Abstract
Objectives
This study aims to evaluate the size-dependent toxicity of spherical silver nanoparticles (Ag NPs) to an endemic benthic organism, Glyptotendipes tokunagai.
Methods
Ag nanoparticles of three nominal sizes (50, 100, and 150 nm) capped with polyvinyl pyrrolidone (PVP-Ag NPs) were used. Their physicochemical properties, acute toxicity (48 hours), and bioaccumulation were measured using third instar larvae of G. tokunagai.
Results
The aggregation and dissolution of PVP-Ag NPs increased with exposure time and concentration, respectively, particularly for 50 nm PVP-Ag NPs. However, the dissolved concentration of Ag ions was not significant compared with the median lethal concentration value for AgNO3 (3.51 mg/L). The acute toxicity of PVP-Ag NPs was highest for the smallest particles (50 nm), whereas bioaccumulation was greatest for the largest particles (150 nm). However, larger PVP-Ag NPs were absorbed and excreted rapidly, resulting in shorter stays in G. tokunagai than the smaller ones.
Conclusions
The size of PVP-Ag NPs significantly affects their acute toxicity to G. tokunagai. In particular, smaller PVP-Ag NPs have a higher solubility and stay longer in the body of G. tokunagai, resulting in higher toxicity than larger PVP-Ag NPs.
Keywords: Bioacummulation, Chironomus, Nanotoxicity, Nanoparticle, Particles size
Introduction
The use of nanomaterials in various commercial products has greatly increased recently, as a consequence of rapid developments in nanotechnology [1,2]. In particular, silver nanoparticles (Ag NPs) that have antibacterial activity are widely used in medical products, mobile devices, cleaning processes, baby care, and textile applications [3-5]. Thus, Ag NPs are likely to enter water bodies and cause adverse effects on aquatic organisms. For example, Ag NPs are known to induce high toxicity to Pseudokirchneriella subcapitata (algae), Daphnia magna (water flea), and Danio rerio (zebrafish) [6,7]. In addition, the impact of NPs in sediment receives more attention, and recent studies have investigated various benthic organisms such as snails and chironomid larvae [8-11]. For instance, Ag NPs have a greater impact on the oxidative stress and detoxification of Chironomus riparius than Ag ions [10].
The toxicity of NPs is largely dependent on their physical and chemical properties, including surface charge and particle size, which affect the dissolution and aggregation of NPs [12]. Positively charged Ag NPs were found to be more toxic to Bacillus cells with a negative charge [13], and smaller Ag NPs showed greater influx rates and bioaccumulation in D. magna [14]. The bioaccumulation of Ag and CuO NPs in a macrobenthic species, Macoma balthica, was also found to depend on the particle size [15]. In general, smaller Ag NPs are dissolved as Ag ions more easily, resulting in greater toxicity [16,17]. However, a previous assessment of the size-dependent uptake or toxicity of Ag NPs to benthic organisms was very limited.
In the present study, the size-dependent toxicity of Ag NPs to Glyptotendipes tokunagai was investigated. Ag NPs were capped with polyvinyl pyrrolidone (PVP) to reduce aggregation. G. tokunagai is a dominant species in urban rivers of Korea that has a short life cycle, a high fecundity, and is easy to culture [18].
Materials and Methods
Chemicals and Test Organisms
PVP-Ag NPs of three nominal sizes (50, 100, and 150 nm) were obtained from Nanotech and Beyond (Yongin, Korea). The PVP-Ag NPs were in a water-based colloid containing 500000 mg/L Ag and about 12% (w/w) PVP as the coating agent. In addition, silver nitrate (AgNO3, 99.9%) was purchased from Kojima Chemicals (Saitama, Japan) and used as the control for Ag ions.
G. tokunagai was collected from Jungrang stream (a branch of Han River in Seoul of Korea) in 2007, and cultured over 30 generations in the laboratory of Prof. Yeon Jae Bae, Korea University, Seoul (Korea). G. tokunagai was reared in aerated tap water at 20±1˚C with a photoperiod of 16 hours/8 hours (light/dark), and Tetra Min (Tetra Werke, Melle, Germany) was provided as food.
