Abstract
Background
Cutaneous angiosarcoma (CAS) is a rare, aggressive vascular sarcoma with a poor prognosis, historically associated with 5-year overall survival (OS) rates between 10 and 30 %.
Methods
This is a single-institution retrospective review of patients treated for CAS from 1999–2011. Demographics, primary tumor characteristics, treatment, and outcomes were analyzed.
Results
A total of 88 patients were identified (median age 70 years and 57 % female). Median tumor size was 3 cm. Median follow-up was 22 months. The 5-year OS and recurrence-free survival (RFS) were 35.2 and 32.3 %, respectively; median was 22.1 months. Also, 36 patients (41 %) received surgery alone, 7 (8 %) received XRT alone, and 41 (47 %) received surgery and XRT. Of the 67 of 88 patients who were disease-free after treatment, 33 (50 %) recurred (median of 12.3 months). Surgery alone had the highest 5-year OS (46.9 %) and RFS (39.9 %) (p = ns). Four presentation groups were identified: (1) XRT induced, n = 30 (34 %), 26 of 30 occurred in females with a prior breast cancer, (2) sporadic CAS on head and neck (H/N), n = 38, (3) sporadic CAS on trunk/extremities, n = 13, and (4) Stewart–Treves n = 7. Those with trunk/extremity CAS had the highest 5-year OS (64.8 %), with H/N CAS having the worst 5-year OS (21.5 %). On MV analysis, only tumor size <5 cm correlated with improved OS (p = 0.014).
Discussion
In this large series, there appears to be a better overall prognosis than historically reported, especially in Stewart–Treves and CAS on trunk or extremities. While surgery alone was associated with better OS and RFS compared with other treatment modalities, this was not statistically significant. Tumor size was a significant prognostic factor for OS.
Introduction
Cutaneous angiosarcoma (CAS) is an aggressive sarcoma of vascular origin. These tumors are rare, accounting for fewer than 2 % of all soft-tissue sarcomas in the United States.1–3 CAS typically presents sporadically on the scalp and face of elderly white males, but can also develop in areas of previous radiotherapy (XRT) for another malignancy and in the presence of chronic lymphedema of the extremity (Stewart–Treves syndrome).1 Historically, CAS has a reported 5-year overall survival (OS) rate between 10 and 30 %.3–5
While radiation-induced CAS of the breast typically presents 5–10 years after XRT, radiation-induced CAS of the head and neck can develop up to 50 years later.6–9 Stewart–Treves angiosarcoma commonly follows axillary lymph node dissection (ALND); the median time interval between ALND and tumor presentation is 5–15 years.10
CAS often presents as what appears to be a “spreading bruise” with poorly defined margins or as multifocal disease. Satellite lesions or multifocal disease may be seen in 41–46 % of patients.2,5 CAS is often confused with other benign entities that present in a similar fashion, especially atypical vascular lesions that are commonly seen after vascular irradiation.11–14
Surgery plus or minus adjuvant or neoadjuvant radiation is the gold standard treatment for those patients with CAS and is the only potentially curative measure.3 However, negative surgical margins can often be difficult to achieve secondary to extensive tumor involvement around vital structures, especially in the head and neck, or in the presence of multifocal disease. Chemotherapy is routinely used in patients with advanced regional or distant metastatic disease.1
The current body of literature on CAS consists of mostly case reports or small single-institution studies specific to CAS involving only 1 anatomic location or 1 type of presentation (XRT induced, sporadic, etc.). In this analysis, we reviewed 88 patients with CAS diagnosed and treated at a single institution (Moffitt Cancer Center, a NIH/NCI-designated comprehensive cancer center in Tampa, FL) for 12 years. We analyzed patient and tumor characteristics as well as treatment algorithms, and we correlate these with outcomes.
Methods
After Institutional Review Board approval, a retrospective review was performed on all patients diagnosed with and treated for CAS at Moffitt Cancer Center between 1999 and 2011. Of the 131 patients seen with a diagnosis of CAS, 88 patients received treatment and serve as the cohort for this analysis.
Patients were divided into four tumor presentation groups: (1) radiation-induced (XRT), (2) lymphedema-associated (Stewart–Treves), and sporadic CAS of the (3) head and neck (H/N) or (4) trunk/extremities. Patient demographics, tumor characteristics (size, margin status), treatment parameters, and outcome data were obtained and analyzed.
