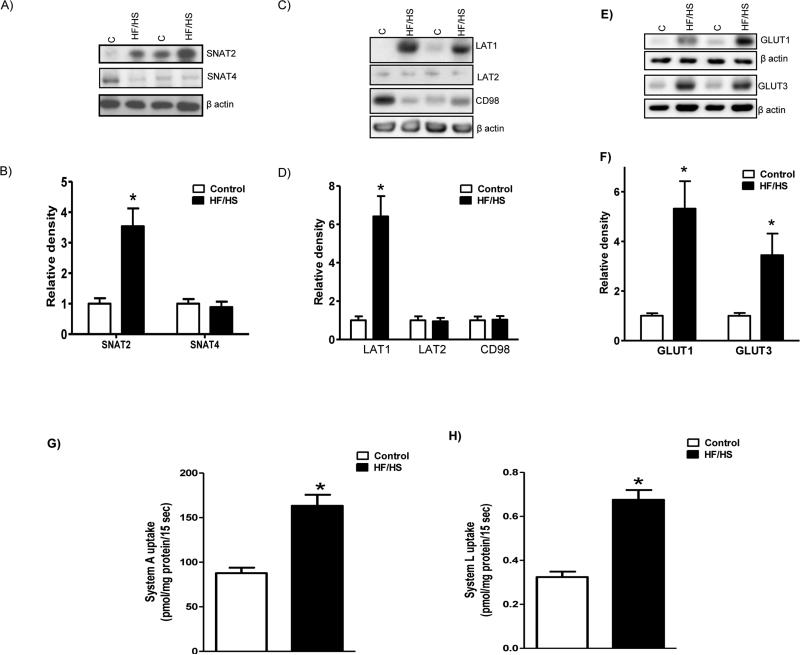
Protein expression of SNAT2 in TPM isolated from placentas of HF/HS diet fed mice was increased (+3.5-fold, p<0.05) compared to controls (n=6; Figure 5A and B); however, no changes could be observed in SNAT4 expression levels (n=6; Figure 5A and B). Feeding a HF/HS diet significantly increased the expression of the System L transporter isoform LAT1 (+6.4 fold, p<0.05) in placental TPM (n=6; Figure 5C and D). In contrast, TPM expression of LAT2 and CD98 were comparable between control and HF/HS group. Furthermore, protein expression of GLUT1 and GLUT3 in TPM isolated from placentas of HF/HS diet fed mice was increased (GLUT-1, +5.3 fold; GLUT3, +3.4 fold, n=6; p<0.05) as compared to control (Figure 5E and F).
Figure 5.
TPM nutrient transporter isoform expression (A-F) and activity (G-H). A and B). Protein expression of System A amino acid transporter isoforms in TPM. Representative Western blots for (A) SNAT2 and 4 in TPM isolated from control and HF/HS diet fed animals at E 18.5. Histogram (B) summarizes the Western blotting data from control (C, n = 6) and HF/HS (n = 6). Equal loading was performed. After normalization to β-actin, the mean density of C samples was assigned an arbitrary value of 1. Values are given as means + SEM; *P < 0.05 vs. control; unpaired Student's t-test.
C and D). Protein expression of System L amino acid transporter isoforms in TPM. Representative Western blots for (C) LAT1, LAT2 and CD98 in TPM isolated from placentas of controls and HF/HS diet fed mice at E 18.5. Histogram (D) summarizes the Western blotting data from control (C, n = 6) and HF/HS (n = 6). Equal loading was performed. After normalization to β-actin, the mean density of C samples was assigned an arbitrary value of 1. Values are given as means + SEM; *P < 0.05 vs. control; unpaired Student's t-test.
E and F). Protein expression of glucose transporter isoforms in TPM. Representative Western blots for (E) GLUT1 and GLUT3 in TPM isolated from placentas of control and HF/HS diet fed mice at E 18.5. Histogram (F) summarizes the Western blotting data from control (C, n = 6) and HF/HS (n = 6). Equal loading was performed. After normalization to β-actin, the mean density of C samples was assigned an arbitrary value of 1. Values are given as means + SEM; *P < 0.05 vs. control; unpaired Student's t-test.
G and H). TPM system A and L activity. System A transport activity (G) was determined as sodium dependent [14C]-MeAIB uptake, and System L transport (H) was measured as [3H]-L-leucine uptake in isolated TPM vescicles. MeAIB, 2-Methylaminoisobutyric acid; Leu, Leucine. Values are given as means + SEM; n=6/group; *P < 0.05 vs. control; unpaired Student's t-test.