Characterization of Polyvinyl Pyrrolidone-Silver Nanoparticles
The morphology of the PVP-Ag NPs was analyzed by transmission electron microscopy (TEM; Tecnai TF20, Austin, TX, USA). The hydrodynamic size and surface charge (zeta potential) Hillsboro, OR, were measured using dynamic light scattering (DLS) and electrophoretic mobility methods, respectively, by a NanoBrook 90Plus Particle Size Analyzer (Brookhaven Instruments, USA). In addition, the dispersion stability of the PVP-Ag NPs was evaluated by measuring surface plasmon resonance (SPR) absorption using a UV vis spectrophotometer (Optizen POP; Mecasys, Daejeon, Korea).
Ag ions released from the PVP-Ag NPs were analyzed using centrifugal ultrafilters with three replicates per treatment [19]. The PVP-Ag NP solution (10 mL) was centrifuged with 10 kDa centrifugal filters (Amicon Ultra-15 centrifugal filter, Millipore Co., Billerica, MA, USA) at 5000 g for 20 minutes. The Ag concentrations in the supernatant were analyzed using an inductively coupled plasma–optical emission spectrophotometer (ICP-OES; Varian Vista PRO, Hayward, CA, USA).
Toxicity and bioaccumulation testing of PVP-Ag NPs
The test medium used in this study was prepared following the US Environmental Protection Agency standard method using moderately hard water (MHW; NaHCO3 =96 mg/L, CaSO4·H2O=60 mg/L, MgSO4=60 mg/L, KCl=4 mg/L) at pH 7.5 with a hardness of 100 mg/L as CaCO3 [20]. The PVP-Ag NP solution was prepared in deionized water (18.2 MΩ cm-1, Esse-UP Water System; Mirae St Co., Anyang, Korea). Acute toxicity tests using G. tokunagai under water-only conditions were conducted according to the Organization for Economic Cooperation and Development standard procedures [20]. Six concentrations of PVP-Ag NPs, ranging from 31.25 to 1000 mg/L, and the control (MHW medium) were prepared. One third instar larva (15 days old) was added to the test solution (10 mL) with two replicates, and each replicate consisted of six individuals. Toxicity tests were conducted at 20±1˚C with a 16 hours light and 8 hours dark photoperiod for 48 hours. After 48 hours of exposure, the mortality of the test organisms was evaluated, and the results are presented in terms of the median lethal concentration (LC50), using the trimmed Spearman-Karber method [21]. G. tokunagai mortality was defined as a lack of response when touched using a fine brush.
Bioaccumulation of PVP-Ag NPs (100 mg/L) in MHW medium with G. tokunagai was observed under the same conditions as the acute toxicity testing. Live individuals were separated at a specific exposure time (1, 2, 4, 8, 12, 24, and 48 hours) and transferred to clean MHW media for 1 hour to remove particles attached to the body and to clear the contents of the gut. The clean larvae were transferred to a 1.5 mL tube, dried at 80˚C, and then weighed (dry weight). The dried larvae were then added to 68% nitric acid (HNO3, Aristar grade), allowed to stand to dissolve the cellular tissue of the organisms, and digested at 110˚C until the acid solution was volatilized. The digestion tube was washed with 2% HNO3, and the washing solutions were transferred to a 15 mL conical tube (SPL Life Science, Pocheon, Korea). The Ag concentrations in the solution were analyzed using an ICP-OES.
Statistical analysis
All statistical analyses were carried out using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). A one-way analysis of variance followed by Tukey’s test was used to identify significant differences between treatments (p<0.05).
Result
Physicochemical Properties of PVP-Ag NPs
TEM images of PVP-Ag NPs with different particle sizes are shown in Figure 1. The PVP-Ag NPs were spherical and the primary particle sizes were 56.57±10.13 nm, 100.06±23.25 nm, and 151.00±39.38 nm for 50 nm, 100 nm, and 150 nm PVP-Ag NPs, respectively. The shape of PVP-Ag NPs, as measured by TEM, was spherical in all cases. The zeta potential and hydrodynamic size of the PVP-Ag NPs in MHW medium are given in Table 1. All PVP-Ag NP samples showed a negative charge, with values larger than -30 mV, which may result in the aggregation of PVP-Ag NPs [22].
Figure 1.
