Abstract
(−)-Epigallocatechin-3-gallate (EGCG), has been shown to inhibit cancer in vivo. EGCG, however, is rapidly methylated by catechol-O-methyl transferase (COMT), which reduces its cancer preventive efficacy. Tolcapone (TOL), is a clinically-used COMT inhibitor. Here, we examined the effect of TOL on the bioavailability of EGCG in male CF-1 mice. Plasma and tissue levels of EGCG and its methyl metabolites were determined following intragastric administration of EGCG (100 mg/kg), TOL (30 mg/kg), or the combination. In mice treated with EGCG, unmethylated plasma EGCG accounted for 63.4 % of the total. Co-administration of TOL increased this fraction to 87.9 %. In the urine, unmethylated EGCG accounted for 29.2 % of the total, whereas treatment with EGCG plus TOL increased this to 81.8 %. Similar effects were observed in the major organs examined. TOL effectively inhibited the methylation of EGCG in vivo. Future studies should examine the cancer preventive effects of the combination.
Keywords: bioavailability, catechol-O-methyltransferase, (−)-epigallocatechin-3-gallate, green tea, tolcapone
1. Introduction
Green tea and its major polyphenolic constituent, (−)-epigallocatechin-3-gallate (EGCG) has been shown to have efficacy in in vitro and animal models of cancer (Yang et al., 2011; Zhong et al., 2012). EGCG-mediated growth inhibitory and pro-apoptotic activity appear to result from its redox properties (Kweon, Adhami, Lee, & Mukhtar, 2006) and/or its ability to interact with specific molecular targets (Artali et al., 2009; Chen et al., 2011; Kavanagh et al., 2001; Yin et al., 1994). Despite strong laboratory evidence, and the fact that green tea intake has been associated with reduced risk for a number of cancers including breast, lung, colon, and liver cancers in a number of epidemiological studies (Ogunleye, Xue, & Michels, 2010; Tang et al., 2009; Ui et al., 2009; Yang et al., 2007), other epidemiological studies have failed to find a protective effect of green tea against cancer. Lifestyle, diet, and genetic factors have been shown to account for part of this variation.
EGCG has been shown to undergo extensive Phase II metabolism, including methylation, which limits the overall oral bioavailability of EGCG (Chen & Sang, 2014; Sang, Lambert, Ho, & Yang, 2011; Ting, Jiang, Ho, & Huang, 2014). Catechol-O-methyltransferase (COMT) rapidly metabolises EGCG to 4″-O-methyl-EGCG (MeEGCG) and 4′,4″-di-O-methyl-EGCG (DiMeEGCG) (Fig. 1). These methylated metabolites have been found to have reduced cancer-related biological activities (Daniel et al., 2006; Fang et al., 2003). COMT is a polymorphic gene and exists as both high activity and low activity forms (Doyle, Goodman, Silber, & Yager, 2004). Therefore, COMT represents a potential genetic confounder for the cancer preventive effects of EGCG.
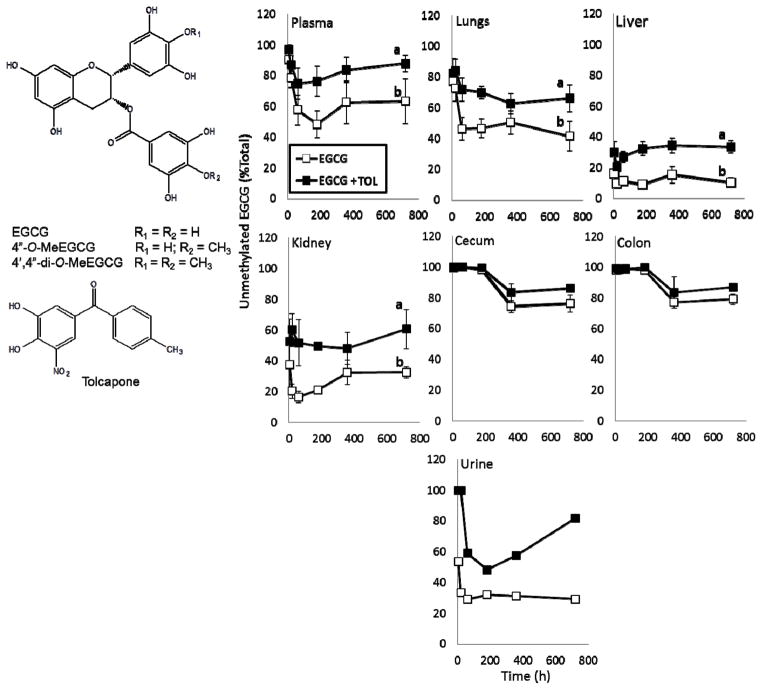
FIGURE 1.