OS was calculated as the time from date of diagnosis to date of death or last contact. Recurrence-free survival (RFS) was only calculated for those who were considered disease free after primary treatment. A disease-free clinical status was established with either negative margins in surgical pathology or a clinical note stating no evidence of disease after primary treatment. RFS was calculated as the time from date considered disease free to date of recurrence, last follow-up, or death. Treatment groups were divided into surgery only, surgery and radiation, and radiation only.
Chemotherapy and Radiation
The majority of patients who were treated with chemotherapy received taxol-based regimens, while a minority of patients received doxorubicin therapy. Radiation was administered in the following manner: preoperative doses were 50 Gy, postoperative doses were 60 Gy, and bulky disease typically received between 66 and 70 Gy.
Statistical Methods
The relationship between clinical and pathological factors and both (1) tumor presentation group and (2) treatment modality were compared, using the χ2 test for categorical clinical and pathological factors, and Wilcoxon rank sum test for continuous clinical and pathological factors, both with the exact method using Monte Carlo estimation. Kaplan–Meier curves and log-rank tests were used for both OS analysis and RFS analysis. Multivariable survival models were fit using Cox proportional hazard models. All p values are 2-sided unless otherwise stated and considered statistically significant at the 0.05 level. All statistical analyses were performed using SAS (version 9.3, SAS Institute, Cary, NC).
Results
Patient and Tumor Characteristics
Of the 88 patients in this series, 50 (57 %) were female and 38 (43 %) were male. The median age at diagnosis was 70 years (range 19–92 years). The majority (95 %) of patients were white. The initial tumor presentation was divided into four groups: 30 XRT induced, 7 Stewart–Treves, 38 head and neck, and 13 sporadic trunk/extremity. The demographics for the patients in each tumor presentation group are found in Table 1.
Table 1. Clinicopathological factors listed by tumor presentation group and treatment group.
| Variable/level | N (%) | N (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Spontaneous head and neck | Spontaneous rest of body | Stewart–Treves syndrome | XRT-induced | p value | Surgery + XRT | Surgery only | XRT only | p value | |
| Age | |||||||||
| <70 years | 14 (34.1 %) | 10 (24.4 %) | 4 (9.8 %) | 13 (31.7 %) | 0.0840 | 20 (51.3 %) | 19 (48.7 %) | 0 (0 %) | 0.0361 |
| ≥70 years | 24 (51.1 %) | 3 (6.4 %) | 3 (6.4 %) | 17 (36.2 %) | 21 (46.7 %) | 17 (37.8 %) | 7 (15.6 %) | ||
| Gender | |||||||||
| Female | 7 (14 %) | 10 (20 %) | 6 (12 %) | 27 (54 %) | <0.0001 | 16 (34 %) | 29 (61.7 %) | 2 (4.3 %) | 0.0002 |
| Male | 31 (81.6 %) | 3 (7.9 %) | 1 (2.6 %) | 3 (7.9 %) | 25 (67.6 %) | 7 (18.9 %) | 5 (13.5 %) | ||
| Tumor size | |||||||||
| <5 cm | 22 (45.8 %) | 5 (10.4 %) | 4 (8.3 %) | 17 (35.4 %) | 0.9683 | 26 (54.2 %) | 20 (41.7 %) | 2 (4.2 %) | 0.6148 |
| ≥5 cm | 14 (46.7 %) | 4 (13.3 %) | 3 (10 %) | 9 (30 %) | 15 (51.7 %) | 11 (37.9 %) | 3 (10.3 %) | ||
| Margin status | |||||||||
| + | 10 (55.6 %) | 3 (16.7 %) | 1 (5.6 %) | 4 (22.2 %) | 0.3511 | 15 (83.3 %) | 3 (16.7 %) | 0 (0 %) | 0.0037 |
| − | 20 (34.5 %) | 8 (13.8 %) | 5 (8.6 %) | 25 (43.1 %) | 25 (43.1 %) | 33 (56.9 %) | 0 (0 %) | ||
| Recurrence status | |||||||||
| No recurrence | 11 (32.4 %) | 6 (17.6 %) | 1 (2.9 %) | 16 (47.1 %) | 0.1370 | 12 (35.3 %) | 21 (61.8 %) | 1 (2.9 %) | 0.0640 |
| Recurrence | 15 (45.5 %) | 3 (9.1 %) | 5 (15.2 %) | 10 (30.3 %) | 20 (60.6 %) | 13 (39.4 %) | 0 (0 %) | ||
| Locoregional recurrence | |||||||||