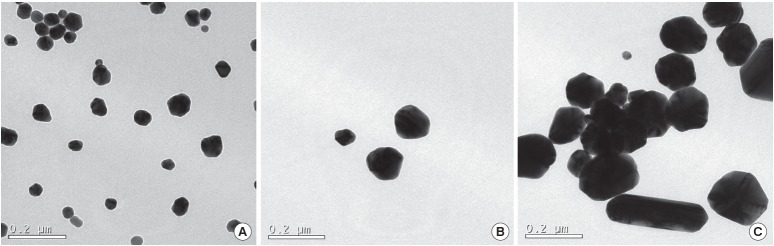
Transmission electron microscopy images of polyvinyl pyrrolidone-silver nanoparticles with nominal particle sizes of (A) 50 nm, (B) 100 nm, and (C) 150 nm.
Table 1.
Hydrodynamic size and zeta potential of PVP-Ag NPs (100 mg/L) in MHW medium as a function of exposure time
| Nominal size (nm) | 50 | 100 | 150 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure time (hr) | 0 | 24 | 48 | 0 | 24 | 48 | 0 | 24 | 48 |
| Hydrodynamic size (nm) | 83.07 | 98.66 | 121.5 | 147.62 | 140.99 | 154.86 | 178.91 | 165.18 | 178.63 |
| 101.1±19.3* | 147.8±6.94* | 174.2±7.85* | |||||||
| Zeta potential (mV) | -2.63 | -4.34 | -4.47 | -7.99 | -5.43 | -6.03 | -7.09 | -10.12 | -5.72 |
PVP-Ag NPs, polyvinyl pyrrolidone-silver nanoparticles; MHW, moderately hard water.
p<0.05.
In fact, hydrodynamic sizes measured by DLS method for 48 hours were larger than the corresponding nominal sizes (101.1±19.3 nm, 147.8±6.94 nm, and 174.2±7.85 nm for 50, 100, and 150 nm PVP-Ag NPs, respectively). In addition, the hydrodynamic sizes increased as the exposure time increased, particularly for 50 nm PVP-Ag NPs. The concentration of Ag ions released from PVP-Ag NPs in MHW medium is shown in Figure 2. The smaller PVP-Ag NPs, particularly for the 50 nm samples, gave dissolved Ag concentrations that were higher than those for the larger particles. Moreover, the solubility increased with increasing exposure concentration.
Figure 2.
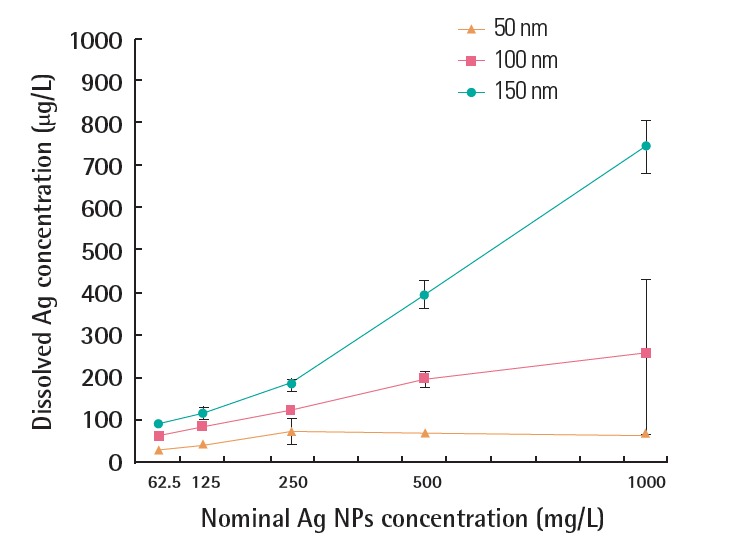
Dissolved concentration of Ag ions released from PVP-Ag NPs in moderately hard water medium after 48 hours exposure. PVP-Ag NPs, polyvinyl pyrrolidone- silver nanoparticles.
Acute toxicity of PVP-Ag NPs to G. tokunagai
The mortality of G. tokunagai exposed to PVP-Ag NPs with different particle sizes is shown in Figure 3. In general, the acute toxicity (48 hours) of PVP-Ag NPs decreased with increasing particle size, so that the LC50 values for 50 nm and 150 nm PVP-Ag NPs were 297.36 and 820.34 mg/L, respectively. No LC50 value was calculated for the 100 nm PVP-Ag NPs, and no acute toxicity was observed for the coating material (PVP).
Figure 3.