Effect of tolcapone (TOL) on the relative proportion of methylated EGCG metabolites in the plasma, tissues, and urine of male CF-1 mice. The chemical structures of EGCG, its major methylated metabolites and TOL are shown. Mice were treated with EGCG (100 mg/kg, i.g.) in the absence or presence of TOL (30 mg/kg, i.g.). Data represent the total unmethylated fraction including glucuronidated and sulphated metabolites. Results are shown as means ± SEM (n = 3 – 6). Different superscript letters (a vs b) indicate that TOL significantly increased the fraction of unmethylated EGCG (P < 0.05).
A case-control study of an Asian-American females found that tea consumers with the low activity form of COMT had reduced risk of developing breast cancer compared to non-consumers (OR = 0.48; 95% CI, 0.29–0.77), whereas tea consumption was not associated with breast cancer risk in women with the high activity form (OR = 1.02; 95% CI, 0.66–1.60) (Wu, Tseng, Van den Berg, & Yu, 2003). These results suggest that decreased methylation of tea polyphenols may enhance their bioavailability and anticancer activity. Indeed, other groups have found that individuals with low activity COMT had decreased GTP methylation (Inoue-Choi et al., 2010; Miller et al., 2012) and increased levels of GTPs (Brown et al., 2011). This finding has not, however been universal (Miller et al., 2012).
Tolcapone (TOL, Fig. 1) is a COMT inhibitor commonly prescribed to Parkinson’s Disease patients to decrease COMT-mediated methylation and inactivation of levodopa, resulting in increased brain levels of levodopa and enhanced management of PD-related dyskinesia and motor fluctuations (Baas et al., 1997; Mueller et al., 2000). We have previously reported that TOL, as well as entacapone (another COMT inhibitor), in combination with EGCG synergistically inhibited the growth of human and murine lung cancer cells in vitro (Forester & Lambert, 2014). The synergistic effects were due in part to inhibition of COMT- mediated methylation of EGCG by cancer cells. TOL produced consistently greater inhibitory effects when combined with EGCG compared to entacapone.
Although initial clinical studies reported that TOL could induce liver toxicity, subsequent clinical studies have not reported additional cases of hepatotoxicity (Ebersbach, Hahn, Lorrain, & Storch, 2010; Lees, Ratziu, Tolosa, & Oertel, 2007; Lew & Kricorian, 2007; Olanow, 2000). Additional analysis of this data has concluded that the risk of hepatotoxic events related to TOL use are small and confined to older (> 60 y old) subjects (Olanow & Watkins, 2007; Watkins, 2000).
Since TOL/EGCG combinations were found to synergistically inhibit growth of lung cancer cells in vitro, in part, by inhibiting methylation of EGCG, we hypothesized that TOL could potently inhibit COMT-mediated methylation of EGCG in vivo. This research would be particularly relevant for human subjects with a high activity COMT genotype, as well as Parkinson’s Disease patients, who may already be co-exposed to TOL and EGCG because of perceived neuroprotective effects of tea consumption (Wang et al., 2013). Herein, we report the effect of co-administration of EGCG and low dose TOL on the plasma and tissue levels of unmethylated EGCG in mice.
2. Materials and methods
2.1 Chemicals
TOL was purchased from Synfine Chemical Co. (99.9% pure, Richmond Hill, ON, Canada). EGCG (98% pure) was purchased from Quality Phytochemicals, LLC (Edison, NJ, USA). All other chemicals were of the highest grade commercially-available.
