Summary
Histaminergic neurons in the tuberomammilary nucleus (TMN) of the hypothalamus form a widely projecting, wake-active network that sustains arousal. Yet most histaminergic neurons contain GABA. Selective siRNA knockdown of the vesicular GABA transporter (vgat, SLC32A1) in histaminergic neurons produced hyperactive mice with an exceptional amount of sustained wakefulness. Ablation of the vgat gene throughout the TMN further sharpened this phenotype. Optogenetic stimulation in the caudate-putamen and neocortex of “histaminergic” axonal projections from the TMN evoked tonic (extrasynaptic) GABAA receptor Cl− currents onto medium spiny neurons and pyramidal neurons. These currents were abolished following vgat gene removal from the TMN area. Thus wake-active histaminergic neurons generate a paracrine GABAergic signal that serves to provide a brake on overactivation from histamine, but could also increase the precision of neocortical processing. The long range of histamine-GABA axonal projections suggests that extrasynaptic inhibition will be coordinated over large neocortical and striatal areas.
Highlights
-
•
Histaminergic axons corelease GABA into the neocortex and striatum
-
•
The released GABA produces slow tonic inhibition
-
•
Reducing vgat expression in histaminergic neurons increases wakefulness
-
•
Histamine-GABA axons will coordinate tonic inhibition over large cortical areas
Hypothalamic histamine neurons are well known as excitatory wake-promoting neurons. But they also contain GABA. In this paper, Yu et al. functionally demonstrate that histaminergic axons in the neocortex corelease a slow paracrine GABA signal to suppress wakefulness.
Introduction
Histaminergic neurons in the tuberomammilary nucleus (TMN), a region of the posterior hypothalamus, help sustain wakefulness (Anaclet et al., 2009; Haas et al., 2008; Lin et al., 1988, 1989; Monnier et al., 1967; Nicholson et al., 1991; Parmentier et al., 2002, 2009; Saper et al., 2010; Wada et al., 1991; Yu et al., 2014). They are the sole source of neuronal histamine and send axons throughout the brain (Bayliss et al., 1990; Panula et al., 1984; Watanabe et al., 1983). TMN neurons become active just after waking and fire at an average rate of about 5 Hz, and their activity is suppressed during sleep (Sakai et al., 2010; Saper et al., 2010; Takahashi et al., 2006). Histamine modulates diverse circuitries (Ellender et al., 2011; Haas et al., 2008; Wada et al., 1991). Histamine can suppress, for example, glutamate or GABA inputs to local circuits by activating hetero-H3 metabotropic receptors on terminals; it can also depolarize cells via H1 and H2 metabotropic receptors or cause phosphorylation of ion channels that influence firing rate (Atzori et al., 2000; Ellender et al., 2011). These effects can occur in the same local circuitry. At the behavioral level, the net effects of histamine’s actions on circuits are enhanced aspects of wakefulness such as cognition, locomotion, feeding, and motivation (Torrealba et al., 2012).
Physiological investigations have focused on the histaminergic aspect of TMN neurons. But these neurons also contain glutamic acid decarboxylase (GAD) enzymes and GABA itself (Airaksinen et al., 1992; Senba et al., 1985; Takeda et al., 1984; Trottier et al., 2002; Vincent et al., 1983). GABA’s presence in histaminergic neurons is conserved, from fish through to humans, suggesting a core function (Sundvik and Panula, 2012; Trottier et al., 2002). It is not known what this function is, and there has been no demonstration that these cells actually release GABA. Optogenetic stimulation of histaminergic fibers in the ventral lateral preoptic (VLPO) area of the hypothalamus found that histamine release stimulated local GABAergic interneurons (Williams et al., 2014), but provided no evidence of GABA release from the histamine fibers.
Varicosities on TMN axons broadcast histamine by volume transmission—histaminergic neurons rarely use synapses (Takagi et al., 1986). So it is likely that if these same TMN axons do release GABA, then this particular source of GABA would also function, similar to histamine, in a paracrine manner that would principally affect extrasynaptic receptors. Because GABA is an electroneutral zwitterion at physiological pH, it has diminished interactions with the extracellular matrix. Thus GABA is well suited for diffusion over long distances (Roberts and Sherman, 1993). Ambient (nonsynaptic) GABA produces sustained inhibitory currents by activating high-affinity extrasynaptic ionotropic GABAA receptors (Brickley et al., 1996; Brickley and Mody, 2012; Lee and Maguire, 2014); this is termed the tonic inhibitory conductance (Gtonic).
In this paper we start by showing that selective pharmacogenetic stimulation of histamine neurons enhances behavioral arousal. We then demonstrate that knocking down or removing vgat gene expression from histaminergic neurons increases locomotion, and causes a substantial increase in wakefulness during the night. Optogenetically stimulating histaminergic fibers in the neocortex and caudate-putamen increases Gtonic onto pyramidal neurons and medium spiny cells, respectively. Eliminating vgat from histaminergic neurons selectively abolishes these evoked tonic inhibitory currents. Thus, during wakefulness, GABA can be deposited widely in neocortical and striatal circuitry by volume transmission. The GABA and histamine TMN components work together to regulate the amount of wakefulness.