TPM nutrient transport activity
TPM system A activity was increased by 1.9-fold (p<0.05) in the HF/HS group compared with control (Figure 5G). Similarly, system L activity was 2.1-fold (p<0.05) higher in the HF/HS group as compared to control (Figure 5H).
DISCUSSION
Babies born to overweight and obese mothers are often large at birth and have an increased risk of developing obesity, diabetes and cardiovascular disease in childhood or in adult life, however the underlying mechanisms are largely unknown. A lack of relevant animal models has hampered progress in this area. Here, we report a novel mouse model of maternal obesity with fetal overgrowth and a maternal endocrine and metabolic profile similar to that of obese pregnant women. The most significant novel findings in this study are that maternal obesity increased (i) the protein expression of specific isoforms of the system A and system L amino acid transporters and glucose transporters (ii) the activity of the system A and L amino acid transport systems in the placental barrier. We propose that an increased placental transfer of nutrients constitutes one of the mechanisms underlying fetal overgrowth in pregnancies complicated by overweight or obesity.
In the current study, HF/HS fed mice had an increased calorie intake in the form of carbohydrates and fat. Although assessments of dietary intake in human pregnancy suffer from considerable under-reporting among overweight and obese women (24), the high calorie intake from fat and carbohydrates in our HF/HS fed mice resembles the diet in overweight/obese women (25), contributing to its relevance for the human condition. Notably, protein intake was not significantly reduced in HF/HS fed animals. This is important because protein malnutrition is common in models of diet-induced obesity and it is well established that maternal protein deficiency in pregnancy results in IUGR (23) and programs the offspring for later metabolic and cardiovascular disease.
Maternal serum leptin and insulin levels were increased and total and HMW adiponectin decreased in HF/HS pregnant mice at E 18.5, consistent with observations in obese pregnant women (11, 26). The markedly higher fasting serum insulin levels in HF/HS dams suggest that this model replicates the insulin resistance characteristic for obese pregnant women (27). Fasting glucose was not elevated in the HF/HS group demonstrating that obese mice are not diabetic. However, they were glucose-intolerant as determined by an increased area under the blood-glucose curve following an intraperitoneal glucose injection. Any degree of abnormal glucose homeostasis in pregnancy (i.e., not just GDM) independently predicts glucose intolerance in the postpartum period (28). The importance of maternal glucose control in determining fetal growth and adiposity was highlighted in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 pregnant women, in which a linear relationship between the maternal plasma glucose following a oral glucose tolerance test and birth weight was observed, even amongst non-diabetic women (29). Furthermore, infant adiposity as determined by the sum of skin-fold thickness, exhibited a similar strong linear relationship with maternal glycemic control (29).
Maternal serum levels of cholesterol and phospholipids were elevated in the HF/HS pregnant mice in general agreement with the metabolic phenotype of the obese pregnant woman (10). Collectively, these data demonstrate that the maternal metabolic phenotype of our novel mouse model resembles the metabolic profile of obese non-diabetic pregnant women.
Fetal growth is critically dependent on the capacity of the placenta to transport nutrients. A significant body of evidence demonstrates that the activity of key placental amino acid transporters is decreased in human IUGR (30) and both placental amino acid and glucose transporters have been reported to be up-regulated in fetal overgrowth. SNAT2 expression in TPM but not SNAT 4 was up-regulated in HF/HS group. These findings are in agreement with other studies of SNAT isoform regulation in the placenta, suggesting that SNAT2 is a highly regulated isoform (23). We recently reported that the activity of the System A amino acid transporter and the protein expression of SNAT2 are increased in placentas of obese Swedish women delivering large babies (9), supporting the relevance of our mouse model. In addition, System L amino acid transporter isoforms LAT1 but not LAT2 and CD98 expression in placental TPM was up regulated in HF/HS group as compared to control.
Glucose is the primary energy substrate for the placenta and the fetus and fetoplacental glucose needs are met entirely by transfer from the maternal circulation (31). In late pregnancy GLUT1 (SLC2A1) is the primary placental glucose transporter in the human (32) whereas both GLUT1 and GLUT3 (SLC2A3) mediate placental glucose transport in the rodent (33). GLUT3 makes a greater contribution to total glucose transporter expression in the placenta as gestation proceeds (34). Interestingly, placental TPM GLUT1 and 3 protein expression was significantly increased in HF/HS group. Fetal glucose stimulates the secretion of pancreatic insulin and IGF-I, the two primary fetal growth hormones, providing a direct link between fetal glucose availability and fetal growth (35). In addition, glucose is readily converted to fat and contributes to fetal fat accumulation, consistent with the possibility that increased glucose availability could increase fetal adiposity.