2.2 Animals and treatment
Male CF-1 mice (4 wk old) were purchased from Charles River Laboratories (Wilmington, MA, USA) and maintained on a 12 h light/dark cycle in gang cages on corn cob bedding Mice had ad libitum access to chow and water. All experiments were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University (IACUC #28962). Following a 2 wk acclimatisation period, mice were separated into groups (n = 6) based on body weight and fasted for 12 h (8 pm – 8 am) prior to treatment. Mice were given a single intragastric dose of EGCG (100 mg/kg), alone or in combination with TOL (30 mg/kg). Both compounds were dissolved in 5% aqueous ethanol. After oral administration of test compounds, mice had ad libitum access to water but were kept without food for the duration of the experiment. At different time points, anesthetised mice were exsanguinated via cardiac puncture. Blood was centrifuged at 3,200 g for 15 min at 4° C, and plasma was aliquoted. Urine from all mice in a particular treatment group was pooled. Both plasma and urine were combined with 0.1 vol. of 20 % ascorbate/0.1 % ethylenediaminetetraacetic acid (EDTA) preservative and frozen at −80°C until analysis. The large intestine, cecum, liver, kidneys, and lungs were removed, rinsed with cold saline (0.9% NaCl in water), snap frozen, and kept at −80°C.
2.3 LC-MS analysis of EGCG and methylated metabolites
EGCG and its metabolites were extracted from plasma and tissue using previously reported methods (Lambert et al., 2003). In brief, tissue samples were homogenised in 5 volumes of buffer containing sodium hydrosulphite buffer (0.3 M sodium hydrosulphite, 0.1 % Na2EDTA in 0.4 M sodium monophosphate buffer (pH 6.8)), methanol, and ethyl acetate (2:1:1, v/v/v) using a mechanical dounce homogenizer and centrifuged for 4 min at 14,500 g. The supernatant was removed and dried for 60 min. Each sample was then resuspended in water. Plasma, tissue extract samples, and urine were then hydrolysed with glucuronidase (250 U/sample) and sulfatase (1 U/sample) enzymes at 37 °C for 45 min, and extracted with dichloromethane and ethyl acetate as previously described. Samples were then re-suspended in 10 % aqueous acetonitrile and analyzed by LC-MS using a previously described method (Lu, Meng, & Yang, 2003). Samples were separated using a Shimadzu HPLC system consisting of two Shimadzu LC-10ADvp pumps and a SIL-10ADvp autoinjector (Shimadzu Scientific, Columbia, MD, USA). Eluent was monitored using a Waters Quattro MicroMass triple quadrupole mass spectrometer (Waters Co., Milford, MA, USA) equipped with an electrospray ionization source in the negative ionization mode. EGCG (457 m/z), MeEGCG (472 m/z) and DiMeEGCG (487 m/z) were monitored and quantified in single ion monitoring mode. These samples represented the sum of EGCG, MeEGCG, and DiMeEGCG and their glucuronide and sulphate conjugates. Duplicate samples were analyzed without glucuronidase/sulfatase treatment to determine the concentrations of unconjugated EGCG, MeEGCG, and DiMeEGCG only.
2.4 Statistical analysis
All values are reported as means ± SEM. Maximal plasma concentration (Cmax), Area under the curve (AUC), and time to Cmax (Tmax) values were calculated using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). Two-tailed Student’s t tests were performed to determine statistical significance of AUC values. A P value < 0.05 was considered statistically significant.
3. Results and Discussion
In the present study, we examined the effect of co-administration of the COMT inhibitor, TOL (30 mg/kg, i.g.), on the methylation and bioavailability of oral EGCG (100 mg/kg, i.g.) in CF-1 mice. The doses of EGCG and TOL used in this study are equivalent to 6 mg/kg and 1.8 mg/kg for a human, respectively, based on allometric scaling (Schneider, Oltmanns, & Hassauer, 2004). This dose of EGCG can be achieved by consuming 2 – 3 cups of green tea. The dose of TOL is well below the recommended initial daily dose for mitigation of PD-related symptoms (100 mg three times daily).
Co-administration of EGCG and TOL significantly increased the fraction of unmethylated total EGCG (including glucuronidated and sulphated metabolites) in the plasma, urine, and a panel of tissue samples derived from treated CF-1 mice compared to mice treated with EGCG only (Fig. 1). Co-treatment with TOL increased the AUC0→720min of unmethylated total EGCG in liver, kidney, plasma, urine, lung, cecum, and colon by 23, 28, 25, 52, 25, 9.8, and 7.6 %, respectively, compared to treatment with EGCG only. The increases in the plasma, lung, liver, and kidney were statistically significant.