Results
Histaminergic Neurons Stimulate Arousal but Are Also GABAergic
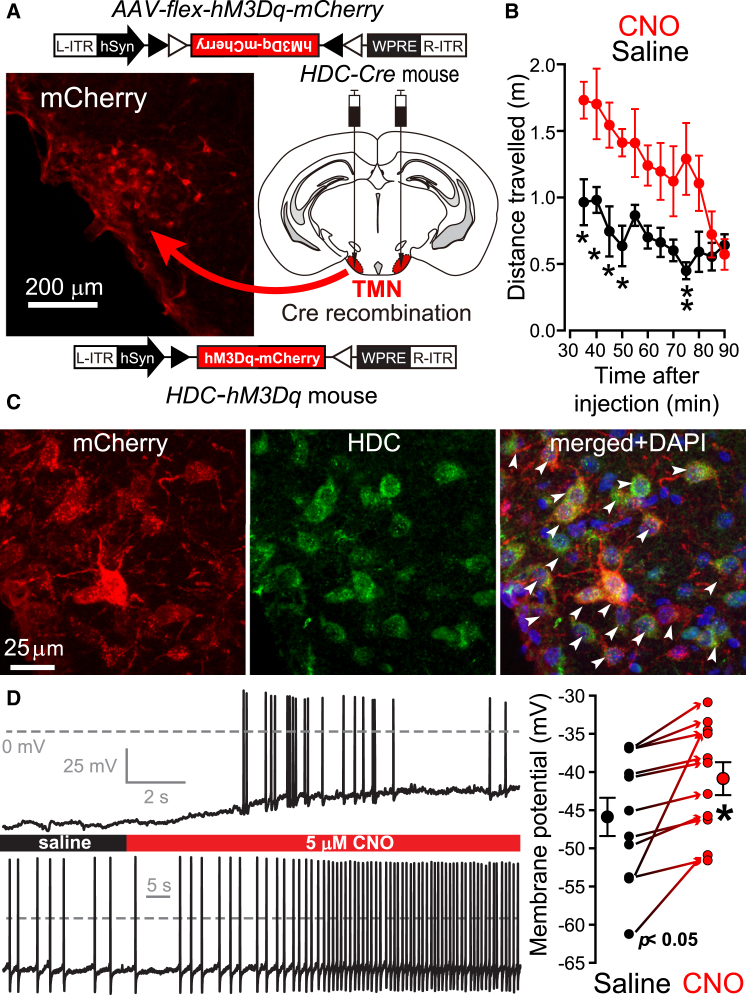
We first tested the excitatory nature of histaminergic neurons on behavior by selectively stimulating them pharmacogenetically in vivo with DREADD hM3Dq-mCherry receptors and clozapine-N-oxide (CNO) (Alexander et al., 2009; Krashes et al., 2011). The hM3Dq receptor is an excitatory metabotropic receptor. Histaminergic neurons can be genetically targeted because of their unique expression of the histidine decarboxylase (hdc) gene, allowing the generation of HDC-Cre mice (Yu et al., 2014; Zecharia et al., 2012). The TMN area of HDC-Cre mice was bilaterally transduced with AAV-flex-hM3Dq-mCherry (Figure 1A) to produce HDC-hM3Dq mice. Intraperitoneal administration of CNO, but not saline, to these HDC-hM3Dq mice significantly increased their motor activity in an open area (Figure 1B) (saline, n = 4; CNO, n = 4, two-way ANOVA and post hoc Bonferroni, ∗p < 0.05; ∗∗p < 0.01; measurements taken 30 min post CNO or saline injection). In these mice, the hM3Dq-mCherry receptor was selectively expressed in most histaminergic neurons (348 mCherry-positive cells out of 405 HDC-positive cells were counted across three animals; 85% ± 3.5% of the HDC-neurons were mCherry positive) (Figure 1C). Using whole-cell recording in acute slices from the TMN area of HDC-hM3Dq mice (n = 4 mice), we demonstrated that CNO significantly (paired t test, p < 0.05) depolarized TMN neurons by ∼5 mV (n = 11 cells, control, −46 ± 3 mV; CNO, −41 ± 2 mV). This was sufficient to elicit action potential (AP) firing in silent cells (5 out of 11) or increase AP firing rate in spontaneously active cells (Figure 1D). Thus, selectively activating histaminergic neurons generated hyperlocomotion and behavioral arousal.
Figure 1.
Pharmacogenetic Activation of Histaminergic Neurons Increased Motor Activity of HDC-hM3Dq Mice
(A) AAV-flex-hM3Dq-mCherry was bilaterally injected into the TMN region of HDC-Cre mice to give hM3Dq-mCherry expression selectively within histaminergic neurons (see inset image).
(B) Activity of HDC-hM3Dq mice in an open field arena 30 min after saline (black trace, n = 4 mice) or CNO (red trace, n = 4 mice) injection. The distance moved was calculated every 5 min and average values (mean ± SEM) plotted over 60 min (∗p < 0.05; ∗∗p < 0.01).
(C) Double-label immunocytochemisty of HDC- and hM3Dq-mCherry-positive neurons with HDC antisera and mCherry antibody; arrowheads indicate examples of double-labeled neurons. DAPI labeling was included to locate all cells.
(D) Two examples of voltage traces recorded from HDC-hM3Dq mice during the application of CNO. The top trace shows an example of a silent neuron that was sufficiently depolarized to fire APs. The bottom trace shows a spontaneously active neuron with increased AP firing in the presence of CNO. On average there was a significant (paired t test, p < 0.05) ∼5 mV depolarization of TMN neurons (n = 11 cells, control, −46 ± 3 mV; CNO, −41 ± 2 mV). The results from each cell are shown on the scatterplot on the right.
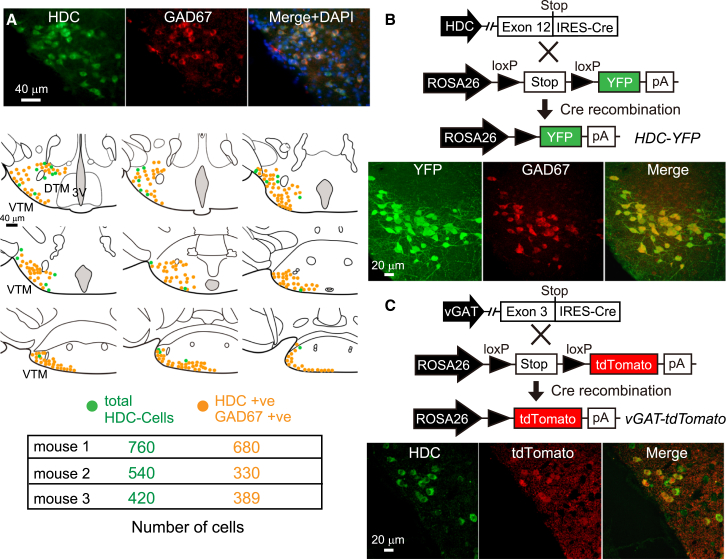
In spite of this excitatory effect on behavior, histaminergic TMN cells contain GABA and its synthetic enzyme GAD67 (gad1) (Airaksinen et al., 1992; Takeda et al., 1984; Vincent et al., 1983). By immunocytochemistry, we found that a majority of HDC-positive neurons costained strongly with GAD67 antisera (1,399 GAD67-positive cells out of 1,720 HDC-positive cells were counted across three animals; 81% ± 10% of the HDC neurons were GAD67 positive; Figure 2A). We corroborated this result genetically by crossing HDC-Cre mice with conditional rosa-lox-stop-lox-YFP mice (Srinivas et al., 2001). In these crosses, 85% ± 5% (n = 3 mice) of YFP-positive neurons in the TMN also expressed GAD67 (Figure 2B). Conventionally, if histaminergic neurons were to release GABA, they should also express the vesicular GABA transporter (vgat, Slc32a1) gene. With the exception of midbrain dopamine neurons (Tritsch et al., 2012, 2014), vGAT has so far proved essential for all neurons that release GABA (Tong et al., 2008; Wojcik et al., 2006); vgat expression, however, has not been demonstrated for HDC cells. Crossing vGAT-Cre mice (Vong et al., 2011) with those containing a rosa-lox-stop-lox-tdTomato allele (Madisen et al., 2010) gave extensive tdTomato/HDC soma costaining in the TMN (Figure 2C). Thus histaminergic neurons express the vgat gene and have the potential to package GABA into vesicles.