| No | 2 (40 %) | 1 (20 %) | 1 (20 %) | 1 (20 %) | 0.9064 | 4 (80 %) | 1 (20 %) | 0 (0 %) | 0.6219 |
| Yes | 13 (46.4 %) | 2 (7.1 %) | 4 (14.3 %) | 9 (32.1 %) | 16 (57.1 %) | 12 (42.9 %) | 0 (0 %) | ||
| Distant metastasis | |||||||||
| No | 27 (42.2 %) | 8 (12.5 %) | 5 (7.8 %) | 24 (37.5 %) | 0.6803 | 27 (43.5 %) | 30 (48.4 %) | 5 (8.1 %) | 0.2557 |
| Yes | 10 (43.5 %) | 5 (21.7 %) | 2 (8.7 %) | 6 (26.1 %) | 14 (63.6 %) | 6 (27.3 %) | 2 (9.1 %) | ||
| Treatment group | |||||||||
| Surgery + XRT | 25 (61 %) | 3 (7.3 %) | 4 (9.8 %) | 9 (22 %) | 0.0007 | ||||
| Surgery only | 5 (13.9 %) | 8 (22.2 %) | 3 (8.3 %) | 20 (55.6 %) | |||||
| XRT only | 6 (85.7 %) | 0 (0 %) | 0 (0 %) | 1 (14.3 %) | |||||
Gross tumor size was recorded in 78 patients and ranged from 0.3 to 38 cm in maximal dimension, with a median tumor size of 3 cm. The median tumor size for sporadic AS of the trunk/extremities was 4.7 cm, contrasting with 2.8 cm for H/N tumors, 2.1 cm for XRT-induced tumors, and 2.0 cm for Stewart–Treves tumors, but these median sizes were not statistically significant.
Of the 30 patients with radiation-induced lesions (XRT), 26 presented on the breast of females receiving prior irradiation for a primary breast tumor, while 4 presented with disease in the H/N. The median latency period between prior radiation and tumor development was 9 years; however, this time to development of CAS was higher in the H/N patients, with a median latency period of 15 years.
All 7 patients with CAS on a lymphedematous extremity (Stewart–Treves) had a history of breast cancer and prior ALND with a median latency period of 14 years.
Treatment
There were three main treatment groups identified, according to treatment modality directed at the primary tumor: 36 patients (41 %) received surgery alone, 7 (8 %) received radiation alone, and 41 (47 %) received surgery and radiation. The remaining 4 patients received chemotherapy alone for palliative measures and were not included in analyses specific to treatment modality. Although these four patients who received only chemotherapy were not considered in this analysis, the administration of chemotherapy was reported in 34 total patients (39 %), and there was no significant difference observed with use of chemotherapy between the different tumor presentations. Radiation was used more often with H/N tumors than all other tumor presentations (p = 0.009).
While age and tumor size did not show a correlation with treatment modality, there was a significant difference in type of treatment used between the 4 tumor presentation groups. Head and neck tumors were more likely to receive surgery and radiation (66 %), while radiation-induced tumors predominantly received surgery alone (67 %) (p = 0.002).
Surgical resection margins were at least 1 cm wherever possible around any visible or palpable tumor, unless bound by critical structures. Graft or flap reconstruction was most often used in the head and neck cases (25 of 30 cases) because of a lack of native tissue to close defects and was also used in 13 of the remaining 47 patients who underwent surgical resection.
Of the 77 patients (87 %) who underwent surgical resection, negative margins were achieved in 59 patients (77 %). Of the 18 patients with positive margins after surgical resection, 8 were able to achieve a clinical status of no evidence of disease (NED) after adjuvant radiation. Table 1 shows clinicopathological factors broken down by primary tumor treatment modality. There was no statistical significance with OS or RFS when analyzing treatment modalities.
Outcomes
Table 2 shows both RFS and OS at 3 and 5 years broken down by clinicopathological and treatment factors. Median follow-up was 22 months for all patients.