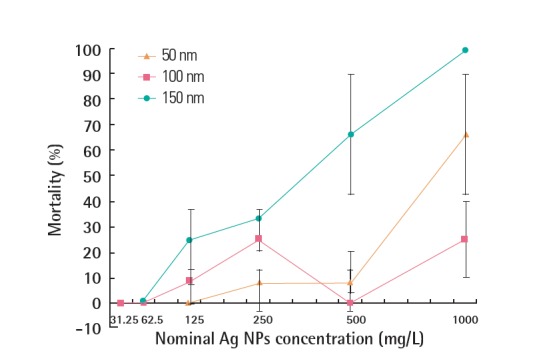
Mortality (48 hours) of G. tokunagai exposed to PVP-Ag NPs as a function of concentration. PVP-Ag NPs, polyvinyl pyrrolidone-silver nanoparticles.
Uptake of PVP-Ag NPs by G. tokunagai during a 48 hours exposure period is shown in Figure 4. Contrary to the results of the acute toxicity tests, the uptake was greater for larger PVP-Ag NPs. In particular, 150 nm Ag NPs were accumulated in a concentration- dependent manner, which was significantly different from those for 50 nm and 100 nm PVP-Ag NPs (p<0.05).
Figure 4.
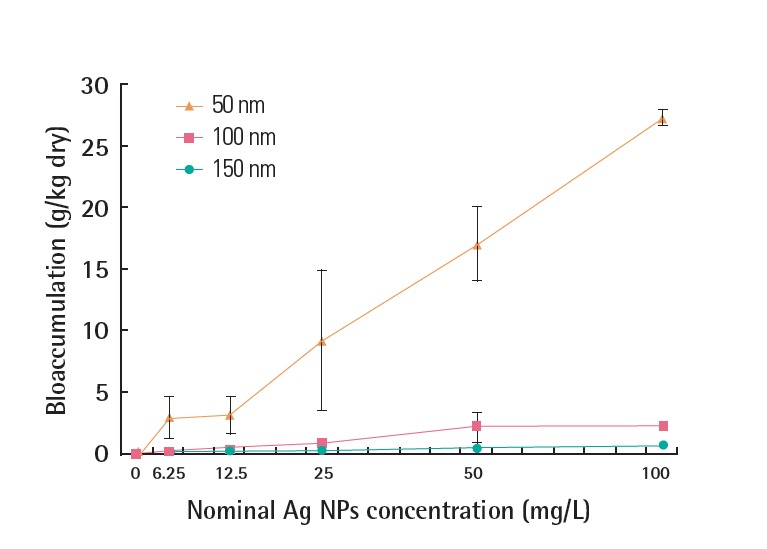
Uptake of PVP-Ag NPs in moderately hard water medium by G. tokunagai after 48 hours exposure. PVP-Ag NPs, polyvinyl pyrrolidone-silver nanoparticles.
Discussion
Dispersion Stability of PVP-Ag NPs
As revealed by DLS measurements (Table 1), the 50 and 100 nm PVP-Ag NPs became larger in MHW medium when compared with the primary particle sizes (Figure 1). In general, ionic strength and pH have no effect on the aggregation of sterically stabilized PVP-Ag NPs [23]. However, electrostatic repulsion may play a role in controlling the stability of PVP-Ag NPs when Ag NPs were partially coated with PVP [24], likely resulting in the aggregation of PVP-Ag NPs in the MHW medium with a higher ionic strength. In addition, the hydrodynamic size of 50 nm PVP-Ag NPs was significantly different from those for 100 and 150 nm PVP-Ag NPs (p<0.05) (Table 1). This indicates that 100 and 150 nm PVP-Ag NPs may show similar behaviors in acute toxicity and bioaccumulation.
UV/Vis absorption spectra were recorded to evaluate the dispersion stability of PVP-Ag NPs in MHW medium (Figure. 5). All of the PVP-Ag NPs showed an absorption peak at about 440 nm, and the intensity of the peak was reduced with increasing primary particle size. The strong absorption peak is a result of the collective oscillations of the metal valence electrons of Ag NPs, known as surface plasmon resonance (SPR) [25]. In general, smaller Ag NPs give a sharp peak with higher intensity [26]. The SPR peak decreased significantly with increasing exposure time, particularly for the 50 nm and 100 nm PVP-Ag NPs, possibly because of the aggregation or sedimentation of PVP-Ag NPs [27]. In general, aggregation increases with increasing collision frequency, which is proportional to the number of particles in a given volume [28]. Considering the same concentration based on the mass of NPs, the number of smaller PVP-Ag NPs should be greater than that of larger PVP-Ag NPs, resulting in a higher possibility of aggregation. These findings suggest that the smaller PVP-Ag NPs were not stable in MHW medium for the exposure period of 48 hours.