Figure 2.
The Majority of Histaminergic Neurons Express GABAergic Markers
(A) Coronal section of the mouse posterior hypothalamus stained with HDC (green) and GAD67 (red) antisera. The diagram summarizes the staining from the whole TMN region: HDC-positive cells (green) and double-positive cells (orange). 3V, third ventricle; VTM, ventral tuberomammillary; DTM, diffuse tuberomammillary.
(B) HDC-Cre mice were crossed with Rosa26-loxP-stop-loxP-YFP mice to generate HDC-YFP mice. TMN sections from HDC-YFP mice were costained with EYFP and GAD67 antisera. Most YFP-positive (HDC neurons) in the TMN were also GAD67-positive.
(C) vGAT-Cre mice were crossed with Rosa26-loxP-stop-loxP-tdTomato mice to generate vGAT-tdTomato mice. Sections from vGAT-tdTomato mice were stained with HDC antisera. Most HDC neurons were tdTomato positive.
Genetic Disruption of GABA Function in Histaminergic Neurons
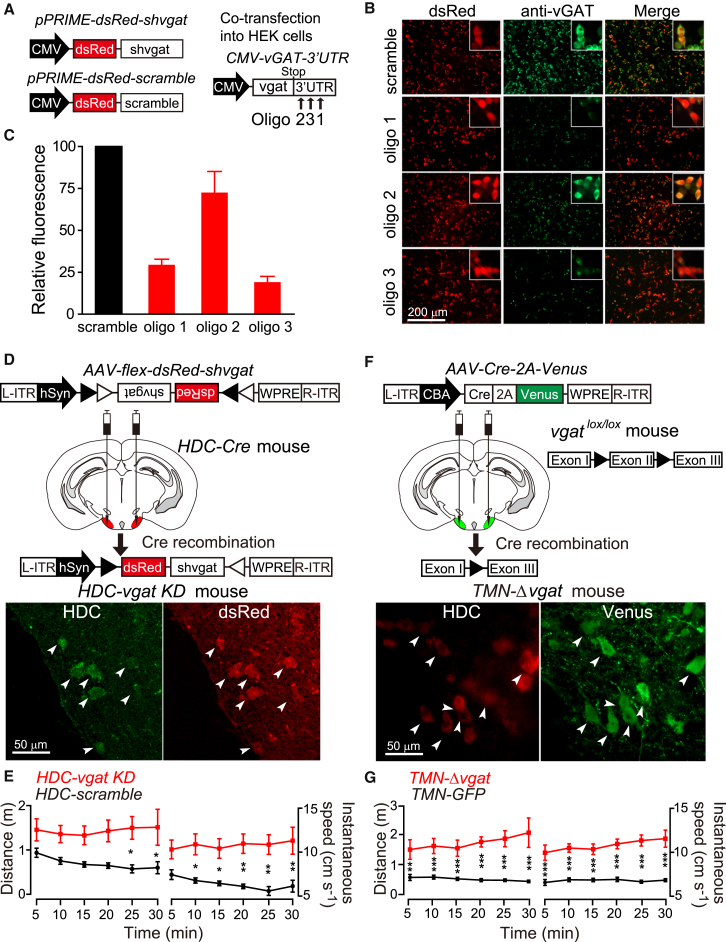
To remove the putative GABAergic function from histaminergic neurons, we considered crossing either vgatlox/lox mice or double gad1lox/lox/gad2lox/lox mice with the HDC-Cre mice. However, the hdc-cre, vgat, gad1, and gad2 genes are all in the same region of mouse chromosome 2, which precluded easy generation of homozygous HDC-Cre/vgatlox/lox or HDC-Cre/gad1lox/lox/gad2lox/lox animals. We therefore developed vgat-selective shRNA constructs to knock down vgat expression (Figure 3 and see Figure S1 available online). For this we placed putative vgat shRNA sequences into a microRNA gene, mir30, in the 3′ untranslated region of a transcript that also encodes dsRed (Stegmeier et al., 2005). These constructs were termed dsRed-shvgat (oligo1, oligo2, or oligo3) (Figure 3A). We tested how well these constructs could reduce vgat reporter gene expression in transfected HEK293 cells by cotransfecting dsRed-shvgat (oligo1, oligo2, or oligo3) with CMV-vGAT, respectively. Two shRNAs strongly knocked down vgat reporter gene expression in HEK cells (Figures 3B and 3C, p < 0.05, t test). Before moving to the more challenging area of the TMN, we used a striatum-based motor assay to demonstrate that the constructs, delivered in AAVs, worked effectively in vivo (Figure S1). To compare the behavior produced by knockdown and knockout of vGAT in the caudate-putamen (CPu), we unilaterally injected AAV-dsRed-shvgat and AAV-Cre-2A-Venus into wild-type and vgatlox/lox mice, respectively, to generate CPu-vgat KD and CPu-Δvgat mice (Figures S1A and S1D). Both CPu-vgat KD and CPu-Δvgat groups had significantly decreased vgat transcript levels (Figures S1B and S1E) and increased ipsilateral turning compared with controls (Figures S1C and S1F).
Figure 3.
Knocking Down and Knocking Out vgat Expression from Histaminergic Neurons in the TMN
(A) Three hairpin (sh) oligonucleotides (sholigo), targeting the vgat transcript, were designed. Each sholigo was separately cloned into the pPRIME-dsRed vector; a scramble shRNA was also cloned into pPRIME-dsRed.
(B) To test the knockdown efficiency of the three shvgat oligonucleotides in vitro, CMV-dsRed-scramble or CMV-dsRed-shvgat (1, 2, or 3) were cotransfected with pCMV-vGAT-3′UTR (a cDNA containing the vgat open reading frame and 3′ untranslated region) into HEK293 cells. Thirty-six hours later, cells were fixed and stained with vGAT antisera. vGAT expression was best reduced with sholigo1 and sholigo3.
(C) Three independent transfections were performed, and relative vGAT immunofluorescence in HEK cells was quantified.
(D) AAV-flex-dsRed-shvgat (oligo3) was bilaterally injected into the TMN region of HDC-Cre mice. Cre recombination produced dsRed-shvgat expression in HDC-positive neurons, and the resulting mice were termed HDC-vgat KD mice. Arrowheads indicate examples of colabeled cells.