Analysis of Cmax and AUC0→720min of unmethylated, unconjugated EGCG (not including glucuronide and sulphate metabolites), showed that co-administation of TOL tended to increase EGCG Cmax in the plasma, liver, kidney, lung, and urine and the AUC0→720min in the liver, kidney, lung, and urine, compared to mice treated with EGCG only (Table 1). By contrast, TOL co-treatment did not affect EGCG Cmax in the cecum or colon, nor did it affect AUC0→720min of EGCG in the plasma, cecum, and colon (Table 1).
Table 1.
Pharmacokinetic parameters of unmethylated, conjugated EGCG in mice in the absence or presence of orally administrated TOL.1
| Sample | Parameter | Units | TOL (−) | TOL (+) |
|---|---|---|---|---|
| Plasma | ||||
| Cmax | nM | 1189.3 ± 314.7 | 1532.2 ± 344.7 | |
| AUC0→720min | nM•min | 245330.8 ± 74700.1 | 154887.7 ± 39082.1 | |
| Tmax | min | 16.7 ± 9.0 | 12.5 ± 3.4 | |
|
| ||||
| Liver | ||||
| Cmax | nmol/g | 2.2 ± 0.9 | 2.7 ± 0.7 | |
| AUC0→720min | (nmol/g)•min | 871.5 ± 401.9 | 1324.8 ± 390.5 | |
| Tmax | min | 200.0 ± 55.1 | 211.7 ± 107.5 | |
|
| ||||
| Lung | ||||
| Cmax | nmol/g | 1.4 ± 1.2 | 23.3 ± 20.8 | |
| AUC0→720min | (nmol/g)•min | 203.3 ± 156.4 | 1002.1 ± 682.1 | |
| Tmax | min | 141.3 ± 83.0 | 167.5 ± 113.6 | |
|
| ||||
| Kidney | ||||
| Cmax | nmol/g | 0.4 ± 0.1 | 2.0 ± 1.7 | |
| AUC0→720min | (nmol/g)•min | 95.7 ± 5.6 | 461.7 ± 358.5 | |
| Tmax | min | 361.7 ± 206.4 | 266.7 ± 227.0 | |
|
| ||||
| Cecum | ||||
| Cmax | nmol/g | 117.7 ± 31.7 | 97.7 ± 19.4 | |
| AUC0→720min | (nmol/g)•min | 41580.2 ± 10560.6 | 39152.8 ± 8338.2 | |
| Tmax | min | 300.8 ± 99.9 | 453.3 ± 127.1 | |
|
| ||||
| Colon | ||||
| Cmax | nmol/g | 63.8 ± 9.6 | 37.6 ± 5.8 | |
| AUC0→720min | (nmol/g)•min | 26301.3 ± 8881.3 | 18631.3 ± 2844.0 | |
| Tmax | min | 420 ± 158.7 | 240 ± 60 | |
|
| ||||
| Urine | ||||
| Cmax | nmol/g | 1631.2 | 4913.7 | |
| AUC0→720min | (nmol/g)•min | 765410 | 1288000 | |
| Tmax | min | 5 | 20 | |
All values are means ± SEM of n = 3 – 6 except urine which was pooled to produce 1 sample per treatment group.
This lack of effect of TOL on the Cmax, and AUC0→720min of unmethylated free EGCG in the cecum and colon may result from two factors. First, the cecum and colon have lower COMT activity than other tissues including the liver and lung, and other Phase II metabolic pathways may be more important to the biotransformation of EGCG in these tissues (Myohanen, Schendzielorz, & Mannisto, 2010). Second, because of the high concentrations of unmethylated, unconjugated EGCG in the contents of the gastrointestinal tract, it is possible that cecum and colon tissue samples have EGCG that is adsorbed to the tissues, but not actually internalized. This represents a confounding effect which may underestimate the impact of TOL co-treatment.