Table 2. Recurrence-free and overall survival at 3 and 5 years.
| Characteristic | No. of patients (%) | Median overall survival (months) | Median recurrence-free survival (months) | Overall survival (%) | Recurrence-free survival (%) | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 3 years | 5 years | 3 years | 5 years | ||||
| Age | |||||||
| <70 years | 41 (47 %) | 55.0 | 28.4 | 65.8 | 43.9 | 47.5 | 39.6 |
| ≥70 years | 47 (53 %) | 34.0 | 21.0 | 45.3 | 27.7 | 34.5 | 25.9 |
| Tumor size | |||||||
| <5 cm | 48 (62 %) | 55.0 | 28.0 | 64.5 | 48.4 | 42.6 | 42.6 |
| ≥5 cm | 30 (38 %) | 26.3 | 7.0 | 34.6 | 11.5 | 36.5 | 24.3 |
| Margin status | |||||||
| + | 18 (24 %) | 20.6 | 28.0 | 34.7 | 34.7 | 28.6 | 28.6 |
| − | 58 (76 %) | 52.8 | 25.4 | 64.3 | 37.9 | 43.8 | 33.4 |
| Tumor presentation | |||||||
| Spontaneous head and neck | 38 (43 %) | 35.5 | 25.4 | 49.3 | 21.5 | 33.7 | 0 |
| Spontaneous trunk/extremities | 13 (15 %) | NEa | NEa | 64.8 | 64.8 | 60.0 | 60.0 |
| Stewart–Treves syndrome | 7 (8 %) | 38.4 | 6.4 | 66.7 | NEa | 0 | 0 |
| XRT-induced | 30 (34 %) | 55.0 | 54.5 | 59.3 | 49.4 | 58.0 | 46.4 |
| Local treatment modality | |||||||
| Surgery + XRT | 41 (47 %) | 37.3 | 16.9 | 58.4 | 30.9 | 27.9 | 27.9 |
| Surgery only | 36 (41 %) | 59.5 | 41.4 | 60.2 | 46.9 | 59.8 | 39.9 |
| XRT only | 7 (8 %) | 23.6 | NEa | 33.3 | 16.7 | – | – |
Unable to be estimated because of small percentage of events (i.e., deaths) in sample
Recurrence-Free Survival
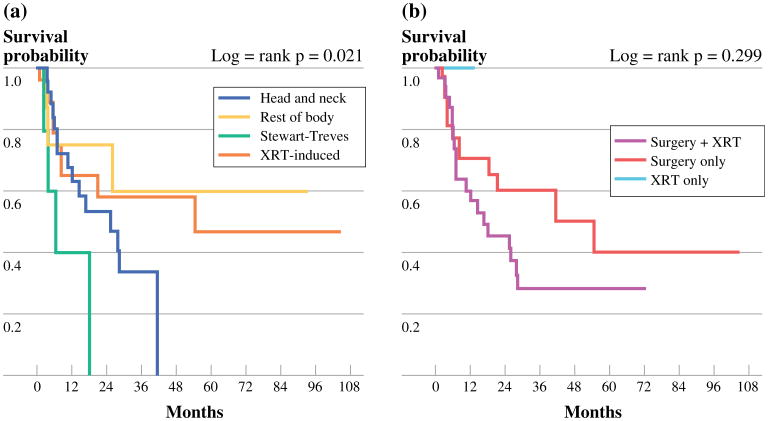
The 3- and 5-year RFS rates were 41 and 32 %, respectively. Of the 67 patients who were rendered NED after treatment, 33 (49 %) experienced recurrence of tumor. The median time period for recurrence was 12.3 months with the latest disease recurrence observed at 8.7 years following treatment. A total of 29 patients recurred with only locoregional disease. At any point in treatment, 23 patients (29 %) developed distant metastases, and 6 patients (7 %) had regional lymph node involvement. The lungs were the most common site of metastatic disease (n = 12) followed by bone metastases (n = 4). Figure 1 shows the RFS Kaplan–Meier curves for both presentation of tumor and treatment modality. At 3 years after definitive treatment, all patients with Stewart– Treves CAS experienced recurrence, and all patients with H/N CAS recurred by 5 years. Patients with Stewart–Treves and H/N CAS experienced more frequent recurrence of tumor than those with radiation-induced or sporadic CAS (p = 0.025). Tumor size <5 cm correlated with improved RFS (p = 0.028). Although not significantly different, age <70 years and a negative margin status all correlated with improved RFS, as seen in Table 2.