(E) HDC-vgat KD mice were more active than HDC-scramble (AAV-flex-dsRed-scramble-injected HDC-Cre) mice in an open field assay (∗p < 0.05; ∗∗p < 0.01).
(F) AAV-Cre-2A-Venus was bilaterally injected into the TMN region of vgatlox/lox mice to generate TMN-Δvgat mice. Sections from virus-injected (TMN-Δvgat) mice were costained with HDC and GFP (Venus) antisera. In the TMN region, GFP expression was in 77% ± 2% of HDC neurons. Arrowheads indicate examples of colabeled cells.
(G) TMN-Δvgat mice ran further than TMN-GFP (AAV-GFP-injected vgatlox/lox) mice in an open field assay (∗∗p < 0.01; ∗∗∗p < 0.001). During each running episode TMN-Δvgat mice also ran faster, as evidenced by instantaneous speed measurements (∗∗p < 0.01; ∗∗∗p < 0.001).
To knock down vgat expression selectively in histaminergic neurons, we placed the dsRed-shvgat and dsRed-scramble cassettes in reverse orientation between heterologous lox sites (a FLEX switch) (Figure 3D), so that expression could only be induced by CRE recombinase (Atasoy et al., 2008). The AAV-flex-dsRed-shvgat or AAV-flex-dsRed-scramble viruses were bilaterally injected into the TMN area of HDC-Cre mice (Figure 3D). The groups of mice are referred to as HDC-vgat KD and HDC-scramble, respectively. We also injected AAV-Cre-2A-Venus and AAV-GFP into the TMN area of vgatlox/lox mice to produce TMN-Δvgat and TMN-GFP mice, respectively (Figure 3F). In TMN-Δvgat mice, the injections covered the TMN area; 77% ± 2% of HDC-positive neurons expressed Cre-2A-Venus (Figure S2). In the TMN, all of the GAD67-positive neurons also contained HDC (Figure 2A). These TMN-Δvgat mice formed an approximate comparison for the HDC-vgat KD mice, with the caveat that some GABAergic cells in the TMN area in addition to the histaminergic cells could be affected. We tested the vgat gene expression levels in the TMN area of HDC-vgat KD and TMN-Δvgat mice by both qPCR on tissue punches and also single-cell qPCR from identified neurons in acute brain slices prepared from the TMN area (Figure S3). The tissue-punch analysis showed that in both TMN-Δvgat and HDC-vgat KD mice, vgat mRNA levels in the TMN were significantly decreased compared with those in TMN-GFP or HDC-scramble mice (TMN-GFP, 1 ± 0.06 versus TMN-Δvgat, 0.5 ± 0.06, t test, ∗∗p < 0.01; HDC-scramble, 1 ± 0.04 versus HDC-vgat KD, 0.76 ± 0.05, t test, ∗p < 0.05) (Figures S3A–S3C). The single-cell qPCR showed that in both TMN-Δvgat and HDC-vgat KD mice, the vgat mRNA levels from identified single neurons were also significantly decreased compared with those in TMN-GFP or HDC-scramble mice (TMN-GFP, 1 ± 0.46 versus TMN-Δvgat, 0.06 ± 0.02, t test, ∗p < 0.05; HDC-scramble, 1 ± 0.24 versus HDC-vgat KD, 0.35 ± 0.17, t test, ∗p < 0.05) (Figures S3D–S3G). The content of histamine in the neocortex and caudate-putamen of HDC-vgat KD and TMN-Δvgat mice was unchanged (see Figure S4).
HDC-vgat KD and TMN-Δvgat Mice Are Hyperactive
We first quantified the general activity of the mice. In an open field assay, HDC-vgat KD mice ran significantly further and had higher instantaneous speeds than the HDC-scramble controls, maintaining a stable and high activity for the 30 min duration of the test (Figure 3E; HDC-scramble, n = 7; HDC-vgat KD, n = 8, two-way ANOVA and post hoc Bonferroni, ∗p < 0.05; ∗∗p < 0.01). Similarly, the TMN-Δvgat mice were continuously more active compared with TMN-GFP control mice (Figure 3G; TMN-GFP, n = 10; TMN-Δvgat, n = 6, two-way ANOVA and post hoc Bonferroni, ∗∗p < 0.01; ∗∗∗p < 0.001). In fact, the activity of the TMN-Δvgat mice, as measured by instantaneous speed and distance covered, tended to increase toward the end of the experiment (Figure 3G).
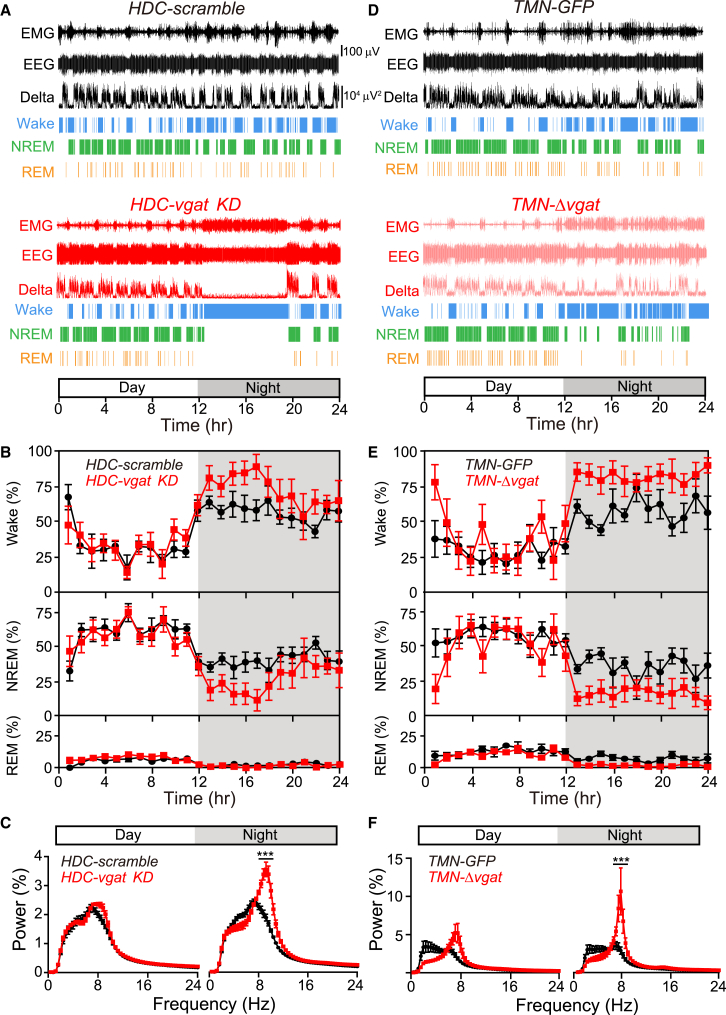
HDC-vgat KD and TMN-Δvgat Mice Have Increased and Sustained Wakefulness during the Night