The lack of effect on the AUC0→720min in the plasma may indicate that inhibition of COMT leads to a compensatory increase in glucuronidation and sulphation. Previously, we reported that EGCG in the plasma exists mainly as glucuronide conjugates in the plasma, but in the unconjugated form in the tissues (Lambert et al., 2003). We examined the effect of TOL on the fraction of unmetabolised EGCG (Fig. 2). Co-treatment with TOL did not significantly affect the levels of unmetabolised EGCG compared to mice treated with EGCG only. These results suggest that although TOL can reduce methylated EGCG metabolites circulating in the plasma, the effect on overall EGCG bioavailability is masked by an increase in glucuronidation. Although the results are unexpected, the outcome may represent an improvement in EGCG bioavailability since these metabolites can be deconjugated to the aglycone forms by various cell types (Bartholome et al., 2010; Lu et al., 2003). These results indicate that a shift from methyl to glucuronide metabolites may facilitate regeneration of the active parent compound in vivo.
FIGURE 2.
Effect of tolcapone (TOL) on the relative proportion of unmetabolised EGCG in the plasma of male CF-1 mice. Mice were treated with EGCG (100 mg/kg, i.g.) in the absence or presence of TOL (30 mg/kg, i.g.). Data represent the only unmethylated, unconjugated EGCG. Results are shown as means ± SEM (n = 3 – 6).
The effects of TOL on EGCG bioavailability may not be unique. Previous studies have shown that quercetin, a common dietary flavonol which has been shown to inhibit COMT, can increase the levels of EGCG found in the lung and kidney by greater than 2-fold (Wang, Heber, & Henning, 2012). Although there is some concern regarding the potential hepatotoxic effects of TOL, this low dose, in conjunction with more recent human studies demonstrating a more favourable safety profile for TOL, would suggest that the present combination may have application in human subjects. Further studies, with appropriate monitoring of liver function, however are needed to validate the utility of the present combination for human subjects (Olanow & Watkins, 2007; Watkins, 2000).
4. Conclusions
Co-administration of the clinically-used COMT inhibitor TOL increased the fraction of unmethylated EGCG in CF-1 mice compared to mice treated with EGCG only. Future studies should explore whether this combination exerts enhanced cancer chemopreventive effects in vivo, the impact of other natural and pharmaceutical inhibitors of COMT, and work to translate this work to human subjections. Development of novel, mechanism-based combinations that improve the bioavailability EGCG and may improve the in vivo biological activity of EGCG has considerable significance to public health.
Highlights.
Epigallocatechin gallate (EGCG) is methylated by catechol methyltransferase (COMT)
We report the effect of tolcapone, a COMT inhibitor, on umetabolized EGCG in mice
Tolcapone increased fraction of unmethylated EGCG in plasma and tissue samples
Acknowledgments
We would like to thank Drs. Sudathip Sae-tan, Tongtong Xu, Yeyi Gu, and Ms. Amy Brownschidle, and Ms. Ling Tao for assisting in harvesting the tissue samples. We would also like to thank Dr. Ryan J. Elias for the use of his LC-MS. This work was funded in part by a grant from the National Institutes of Health (AT004678) and the American Institute for Cancer Research (10A102) to JDL.
Footnotes
Abbreviations: AUC, area under the curve; CI, confidence interval; COMT, catechol-O-methyltransferase; DiMeEGCG, 4′,4″-di-O-methyl-(−)-epigallocatechin-3-gallate; EGCG, (−)-epigallocatechin-3-gallate; GTP, green tea polyphenol; i.g., intragastric; LC-MS, liquid chromatography mass spectrometry; MeEGCG, 4″-O-methyl-(−)-epigallocatechin-3-gallate; OR, odds ratio; TOL, tolcapone
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artali R, Beretta G, Morazzoni P, Bombardelli E, Meneghetti F. Green tea catechins in chemoprevention of cancer: A molecular docking investigation into their interaction with glutathione s-transferase (gst p1-1) J Enzyme InhibMed Chem. 2009;24:287–95. doi: 10.1080/14756360802177282. [DOI] [PubMed] [Google Scholar]