Figure 5.
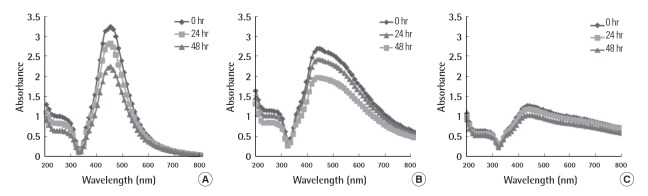
UV/Vis absorption spectra of (A) 50 nm, (B) 100 nm, and (C) 150 nm polyvinyl pyrrolidone-silver nanoparticles in moderately hard water medium as a function of exposure time.
Toxicity of PVP-Ag NPs to G. tokunagai
The solubility of the 50 nm PVP-Ag NPs was much higher compared with the solubility of the 100 nm and 150 nm PVP-Ag NPs, possibly owing to the larger surface area of the smaller 50 nm PVP-Ag NPs [29]. In addition, 50 nm PVP-Ag NPs showed significantly higher acute toxicity to G. tokunagai compared with the 100 nm and 150 nm particles, which is in line with the general fact that smaller particles are more toxic than larger ones [30]. As indicated in Figure 2, the dissolved concentration of Ag released from PVP-Ag NPs was far below the LC50 values for AgNO3 (3.51 mg/L) determined in this study. This suggests that the acute toxicity of PVP-Ag NPs to G. tokunagai is probably not attributable to Ag ions, but to Ag NPs. However, the dissolution of PVP-Ag NPs in the gut of G. tokunagai cannot be ruled out and these Ag ions may contribute to the toxicity observed in this study. Considering that Ag ions can inhibit Na+/K+-ATPase activity in biological membranes, whereas Ag NPs may induce membrane deformation and DNA damage [31], the toxicity mechanism should be further studied in order to identify their relative contributions to the observed toxicity of PVP-Ag NPs.
The uptake of PVP-Ag NPs in G. tokunagai showed the opposite pattern to the acute toxicity results (Figure 3), in which bioaccumulation in G. tokunagai was greater for the 150 nm PVP-Ag NPs (Figure 4). This is also contrary to the result that smaller Ag NPs were accumulated more in D. magna [14]. G. tokunagai, a deposit feeder in sediment, ingests nutrients from particles suspended in sediment, whereas D. magna, a filter feeder, obtains nutrients from water. Thus, theses different feeding habits may be related to their different uptake results. The uptake of PVP-Ag NPs by G. tokunagai as a function of time is shown in Figure 6. The larger PVP-Ag NPs were absorbed and excreted rapidly, resulting in a shorter stay in G. tokunagai. These findings suggest that the higher toxicity of smaller PVP-Ag NPs could be attributed to the longer retention time. In addition, the higher solubility of smaller PVP-Ag NPs may also lead to the observed toxicity difference.
Figure 6.
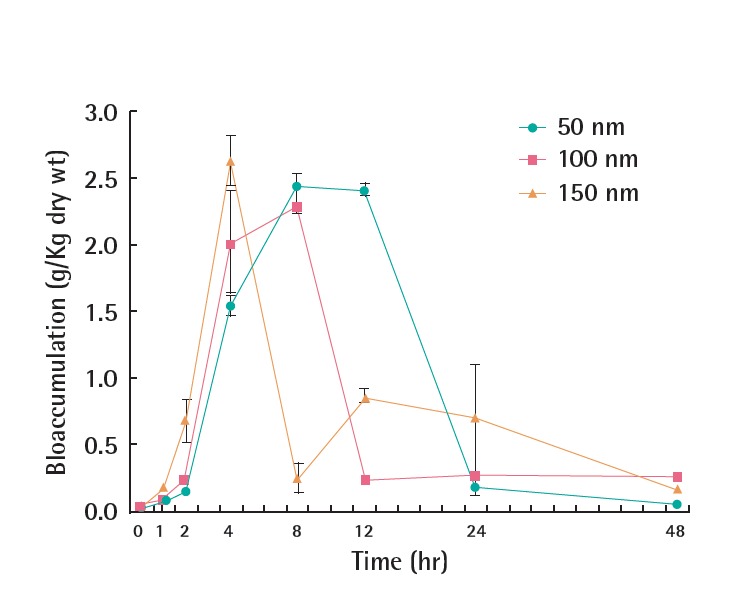
Uptake of polyvinyl pyrrolidone-silver nanoparticles (100 mg/L) by G. tokunagai in moderately hard water medium as a function of exposure time.