We used EEG/EMG analysis to assess the sleep-wake cycle (Figures 4 and S5A). The HDC-vgat KD and TMN-Δvgat mice exhibited similar changes in their sleep-wake behavior, largely confined to the night. First we describe the HDC-vgat KD (n = 6) and HDC-scramble (n = 6) groups (Figures 4A–4C and S5B). For the first half of the night, HDC-vgat KD mice had significantly more wake than the HDC-scramble controls (Figures 4A, 4B, and S5B) (wake, HDC-scramble, 400 ± 13 min versus HDC-vgat KD, 511 ± 15 min, t test, ∗∗∗p < 0.001). The example in Figure 4A shows the EEG from an HDC-vgat KD mouse that was awake continuously for 6 hr during the first half of the night. In fact, during the night, HDC-vgat KD mice had only about 65% of normal NREM sleep compared with HDC-scramble mice (NREM, HDC-scramble, 293 ± 12 min versus HDC-vgat KD, 190 ± 14 min, t test, ∗∗∗p < 0.001). Their REM sleep was also slightly decreased (HDC-scramble, 22 ± 2 min versus HDC-vgat KD, 17 ± 2 min, t test, ∗p < 0.05). The EEG θ power of HDC-vgat KD mice was significantly increased selectively during the night (Figure 4C) (two-way ANOVA and post hoc Bonferroni, ∗∗∗p < 0.001), consistent with the heightened wakefulness of HDC-vgat KD mice (Figure 4B). In the day, both the HDC-vgat KD and HDC-scramble mice showed a similar sleep-wake pattern—with the same amount of NREM sleep and wake (Figures 4A, 4B, and S5B; wake, HDC-scramble (n = 6), 243 ± 10 min versus HDC-vgat KD (n = 6), 252 ± 18 min, t test, p > 0.05; NREM, HDC-scramble (n = 6), 425 ± 10 min versus HDC-vgat KD (n = 6), 409 ± 12 min, t test, p > 0.05).
Figure 4.
GABA Release from Histaminergic Neurons Governs the Amount of Sleep
(A) Continuous EMG, EEG, delta power, wake, NREM sleep, and REM sleep scoring data recorded for an HDC-scramble and an HDC-vgat KD mouse for 24 hr.
(B) The graphs illustrate the average 24 hr sleep scoring (percentage of wake, NREM, or REM sleep) for HDC-scramble (black trace) and HDC-vgat KD (red trace) mice; bars, SEM.
(C) Comparison of the power spectra of wake obtained from EEG data recorded during the day and night for the HDC-scramble (black trace) and HDC-vgat KD (red trace) mice.
(D) Continuous EMG, EEG, delta power, wake, NREM sleep, and REM sleep scoring data recorded from TMN-GFP and TMN-Δvgat mice.
(E) The graphs illustrate the average 24 hr sleep scoring (percentage of wake, NREM, or REM sleep) for TMN-GFP (black trace) and TMN-Δvgat (red trace) mice; bars, SEM.
(F) Comparison of the power spectra of wake obtained from EEG data recorded during the day and night for the TMN-GFP (black trace) and TMN-Δvgat (red trace) mice.
Similar to HDC-vgat KD mice, TMN-Δvgat mice had a strong “sustained wakefulness” phenotype confined to the night (Figures 4D, 4E, and S5C). Compared with the TMN-GFP controls (n = 5), TMN-Δvgat (n = 7) animals were awake nearly the whole night (TMN-GFP, 411 ± 9 min versus TMN-Δvgat, 586 ± 27 min, t test, ∗∗∗p < 0.001). Conversely, these TMN-Δvgat mice exhibited little NREM sleep during the dark period (Figures 4E and S5C; TMN-GFP, 258 ± 12 min versus TMN-Δvgat, 118 ± 25 min, t test, ∗∗p < 0.01). Their amount of REM sleep was also decreased (TMN-GFP, 48 ± 9 min versus TMN-Δvgat, 13 ± 3 min, t test, ∗∗p < 0.01). Similar to the HDC-vgat KD mice, selectively during the night, TMN-Δvgat mice had a strong increase of their maximum θ power frequency (Figure 4F). Thus both the HDC-vgat KD and TMN-Δvgat mice slept less. Their loss of sleep during the dark phase was not balanced by a corresponding increase in sleep duration during the day (two-way ANOVA and post hoc Bonferroni, ∗∗∗p < 0.001).
After Sleep Deprivation, HDC-vgat KD and TMN-Δvgat Mice Still Sleep Less Than Control Mice, Maintaining Their Hyperactive State
Given the lack of sleep displayed by the HDC-vgat KD and TMN-Δvgat mice during a typical 24 hr cycle, we investigated how they behaved after sleep deprivation. Compared with control mice, would they demonstrate more recovery sleep to compensate for general lack of sleep during the normal sleep-wake cycle? Mice were sleep deprived for 5 hr during the beginning of the day by presenting them with novel objects. After sleep deprivation, mice were allowed recovery sleep in their home cages (Figure S6). Compared with the control mice, HDC-vgat KD mice had less NREM recovery sleep (Figures S6A and S6B), but the rate at which they reaccumulated their lost NREM sleep (∼12.5 extra minutes NREM sleep recovered/hour) was similar to controls (Figure S6C). TMN-Δvgat mice behaved more extremely (Figures S6D and S6E). After sleep deprivation they were awake longer than control sleep-deprived mice and some 16 hr after sleep deprivation were spending most of their time awake (Figure S6E). However, as for the TMN-Δvgat mice, the rate at which they reaccumulated their lost NREM sleep (∼12 extra minutes NREM sleep recovered/hour) was similar to controls (Figure S6F). Thus HDC-vgat KD and TMN-Δvgat mice sleep less than control mice, maintaining their hyperactive state even after sleep deprivation.