- Baas H, Beiske AG, Ghika J, Jackson M, Oertel WH, Poewe W, Ransmayr G, Auff E, Volc D, Dupont E, Mikkelsen B, Wermuth L, WommPetersen J, Benecke R, Eichhom T, Kolbe H, Oertel W, Schimrigk K, Olsson JE, Palhagen S, Burgunder JM, Ghika A, Regli F, Steck A, Medcalf P. Catechol-o-methyltransferase inhibition with tolcapone reduces the “wearing off” phenomenon and levodopa requirements in fluctuating parkinsonian patients. J Neurol Neurosurg Psych. 1997;63:421–8. doi: 10.1136/jnnp.63.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholome R, Haenen G, Hollman CH, Bast A, Dagnelie PC, Roos D, Keijer J, Kroon PA, Needs PW, Arts IC. Deconjugation kinetics of glucuronidated phase ii flavonoid metabolites by beta-glucuronidase from neutrophils. Drug Metab Pharmacokinet. 2010;25:379–87. doi: 10.2133/dmpk.dmpk-10-rg-002. [DOI] [PubMed] [Google Scholar]
- Brown AL, Lane J, Holyoak C, Nicol B, Mayes AE, Dadd T. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Brit J Nutr. 2011;106:1880–9. doi: 10.1017/S0007114511002376. [DOI] [PubMed] [Google Scholar]
- Chen HD, Sang SM. Biotransformation of tea polyphenols by gut microbiota. J Funct Foods. 2014;7:26–42. [Google Scholar]
- Chen PN, Chu SC, Kuo WH, Chou MY, Lin JK, Hsieh YS. Epigallocatechin-3 gallate inhibits invasion, epithelial-mesenchymal transition, and tumor growth in oral cancer cells. J Agric Food Chem. 2011;59:3836–44. doi: 10.1021/jf1049408. [DOI] [PubMed] [Google Scholar]
- Daniel KG, Landis-Piwowar KR, Chen D, Wan SB, Chan TH, Dou QP. Methylation of green tea polyphenols affects their binding to and inhibitory poses of the proteasome beta5 subunit. Int J Mol Med. 2006;18:625–32. [PubMed] [Google Scholar]
- Doyle AES, Goodman JE, Silber PM, Yager JD. Catechol-o-methyltransferase low activity genotype (comtll) is associated with low levels of comt protein in human hepatocytes. Cancer Lett. 2004;214:189–95. doi: 10.1016/j.canlet.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Hahn K, Lorrain M, Storch A. Tolcapone improves sleep in patients with advanced parkinson’s disease (pd) Arch Gerontol Geriatr. 2010;51:e125–8. doi: 10.1016/j.archger.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Fang MZ, Wang YM, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- Forester SC, Lambert JD. Synergistic inhibition of lung cancer cell lines by ()-epigallocatechin-3-gallate in combination with clinically used nitrocatechol inhibitors of catechol-o-methyltransferase. Carcinogenesis. 2014;35:365–72. doi: 10.1093/carcin/bgt347. [DOI] [PubMed] [Google Scholar]
- Inoue-Choi M, Yuan J, Yang CS, Van Den Berg DJ, Lee M, Gao Y, Yu MC. Genetic association between the comt genotype and urinary levels of tea polyphenols and their metabolites among daily green tea drinkers. Int J Mol Epidemiol Genet. 2010;1:114–23. [PMC free article] [PubMed] [Google Scholar]
- Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001;82:387–98. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- Kweon MH, Adhami VM, Lee JS, Mukhtar H. Constitutive overexpression of nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J Biol Chem. 2006;281:33761–72. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Lee MJ, Lu H, Meng XF, Ju J, Hong J, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133:4172–7. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Ratziu V, Tolosa E, Oertel WH. Safety and tolerability of adjunctive tolcapone treatment in patients with early parkinson’s disease. J Neurol Neurosurg Psych. 2007;78:944–8. doi: 10.1136/jnnp.2006.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew MF, Kricorian G. Results from a 2-year centralized tolcapone liver enzyme monitoring program. Clin Neuropharmacol. 2007;30:281–6. doi: 10.1097/WNF.0b013e318149f290. [DOI] [PubMed] [Google Scholar]
- Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. Glucuronides of tea catechins: Enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003;31:452–61. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- Lu H, Meng XF, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-o-methyltransferase by (−)-epigallocatechin gallate. Drug Metabol Dispos. 2003;31:572–9. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Jackson KG, Dadd T, Mayes AE, Brown AL, Lovegrove JA, Minihane AM. The impact of the catechol-o-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol Nutr Food Res. 2012;56:966–75. doi: 10.1002/mnfr.201100726. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Jackson KG, Dadd T, Nicol B, Dick JL, Mayes AE, Brown AL, Minihane AM. A preliminary investigation of the impact of catechol-o-methyltransferase genotype on the absorption and metabolism of green tea catechins. Eur J Nutr. 2012;51:47–55. doi: 10.1007/s00394-011-0189-0. [DOI] [PubMed] [Google Scholar]
- Mueller T, Woitalla D, Schulz D, Peters S, Kuhn W, Przuntek H. Tolcapone increases maximum concentration of levodopa: Short communication. J Neural Trans. 2000;107:113–9. doi: 10.1007/s007020050010. [DOI] [PubMed] [Google Scholar]
- Myohanen TT, Schendzielorz N, Mannisto PT. Distribution of catechol-o-methyltransferase (comt) proteins and enzymatic activities in wild-type and soluble comt deficient mice. J Neurochem. 2010;113:1632–43. doi: 10.1111/j.1471-4159.2010.06723.x. [DOI] [PubMed] [Google Scholar]
- Ogunleye AA, Xue F, Michels KB. Green tea consumption and breast cancer risk or recurrence: A meta-analysis. Breast Cancer Research and Treatment. 2010;119:477–84. doi: 10.1007/s10549-009-0415-0. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Tolcapone and hepatotoxic effects. Tasmar advisory panel. Arch Neurol. 2000;57:263–7. doi: 10.1001/archneur.57.2.263. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Watkins PB. Tolcapone: An efficacy and safety review (2007) Clin Neuropharmacol. 2007;30:287–94. doi: 10.1097/wnf.0b013e318038d2b6. [DOI] [PubMed] [Google Scholar]
- Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Schneider K, Oltmanns J, Hassauer M. Allometric principles for interspecies extrapolation in toxicological risk assessment--empirical investigations. Regul Toxicol Pharmacol. 2004;39:334–47. doi: 10.1016/j.yrtph.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Tang NP, Wu YM, Zhou B, Wang B, Yu RB. Green tea, black tea consumption and risk of lung cancer: A meta-analysis. Lung Cancer. 2009;65:274–83. doi: 10.1016/j.lungcan.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Ting YW, Jiang Y, Ho CT, Huang QR. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J Funct Foods. 2014;7:112–28. [Google Scholar]
- Ui A, Kuriyama S, Kakizaki M, Sone T, Nakaya N, Ohmori-Matsuda K, Hozawa A, Nishino Y, Tsuji I. Green tea consumption and the risk of liver cancer in japan: The ohsaki cohort study. Cancer Causes & Control. 2009;20:1939–45. doi: 10.1007/s10552-009-9388-x. [DOI] [PubMed] [Google Scholar]
- Wang PW, Heber D, Henning SM. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012;3:635–42. doi: 10.1039/c2fo10254d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xie CL, Wang WW, Lu L, Fu DL, Wang XT, Zheng GQ. Epidemiology of complementary and alternative medicine use in patients with parkinson’s disease. J Clin Neurosci. 2013;20:1062–7. doi: 10.1016/j.jocn.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Watkins P. COMT inhibitors and liver toxicity. Neurol. 2000;55:S51–S2. [PubMed] [Google Scholar]
- Wu AH, Tseng CC, Van den Berg D, Yu MC. Tea intake, comt genotype, and breast cancer in asian-american women. Cancer Res. 2003;63:7526–9. [PubMed] [Google Scholar]
- Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res. 2011;64:113–22. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Yang G, Shu XO, Li HL, Chow WH, Ji BT, Zhang XL, Gao YT, Zheng W. Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev. 2007;16:1219–23. doi: 10.1158/1055-9965.EPI-07-0097. [DOI] [PubMed] [Google Scholar]
- Yin PZ, Zhao JY, Cheng SJ, Hara Y, Zhu QF, Liu ZG. Experimental studies of the inhibitory effects of green tea catechin on mice large intestinal cancers induced by 1,2-dimethylhydrazine. Cancer Lett. 1994;79:33–8. doi: 10.1016/0304-3835(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Chiou YS, Pan MH, Ho CT, Shahidi F. Protective effects of epigallocatechin gallate (EGCG) derivatives on azoxymethane-induced colonic carcinogenesis in mice. J Funct Foods. 2012;4:323–30. [Google Scholar]