We have previously reported that female C57/BL6 mice fed a high-fat diet before and during pregnancy did not develop obesity (21). However, high fat diet alone stimulated fetal growth and increased placental protein expression of glucose transporter-1 and sodium-coupled neutral amino acid transporter-2 in mice (21). In the present study, feeding mice a high fat and high sugar diet during preconception and conception resulted in maternal obesity and caused fetal overgrowth. Furthermore, maternal protein malnutrition in the rat results in down-regulation of placental amino acid transporters and IUGR in late gestation. The increase in placental TPM nutrient transporter isoform expression and fetal growth in response to a HF/HS diet in our mouse model resembles our previous reports in pregnant women in which fetal overgrowth in women with type 1 diabetes was associated with increased glucose transporter activity and GLUT 1 expression and increased system A amino acid transporter activity in isolated syncytiotrophoblast plasma membranes (6, 8). Collectively, these reports and the current study suggest that the expression of placental nutrient transporters is positively correlated with fetal growth, consistent with the possibility that changes in placental nutrient transport directly contribute to altered fetal growth and programming of metabolic disease in the offspring.
Almost one third of women of reproductive age are obese with significant socioeconomic costs directly attributable to the increased risk of maternal and neonatal complications (36). Life-style changes and anti-obesity drugs result in only modest weight reduction in obese individuals (37) and bariatric surgery is the only treatment with well-documented long-term weight loss (38). However, surgical treatment is a realistic option only for a small fraction of obese individuals. There is, therefore, an immediate need for novel approaches to prevent and/or treat obesity. Measures to alleviate fetal overgrowth and/or adiposity in obese women represent an early intervention strategy that could contribute to a decrease in the prevalence of obesity and diabetes in future generations. Thus, it is essential that a new model of maternal obesity is developed in the mouse, because it is the only species that currently allows systematic cause-and-effect studies of the molecular mechanisms underlying programming of metabolic syndrome in offspring of obese mothers. As with all animal studies, extrapolation of findings in this mouse model to obese pregnant women has to be done with caution. For example, the mouse has a large litter and there are significant structural differences in the placentas of humans and mice introducing some limitations in using the mouse as a model for human pregnancy. However, the extensive functional similarities between the two species (39) suggest that this novel mouse model of maternal obesity will be a valuable tool to advance our understanding of regulation placental function and fetal growth in pregnancies complicated by obesity.
Supplementary Material
There is an association between maternal obesity, large size at birth and metabolic syndrome in childhood. This creates a vicious, detrimental cycle of intrauterine transmission of obesity from the mother to her children. The mechanisms by which exposure to the abnormal metabolic environment of obese mothers leads to fetal overgrowth and metabolic disease in the offspring are not well understood.
We developed a novel mouse model of maternal obesity by feeding a diet high in saturated fat, cholesterol and simple sugars, resembling a diet common in Western societies. This resulted in fetal overgrowth associated with maternal metabolic alterations similar to that observed in the pregnant woman with increased BMI.
The protein expression of specific glucose and amino acid transporter isoforms and amino acid transport activity were markedly elevated in placentas of obese dams, suggesting that increased placental nutrient transport contributes to fetal overgrowth in response to maternal obesity.
Acknowledgements
This study was supported by grants from NIH (OD016724). The analysis of serum lipids was performed by the Mouse Metabolic Phenotyping Center (DK59630) at University of Cincinnati.