Histaminergic Axons in Neocortex and Striatum Release Paracrine GABA
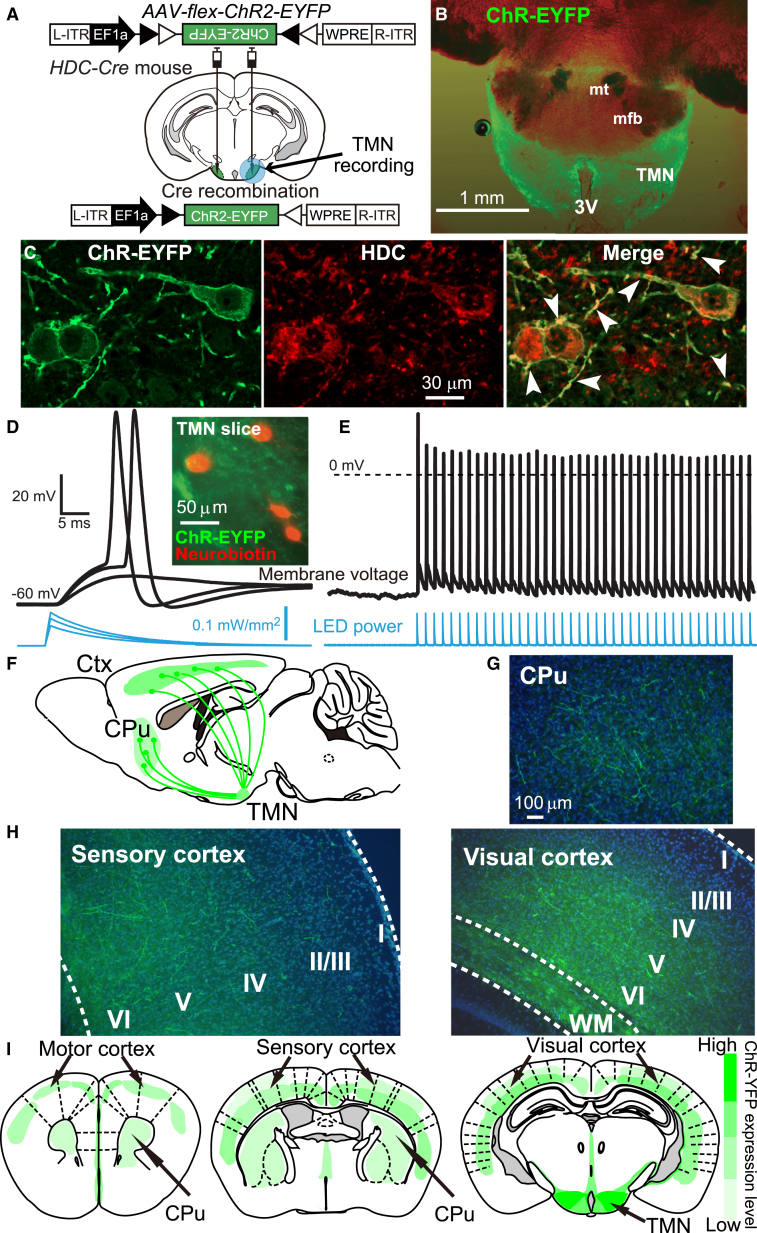
Histamine neurons can globally coordinate behavioral states because their axons spread and diverge throughout the brain (Panula et al., 1989; Takagi et al., 1986; Wada et al., 1991). Can the axonal projections of histaminergic neurons release GABA, and does this release require vGAT? The behavioral results with HDC-vgat KD and TMN-Δvgat mice imply that this is the case. We addressed this directly using optogenetic stimulation in two types of mice: HDC-Channel-Rhodopsin(ChR) mice and TMN-Δvgat/HDC-ChR mice. To make HDC-ChR mice, the TMN area of HDC-Cre mice was bilaterally injected with AAV-flex-ChR2H134R-EYFP (Figures 5A and 5B). A TMN acute slice from HDC-ChR mice with ChR-EYFP primary fluorescence is shown in Figure 5B. The ChR-EYFP fusion protein was enriched in the membrane of HDC-neurons, including their axons (Figures 5B and 5C). Brief 1 ms stimuli were delivered to the LED every 200 ms, resulting in a 460 nm 0.1 mW/mm2 peak light pulse that decayed with a time course of 10 msec. A continuous 5 Hz protocol was delivered for 3 min to HDC-ChR-EYFP-positive neurons in acute TMN slices (Figure 5D). This ChR stimulation protocol was capable of entraining action potential firing at 5 Hz (Figure 5E), corresponding to wake-active firing rates of TMN neurons (Takahashi et al., 2006). The ChR-EYFP fusion protein is an excellent substrate for anterograde axonal transport (Petreanu et al., 2007; Tye and Deisseroth, 2012). EYFP-positive fibers, marking the presence of ChR, were present in both the caudate-putamen (Figures 5F and 5G) and all neocortical areas examined e.g., sensory, motor, and visual neocortex (Figures 5H and 5I). The TMN-Δvgat/HDC-ChR mice have light-sensitive HDC neurons with deleted vgat gene expression. To make these, we coinjected AAV-Cre-2A-Venus and AAV-flex-ChR2-EYFP bilaterally into the TMN area of vgatlox/lox mice (Figures 6A and 6B).
Figure 5.
Selective ChR2-EYFP Expression in Histaminergic Neurons and Tracing Their Axons to Neocortex and Striatum
(A) AAV-flex-ChR2-EYFP was bilaterally injected into the TMN region of HDC-Cre mice. The blue circle indicates the area of the slice that was optically stimulated.
(B) Cre recombination produced ChR2-EYFP expression within HDC-expressing neurons. Shown is a combined bright-field and primary fluorescence photograph, taken at low magnification, of a freshly cut coronal brain slice used for electrophysiological recording from TMN neurons.
(C) ChR-EYFP expression in the TMN imaged with antisera to GFP (green) and HDC (red), with arrowheads indicating ChR2 expression in processes.
(D) The duration of the LED power output (blue trace) measured from the objective lens, and the membrane voltage recorded (black trace) from the soma of a HDC-ChR2-EYFP neuron. Increasing the LED power depolarized the membrane to generate action potentials. Inset image: four neurons recorded from this slice with cofluorescence for ChR-EYFP (green) and postrecording neurobiotin fill (red).
(E) The same cell as (D) firing action potentials with 5 Hz light stimulation.
(F) Schematic of the fibers (axons) which, following AAV-flex-ChR2-EYFP injection into the TMN of HDC-Cre mice, transported ChR-EYFP from the HDC-ChR2-EYFP soma into the neocortex and caudate-putamen.
(G and H) Low-power photographs of the ChR2-EYFP-positive fibers in the caudate-putamen (CPu) (G) and sensory and visual cortex (H). Blue, DAPI; I, layer I; II, layer II; III, layer III; IV, layer IV; V, layer V; VI, layer VI; WM, white matter.
(I) Schematic of the ChR2-EYFP fiber distribution in the caudate-putamen (CPu) and neocortex.