Fig 1.
a RFS tumor presentation groups. b RFS treatment groups
Overall Survival
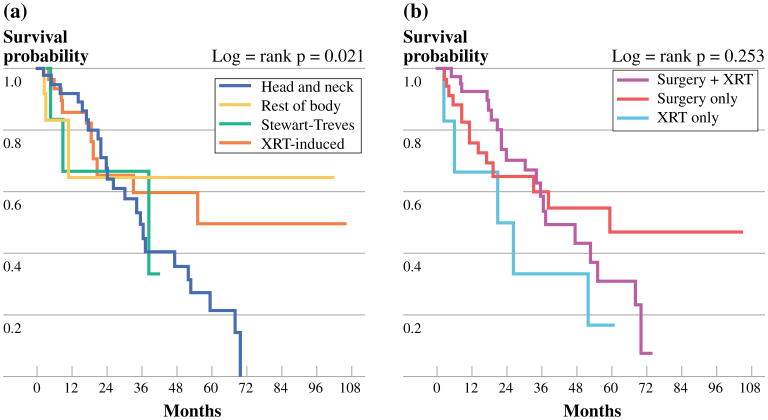
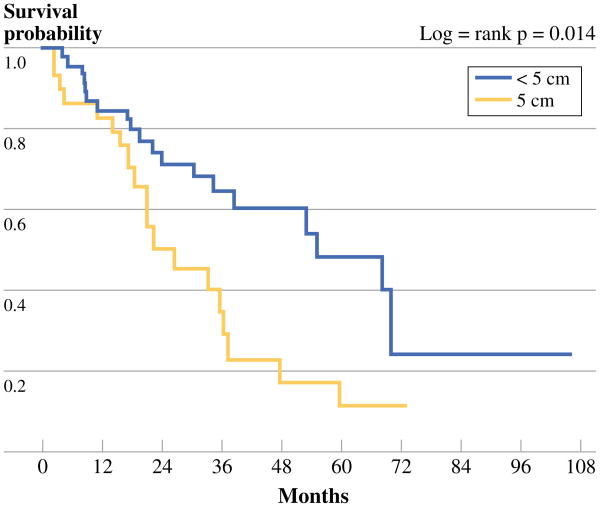
A total of 42 patients (48 %) died from CAS at a median survival of 22.1 months. The 3- and 5-year OS rates were 55 and 35 %, respectively. H/N CAS had the lowest 3-year OS (49.3 %), while Stewart–Treves CAS had the highest (66.7 %). Figure 2 shows the OS Kaplan–Meier curves for both presentation of tumor and treatment modality. Like RFS, age <70 years, a negative margin status, and surgery alone as treatment all correlated with OS although not statistically significantly (Table 2). Interestingly, those patients with primary tumor size <5 cm had a significantly improved OS on multivariable analysis (p = 0.023). Figure 3 shows Kaplan–Meier survival curves based on tumor size <5 and ≥5 cm.
Fig 2.
a OS tumor presentation groups. b OS treatment groups
Fig 3. OS tumor size.
Discussion
CAS is a rare and aggressive malignancy that shows marked heterogeneity in presentation and etiology. In this series of 88 patients, the 5-year OS was 35 % for all patients. The most commonly observed presentation of CAS was on the head and neck of white males older than 70 years of age, accounting for 75 % of patients with sporadic CAS. The remaining tumors were predominantly found in females (89 %) around 70 years of age with a history of breast cancer who underwent either whole breast irradiation, ALND, or both. These demographic findings are similar to previous reports.2,3,15,16
It is well known that radiation can cause CAS, and the risk of developing CAS increases with higher doses and time elapse after administration of XRT.17 While the majority of radiation-induced tumors occurred in the breast (86 %), 4 patients developed tumor associated with previous radiation in the head and neck. Chronic lymphedema is another classically described cause of CAS. The direct causation between lymphedema and the development of CAS is unknown; however, it is hypothesized this is due to either a systemic carcinogenic factor or a local immunodeficiency due to the disruption in the lymphatic system.10 In this series, 7 cases of CAS developing in an edematous extremity were identified. Other than previous XRT and chronic lymphedema, the etiology of CAS remains unclear.