In summary, the toxicity of PVP-Ag NPs was very dependent on the particle size. Particularly, smaller PVP-Ag NPs were more toxic to G. tokunagai compared to larger particle, possibly owing to their prolonged stay and higher dissolution in the body. However, the toxicity mechanism of PVP-Ag NPs should be further studied in order to identify the role of Ag ions and NPs more clearly.
Acknowledgments
This study was supported by the research project for Environmental Risk Assessment of Manufactured Nanomaterials (KK-1303-03) funded by the Korea Institute of Toxicology, and by the Korea Ministry of Environment through the project of “Development of integrated model for climate change impact and vulnerability assessment.” The authors would like to thank anonymous reviewers for their valuable comments.
Footnotes
The authors have no conflicts of interest with material presented in this paper.
References
- 1.Kaegi R, Voegelin A, Ort C, Sinnet B, Thalmann B, Krismer J, et al. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res. 2013;47(12):3866–3877. doi: 10.1016/j.watres.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 2.Hendren CO, Badireddy AR, Casman E, Wiesner MR. Modeling nanomaterial fate in wastewater treatment: Monte Carlo simulation of silver nanoparticles (nano-Ag) Sci Total Environ. 2013;449:418–425. doi: 10.1016/j.scitotenv.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 3.Perelshtein I, Applerot G, Perkas N, Guibert G, Mikhailov S, Gedanken A. Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology. 2008;19(24):245705. doi: 10.1088/0957-4484/19/24/245705. [DOI] [PubMed] [Google Scholar]
- 4.Farkas J, Peter H, Christian P, Gallego Urrea JA, Hassellöv M, Tuoriniemi J, et al. Characterization of the effluent from a nanosilver producing washing machine. Environ Int. 2011;37(6):1057–1062. doi: 10.1016/j.envint.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Golovina NB, Kustov LM. Toxicity of metal nanoparticles with a focus on silver. Mendeleev Commun. 2013;23(2):59–65. [Google Scholar]
- 6.Ribeiro F, Gallego-Urrea JA, Jurkschat K, Crossley A, Hassellöv M, Taylor C, et al. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci Total Environ. 2014;467:232–241. doi: 10.1016/j.scitotenv.2013.06.101. [DOI] [PubMed] [Google Scholar]
- 7.Asghari S, Johari SA, Lee JH, Kim YS, Jeon YB, Choi HJ, et al. Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J Nanobiotechnology. 2012;10:14. doi: 10.1186/1477-3155-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pang C, Selck H, Misra SK, Berhanu D, Dybowska A, Valsami- Jones E, et al. Effects of sediment-associated copper to the depositfeeding snail, Potamopyrgus antipodarum: a comparison of Cu added in aqueous form or as nano- and micro-CuO particles. Aquat Toxicol. 2012;107:114–122. doi: 10.1016/j.aquatox.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Nair PM, Park SY, Lee SW, Choi J. Differential expression of ribosomal protein gene, gonadotrophin releasing hormone gene and Balbiani ring protein gene in silver nanoparticles exposed Chironomus riparius. Aquat Toxicol. 2011;101(1):31–37. doi: 10.1016/j.aquatox.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Nair PM, Park SY, Choi J. Evaluation of the effect of silver nanoparticles and silver ions using stress responsive gene expression in Chironomus riparius. Chemosphere. 2013;92(5):592–599. doi: 10.1016/j.chemosphere.2013.03.060. [DOI] [PubMed] [Google Scholar]
- 11.Pang C, Selck H, Banta GT, Misra SK, Berhanu D, Dybowska A, et al. Bioaccumulation, toxicokinetics, and effects of copper from sediment spiked with aqueous Cu, nano-CuO, or micro-CuO in the deposit-feeding snail, Potamopyrgus antipodarum. Environ Toxicol Chem. 2013;32(7):1561–1573. doi: 10.1002/etc.2216. [DOI] [PubMed] [Google Scholar]
- 12.Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, et al. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem. 2008;27(9):1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 13.El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM. Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol. 2011;45(1):283–287. doi: 10.1021/es1034188. [DOI] [PubMed] [Google Scholar]