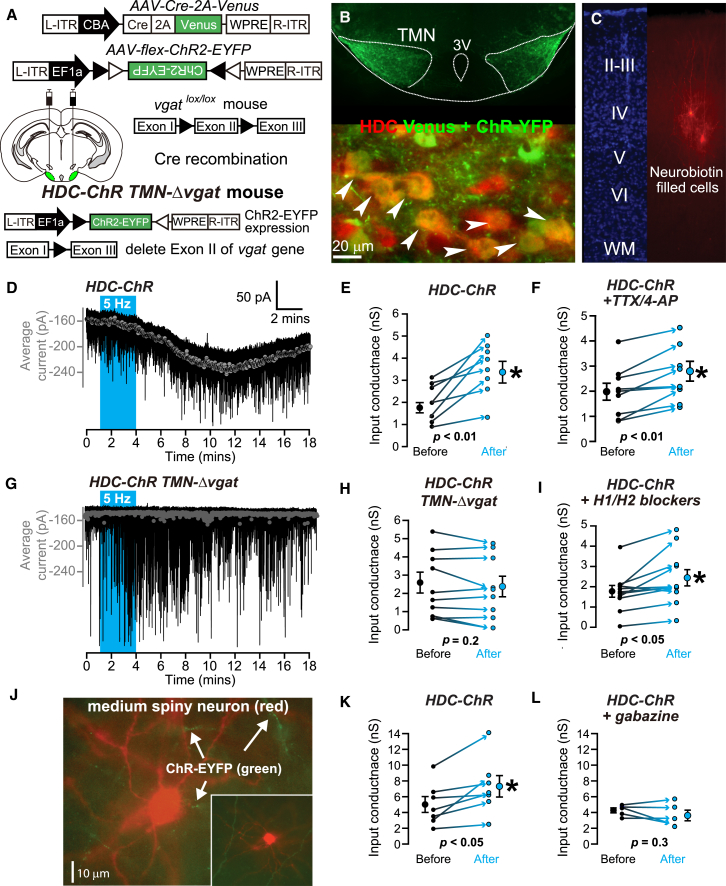
Figure 6.
Histaminergic Axons Produce Slow GABAergic Responses in Visual Cortex Pyramidal and Striatal Neurons
(A) Double injection of AAV-Cre-Venus and AAV-flex-ChR2-EYFP into the TMN of vgatlox/lox mice to make light-sensitive histaminergic neurons that lack vGAT (HDC-ChR TMN-Δvgat mouse).
(B) The top photomicrograph shows combined expression of the two AAV transgenes in the bilateral TMN area (coronal section). The lower picture shows double staining in the TMN with HDC and EGFP antisera.
(C) Two layer IV pyramidal neurons filled with neurobiotin/Alexa 555 (red) postrecording.
(D) Electrophysiological data from a HDC-ChR mouse. The gray symbols superimposed upon the current trace (black line) show the average holding current calculated for every 1 s epoch during the entire recording. An increase in the holding current begins during the 5 Hz optogenetic stimulation but takes minutes to reach its peak response following termination of the stimuli.
(E) Scatterplot for all recordings made from the HDC-ChR mice. The lines indicate paired recordings made before and after the 5 Hz optogenetic stimulation. On average, Gtonic significantly increased by 1.1 ± 0.3 nS (paired t test, p < 0.01; n = 8) in the HDC-ChR slices.
(F) Scatterplot for all recordings made from the HDC-ChR mice in the presence of TTX and 4-AP. On average, Gtonic significantly increased by 0.5 ± 0.1 nS (paired t test, p < 0.005; n = 9) in the HDC-ChR slices.
(G) Electrophysiological data from a HDC-ChR TMN-Δvgat mouse. The gray symbols superimposed upon the current trace (black line) show the average holding current calculated every 1 s epoch for the entire recording. No change in the holding current occurred in response to the 5 Hz optogenetic stimulation, but there was an increase in the frequency of spontaneous synaptic activity (transient downward deflections).
(H) Scatterplots for recordings made from the HDC-ChR TMN-Δvgat mice. The lines indicate the paired recordings made before and after the 5 Hz optogenetic stimulation. Gtonic was reduced by −0.2 ± 0.1 nS in the TMN-Δvgat/HDC-ChR mice (paired t test, p = 0.225, n = 9).
(I) Scatterplots for recordings made from the HDC-ChR mice in the presence of H1 (pyrilamine) and H2 (ranitidine) blockers. Gtonic increased by 0.6 ± 0.2 nS (paired t test, p < 0.05; n = 11).
(J) Medium spiny neuron (red, neurobiotin fill postrecording) from the caudate-putamen of a HDC-ChR mouse; several ChR-EYFP-positive axons (green) are in the vicinity of the cell.
(K and L) Scatterplots for all recordings made from medium spiny neurons in the HDC-ChR mice in control conditions and with the GABAA receptor antagonist gabazine (10 μM). Gtonic increased by 1.9 ± 0.6 nS (paired t test, p < 0.05; n = 7) in control conditions and decreased by −0.8 ± 0.5 nS in the presence of gabazine (paired t test, p = 0.3; n = 6).
We carried out functional circuit mapping by light-stimulating the ChR-EYFP labeled fibers in the neocortex (Figure 6C) and caudate-putamen (Figure 6J) prepared from TMN-Δvgat/HDC-ChR and HDC-ChR mice. Stimulation of HDC-ChR neocortical slices with brief light pulses delivered at 5 Hz generated slow and sustained increases in the holding current recorded, in whole-cell mode, from pyramidal cells (Figure 6D). This current resembled the Gtonic produced by activation of extrasynaptic GABAA receptors (Brickley et al., 1996; Brickley and Mody, 2012; Bright et al., 2007; Wisden et al., 2002). On average, Gtonic significantly increased by 1.1 ± 0.3 nS (paired t test, p < 0.01; n = 8) in the HDC-ChR slices (Figure 6E). In the example shown (Figure 6D), the enhancement of Gtonic was apparent after a few minutes of 5 Hz continuous optical stimulation, consistent with GABA diffusing to its target receptors from distant release sites (i.e., a paracrine action). To determine whether the GABA was being released directly from HDC terminals or if this required an interneuron intermediate, we repeated these experiments in the presence of TTX (1 μM) and 4-AP (100 μM) (Root et al., 2014). A robust increase in Gtonic was observed, and on average it significantly increased by 0.5 ± 0.1 nS (paired t test, p < 0.01; n = 9) in the HDC-ChR slices (Figure 6F). In contrast, there was no increase in Gtonic in TMN-Δvgat/HDC-ChR slices following 5 Hz light stimulation (Figure 6G). On average Gtonic was slightly reduced by −0.2 ± 0.1 nS (n = 9) in the TMN-Δvgat/HDC-ChR mice (paired t test, p = 0.225, n = 9; Figure 6H). This suggests that vesicular GABA was being directly released from the histaminergic fibers using GABAergic (vGAT-dependent) vesicles to cause the increase in Gtonic. The results also imply that the Gtonic is not caused by activating any putative histamine-gated chloride channels (Fleck et al., 2012).