In the current series, CAS patients were found to have a slightly better prognosis than that which is historically reported, with a 3- and 5-year survival of 55 and 35 %, respectively.
While advanced age has historically been associated with a worse prognosis, our study did not show a statistically significant improvement in either OS or RFS with patients <70 years of age. This is likely explained with the small standard deviation observed among ages at diagnosis in this study (STD = 14.4). Tumor size is another characteristic of CAS shown in literature to correlate with disease outcome, a tumor size <5 cm indicating an improved prognosis.2 Our results were consistent with this finding as tumors smaller than 5 cm had a statistically significant improvement in OS (p = 0.014) on multivariable analysis. This analysis accounted for different treatment modalities that might have been used for different tumor sizes.
While tumor size did not correlate with treatment modality, there was a significant difference with treatment used for the 4 tumor presentations studied (p = 0.0007). Surgery + XRT was predominantly used in lesions of the head and neck. Meanwhile, surgery alone was the primary mode of treatment in the remaining tumors, especially in patients with radiation-induced tumors. In these patients with a history of radiation, surgery alone had a 2:1 prevalence over the other treatment combinations (XRT only, surgery + XRT).
In this series, patients with CAS of the head and neck experienced positive margins 50 % of the time, while those with radiation-induced tumors only had positive margins 16 % of the time. Difficulty obtaining wide margins secondary to tumor involvement or juxtaposed to vital structures and frequency of multifocal disease in the head and neck region limit aggressive resection.2,16 This anatomic limitation might explain why patients with CAS of the head and neck had lower OS and RFS at 3 and 5 years than those with Stewart–Treves CAS who had the highest 3- and 5-year OS. Interestingly, although patients with Stewart–Treves CAS all recurred within 3 years there was an improved OS. This could likely be explained by more aggressive treatment interventions unique to the extremity that may prolong survival, such as forequarter amputation for advanced tumors that were localized and isolated limb infusions/perfusions.
The implication of surgical margin status on outcome of CAS is debated in the current literature. Morgan et al.18 reported a series of head and neck CAS, showing a correlation between positive surgical margins and decreased OS. However, multiple studies, including this review, have not shown such a correlation.2,5,19,20 Furthermore, it has also been reported that intraoperative “negative” margins have often shown disease on final permanent section.5
There was no significant difference in rates of both locoregional recurrence and distant metastasis among the different tumor presentations or treatment modalities studied. This is consistent with other institutional reviews of this aggressive malignancy.1,15,16
Because of the difficulty obtaining “true” negative surgical margins and the high rate of recurrence and metastasis associated with CAS, there is current debate about the role surgery plays in treatment of this disease. In 2008, Buschmann et al.21 reported on a series of 19 patients with sHN CAS and suggested surgery should be limited to a debulking procedure that allows for permanent defect cover in order to reduce both patient morbidity and extended surgical recovery time that may delay the ability to start adjuvant therapy. Guadagnolo et al.2 also reported on the extent of surgical resection, stating standard of treatment at their institution for H/N CAS consists of neoadjuvant chemotherapy before gross resection of tumor without attempt at negative margins followed by radiotherapy. Although not significant on MV analysis, surgical intervention still provides the greatest potential for improved 3- and 5-year disease-free survival and OS, and we recommend aggressive surgical intervention with radiotherapy if possible.
The limitations of the current study relate to the small total cohort, further broken down into subgroups, which negatively impacts the statistical analysis as a result of the small sample size of each cohort.
The effect of chemotherapy on recurrence and survival with CAS is unclear.1 Case reports and small studies continue to show a response of CAS to chemotherapy; however, there is insufficient evidence that any of these treatments improve disease outcome.1–3,22 However, these results may be explained in part by selection bias as chemotherapy has historically only been used in patients with advanced CAS, and there is a need for further study of therapeutic and targeted agents for CAS.