Footnotes
Conflict of interest: The authors declare no conflict of interest
References
- 1.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet. 2011;2:27. doi: 10.3389/fgene.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano PM. Obesity and pregnancy - the propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88:3505–3506. doi: 10.1210/jc.2003-031046. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 5.Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989-2007. International journal of obesity. 2010;34:420–428. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- 6.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- 7.Kuruvilla AG, D'Souza SW, Glazier JD, Mahendran D, Maresh MJ, Sibley CP. Altered activity of the system A amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J Clin Invest. 1994;94:689–695. doi: 10.1172/JCI117386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol. 1999;180:163–168. doi: 10.1016/s0002-9378(99)70169-9. [DOI] [PubMed] [Google Scholar]
- 9.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98:105–113. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 11.Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Hulthen-Rossander L, Powell TL, et al. Maternal hormones linking maternal bodymass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87:1743–1749. doi: 10.1093/ajcn/87.6.1743. [DOI] [PubMed] [Google Scholar]
- 12.Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport. A review. Placenta. 2007;28:763–774. doi: 10.1016/j.placenta.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88:1205–1211. doi: 10.1210/jc.2002-021332. [DOI] [PubMed] [Google Scholar]
- 14.Jones HN, Jansson T, Powell TL. IL-6 stimulates System A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell. 2009;297:C1228–1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 15.Jones HN, Jansson T, Powell TL. Full length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes. 2010;59:1161–1170. doi: 10.2337/db09-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55:76–82. doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Long NM, Hein SM, Ma Y, Nathanielsz PW, Ford SP. Maternal obesity in ewes results in reduced fetal pancreatic beta-cell numbers in late gestation and decreased circulating insulin concentration at term. Domest Anim Endocrinol. 2011;40:30–39. doi: 10.1016/j.domaniend.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456–2464. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuyama H, Hiramatsu Y. Treatment with constitutive androstane receptor ligand during pregnancy prevents insulin resistance in offspring from high-fat diet-induced obese pregnant mice. Am J Physiol Endocrinol Metab. 2012;303:E293–300. doi: 10.1152/ajpendo.00167.2012. [DOI] [PubMed] [Google Scholar]
- 20.Mazzucco MB, Higa R, Capobianco E, Kurtz M, Jawerbaum A, White V. Saturated fat-rich diet increases fetal lipids and modulates LPL and leptin receptor expression in rat placentas. J Endocrinol. 2013;217:303–315. doi: 10.1530/JOE-13-0021. [DOI] [PubMed] [Google Scholar]
- 21.Jones HN, Woollett LP, Barbour N, Prasad PP, Powell TL, Jansson T. High fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/Bl6 mice. FASEB J. 2009;23:271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusinski LC, Jones CJ, Baker PN, Sibley CP, Glazier JD. Isolation of plasma membrane vesicles from mouse placenta at term and measurement of system A and system beta amino acid transporter activity. Placenta. 2010;31:53–59. doi: 10.1016/j.placenta.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology. 2011;152:1119–1129. doi: 10.1210/en.2010-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan CA, McAuliffe FM. Maternal nutrient intakes and levels of energy underreporting during early pregnancy. Eur J Clin Nutr. 2012;66:906–913. doi: 10.1038/ejcn.2012.15. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert PA, Khokhar S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr Rev. 2008;66:203–215. doi: 10.1111/j.1753-4887.2008.00025.x. [DOI] [PubMed] [Google Scholar]
- 26.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Plasma adiponectin concentrations in non pregnant, normal pregnancy and overweight pregnant women. J Perinat Med. 2007:522–531. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140:365–371. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31:2026–2031. doi: 10.2337/dc08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, et al. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Prendergast CH, Parker KH, Gray R, Venkatesan S, Bannister P, Castro-Soares J, et al. Glucose production by the human placenta in vivo. Placenta. 1999;20:591–598. doi: 10.1053/plac.1999.0419. [DOI] [PubMed] [Google Scholar]
- 32.Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77:1554–1562. doi: 10.1210/jcem.77.6.8263141. [DOI] [PubMed] [Google Scholar]
- 33.Ganguly A, Collis L, Devaskar SU. Placental glucose and amino acid transport in calorie-restricted wild-type and Glut3 null heterozygous mice. Endocrinology. 2012;153:3995–4007. doi: 10.1210/en.2011-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y, Zhuo Y, Fang ZF, Che LQ, Wu D. Effect of maternal dietary energy types on placenta nutrient transporter gene expressions and intrauterine fetal growth in rats. Nutrition. 2012;28:1037–1043. doi: 10.1016/j.nut.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol. 1954:3303–42. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- 36.Chu SY, Bachman DJ, Callaghan WM, Whitlock EP, Dietz PM, Berg CJ, et al. Association between obesity during pregnancy and increased use of health care. N Engl J Med. 2008;358:1444–1453. doi: 10.1056/NEJMoa0706786. [DOI] [PubMed] [Google Scholar]
- 37.Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Schmid CH, et al. Long-term effectiveness of weight-loss interventions in adults with pre-diabetes: a review. Am J Prev Med. 2005;28:126–139. doi: 10.1016/j.amepre.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 39.Dilworth MR, Sibley CP. Review: Transport across the placenta of mice and women. Placenta. 2013;34(Suppl):S34–39. doi: 10.1016/j.placenta.2012.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.