- 14.Zhao CM, Wang WX. Size-dependent uptake of silver nanoparticles in Daphnia magna. Environ Sci Technol. 2012;46(20):11345–11351. doi: 10.1021/es3014375. [DOI] [PubMed] [Google Scholar]
- 15.Dai L, Syberg K, Banta GT, Selck H, Forbes VE. Effects, uptake, and depuration kinetics of silver oxide and copper oxide nanoparticles in a marine deposit feeder, Macoma balthica. ACS Sustain Chem Eng. 2013;1(7):760–767. [Google Scholar]
- 16.Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A. 2012;100(4):1033–1043. doi: 10.1002/jbm.a.34053. [DOI] [PubMed] [Google Scholar]
- 17.Park Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briedé JJ, et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011;32(36):9810–9817. doi: 10.1016/j.biomaterials.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 18.Baek MJ, Yoon TJ, Bae YJ. Development of Glyptotendipes tokunagai (Diptera: Chironomidae) under different temperature conditions. Environ Entomol. 2012;41(4):950–958. doi: 10.1603/EN14052. [DOI] [PubMed] [Google Scholar]
- 19.Reed RB, Ladner DA, Higgins CP, Westerhoff P, Ranville JF. Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ Toxicol Chem. 2012;31(1):93–99. doi: 10.1002/etc.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Organization for Economic Cooperation and Development (OECD) OECD guidelines for testing of chemicals, section 2. Test no. 235: chironomus sp., acute immobilisation Test. 2011 [cited 2014 Jan 23]. Available from: http://www.oecd-ilibrary.org/environment/oecdguidelines-for-the-testing-of-chemicals-section-2-effects-on-bioticsystems_20745761.
- 21.United States Environmental Protection Agency Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. [cited 2014 Jan 23]. Available from: http://water.epa.gov/scitech/methods/cwa/wet/disk2_index.cfm.
- 22.Jo HJ, Choi JW, Lee SH, Hong SW. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: the importance of their dissolved fraction varying with preparation methods. J Hazard Mater. 2012;228:301–308. doi: 10.1016/j.jhazmat.2012.05.066. [DOI] [PubMed] [Google Scholar]
- 23.Sharma VK, Siskova KM, Zboril R, Gardea-Torresdey JL. Organiccoated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. Adv Colloid Interface Sci. 2014;204:15–34. doi: 10.1016/j.cis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Huynh KA, Chen KL. Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ Sci Technol. 2011;45(13):5564–5571. doi: 10.1021/es200157h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stebounova LV, Guio E, Grassian VH. Silver nanoparticles in simulated biological media: a study of aggregation, sedimentation, and dissolution. J Nanopart Res. 2011;13(1):233–244. [Google Scholar]
- 26.Tejamaya M, Römer I, Merrifield RC, Lead JR. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol. 2012;46(13):7011–7017. doi: 10.1021/es2038596. [DOI] [PubMed] [Google Scholar]
- 27.Zook JM, Long SE, Cleveland D, Geronimo CL, MacCuspie RI. Measuring silver nanoparticle dissolution in complex biological and environmental matrices using UV-visible absorbance. Anal Bioanal Chem. 2011;401(6):1993–2002. doi: 10.1007/s00216-011-5266-y. [DOI] [PubMed] [Google Scholar]
- 28.Suttiponparnit K, Jiang J, Sahu M, Suvachittanont S, Charinpanitkul T, Biswas P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticledispersion properties. Nanoscale Res Lett. 2011;6:27. doi: 10.1007/s11671-010-9772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobias J, Bernier-Latmani R. Silver release from silver nanoparticles in natural waters. Environ Sci Technol. 2013;47(9):4140–4146. doi: 10.1021/es304023p. [DOI] [PubMed] [Google Scholar]
- 30.Scanlan LD, Reed RB, Loguinov AV, Antczak P, Tagmount A, Aloni S, et al. Silver nanowire exposure results in internalization and toxicity to Daphnia magna. ACS Nano. 2013;7(12):10681–10694. doi: 10.1021/nn4034103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao CM, Wang WX. Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem. 2011;30(4):885–892. doi: 10.1002/etc.451. [DOI] [PubMed] [Google Scholar]