References
- 1.Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241–7. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Guadagnolo BA, Zagars GK, Araujo D, Ravi V, Shellenberger TD, Sturgis EM. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661–7. doi: 10.1002/hed.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Mendenhall CM, Werning JW, Reith JD, Mendenhall NP. Cutaneous angiosarcoma. Am J Clin Oncol. 2006;29:524–8. doi: 10.1097/01.coc.0000227544.01779.52. [DOI] [PubMed] [Google Scholar]
- 4.Morrison WH, Byers RM, Garden AS, Evans HL, Ang KK, Peters LJ. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319–27. doi: 10.1002/1097-0142(19950715)76:2<319::aid-cncr2820760224>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Pawlik TM, Paulino AF, McGinn CJ, Baker LH, Cohen DS, Morris JS, et al. Cutaneous angiosarcoma of the scalp: a multi-disciplinary approach. Cancer. 2003;98:1716–26. doi: 10.1002/cncr.11667. [DOI] [PubMed] [Google Scholar]
- 6.Gladdy RA, Qin LX, Moraco N, Edgar MA, Antonescu CR, Alektiar KM, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010;28:2064–9. doi: 10.1200/JCO.2009.25.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jallali N, James S, Searle A, Ghattaura A, Hayes A, Harris P. Surgical management of radiation-induced angiosarcoma after breast conservation therapy. Am J Surg. 2012;203:156–61. doi: 10.1016/j.amjsurg.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Lindford A, Bohling T, Vaalavirta L, Tenhunen M, Jahkola T, Tukiainen E. Surgical management of radiation-associated cutaneous breast angiosarcoma. J Plast Reconstr Aesthet Surg. 2011;64:1036–42. doi: 10.1016/j.bjps.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907–21. doi: 10.1002/1097-0142(19811015)48:8<1907::aid-cncr2820480832>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Schwartz RA. Stewart–Treves syndrome: Pathogenesis and management. J Am Acad Dermatol. 2012;67:1342–8. doi: 10.1016/j.jaad.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983–96. [PubMed] [Google Scholar]
- 12.Kast DR, Sammons D, Nixon RM, Geiss DF. Cutaneous angiosarcoma masquerading as herpes zoster. Cutis. 2012;90:42–5. [PubMed] [Google Scholar]
- 13.Patel RM, Billings SD. Cutaneous soft tissue tumors that make you say, “oh $*&%!”. Adv Anat Pathol. 2012;19:320–30. doi: 10.1097/PAP.0b013e31826661d1. [DOI] [PubMed] [Google Scholar]
- 14.Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943–50. doi: 10.1097/pas.0b013e31815bf8fe. [DOI] [PubMed] [Google Scholar]
- 15.Abraham JA, Hornicek FJ, Kaufman AM, Harmon DC, Springfield DS, Raskin KA, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953–67. doi: 10.1245/s10434-006-9335-y. [DOI] [PubMed] [Google Scholar]
- 16.Espat NJ, Lewis JJ, Woodruff JM, Antonescu C, Xia J, Leung D, et al. Confirmed angiosarcoma: prognostic factors and outcome in 50 prospectively followed patients. Sarcoma. 2000;4:173–7. doi: 10.1155/2000/575781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson E, Neugut AI, Wylie P. Clinical aspects of postirradiation sarcomas. J Natl Cancer Inst. 1988;80:233–40. doi: 10.1093/jnci/80.4.233. [DOI] [PubMed] [Google Scholar]
- 18.Morgan MB, Swann M, Somach S, Eng W, Smoller B. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867–74. doi: 10.1016/j.jaad.2003.10.671. [DOI] [PubMed] [Google Scholar]
- 19.Lydiatt WM, Shaha AR, Shah JP. Angiosarcoma of the head and neck. Am J Surg. 1994;168:451–4. doi: 10.1016/s0002-9610(05)80097-2. [DOI] [PubMed] [Google Scholar]
- 20.Ward JR, Feigenberg SJ, Mendenhall NP, Marcus RB, Jr, Mendenhall WM. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873–8. doi: 10.1002/hed.10276. [DOI] [PubMed] [Google Scholar]
- 21.Buschmann A, Lehnhardt M, Toman N, Preiler P, Salakdeh MS, Muehlberger T. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399–403. doi: 10.1097/SAP.0b013e31816b31f8. [DOI] [PubMed] [Google Scholar]
- 22.Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single center experience. J Dermatol Treat. 2013 doi: 10.3109/09546634.2012.754839. [DOI] [PubMed] [Google Scholar]