We next confirmed, independently of the HDC-specific vgat knockouts, that histamine release was not responsible for stimulating GABAergic interneurons to release GABA. We repeated the 5 Hz light stimulation of HDC-ChR fibers in the presence of the H1 receptor antagonist pyrilamine (20 μM) and the H2 receptor antagonist ranitidine (5 μM). A significant increase in Gtonic (0.6 ± 0.2 nS, n = 11; paired t test, p < 0.05) still occurred (Figure 6I), and as before, the increase was delayed after the onset of light stimulation. Applying H1/H2 receptor antagonists further suggested that there was no intermediary GABAergic interneuron involved that first required excitation by histamine, and which then released GABA to increase Gtonic. Instead, GABA release in the neocortex from TMN axons was vGAT dependent.
ChR-EYFP-positive fibers were also present in the caudate putamen of HDC-ChR mice (Figure 6J). During 5 Hz light stimulation, there was a gradual increase in Gtonic (1.9 ± 0.6 nS, n = 7; paired t test, p < 0.05) onto medium spiny neurons (Figure 6K). When the GABAA receptor antagonist gabazine (100 μM) was present during the stimulation, there was no increase in Gtonic; in fact, on average it decreased by −0.8 ± 0.5 nS (n = 6) (Figure 6L).
GABA Released from Histaminergic Axons Does Not Increase Phasic Inhibition, but Histamine Increases Synaptic Drive
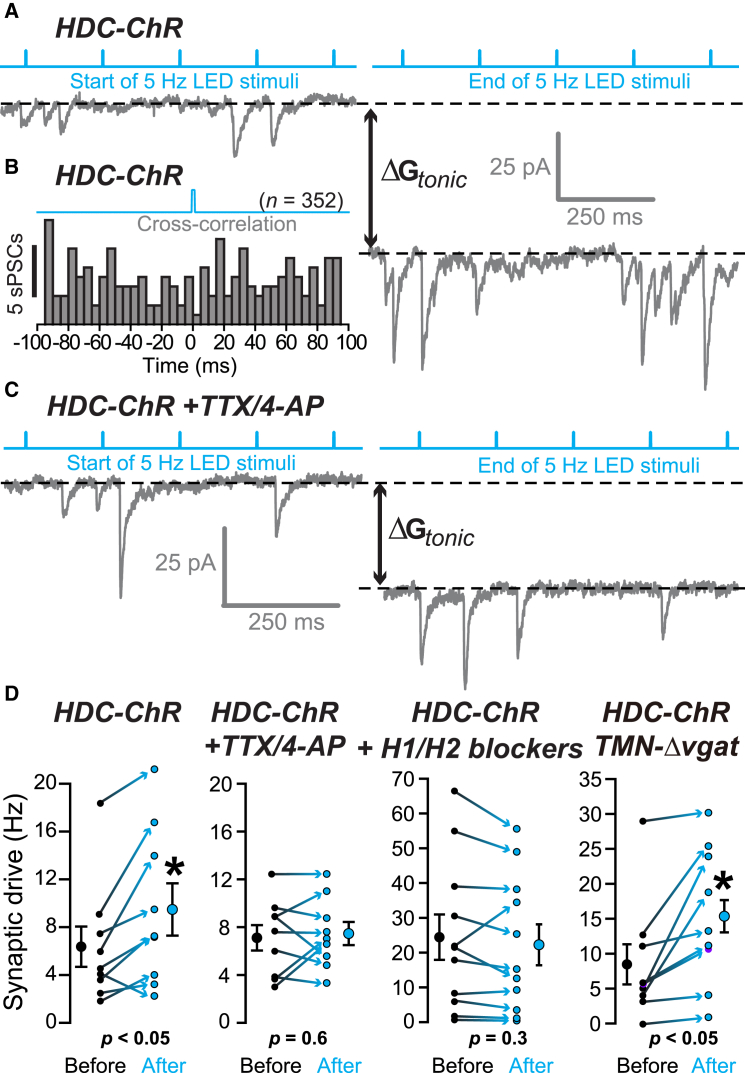
We examined if GABA release from “GABA-histaminergic” axons in neocortex of HDC-ChR mice increased phasic (synaptic) GABAA receptor activation, in addition to its ability to raise Gtonic. The timing of sPSCs was analyzed relative to the LED trigger (Figure 7A). By cross-correlation analysis, sPSCs were not phase locked with ChR stimulation (Figure 7B) (n = 9 recordings from four HDC-ChR mice). However, the sPSC frequency significantly increased in these neocortical slices from 6.4 ± 1.7 Hz (n = 9) to 9.5 ± 2.2 Hz (paired t test, p < 0.05). This increase in asynchronous sPSC frequency was not observed when these experiments were repeated in the presence of TTX/4-AP (Figure 7C), although Gtonic did significantly increase (see Figure 6F). A similar increase in sPSC frequency was observed in the TMN-Δvgat/HDC-ChR mice, where the sPSC frequency increased from 8.7 ± 2.8 Hz (n = 9) to 15.6 ± 3.3 Hz (paired t test, p < 0.05). However, in these mice there was no increase in Gtonic (see Figure 6H). Does this light-evoked increase in sPSC rate depend on histamine release? In the presence of the H1 receptor antagonist pyrilamine (20 μM) and the H2 receptor antagonist ranitidine (5 μM), the ChR-stimulated increase in the rate of asynchronous sPSCs was blocked at the start and at the end of stimulation (Figure 7D; 23.9 ± 6.0 Hz [start] to 21.9 ± 5.4 Hz [end]; n = 11, paired t test, p = 0.3). For neocortical layer neurons, these data highlight the independent nature of the two signals from histaminergic-GABA axons originating from the TMN: an increased synaptic drive generated by histamine release and an increased Gtonic generated by GABA release.
Figure 7.
Activated Neocortical “GABA-Histamine” Axons Increase Synaptic Drive and Gtonic by Independent Mechanisms
(A) Whole-cell voltage-clamp recording from a pyramidal cell in layer IV of visual cortex during 5 Hz LED stimulation of axon fibers from an HDC-ChR mouse. The left-hand trace is from the start, and the right-hand trace is at the end of, the 5 Hz LED stimuli. The increased holding current between the first and last trace was used to calculate the increase in tonic conductance (ΔGtonic).
(B) Cross-correlation analyses between the LED trigger and the occurrence of sPSCs. There was no peak in the histogram, consistent with a lack of LED-triggered PSCs.
(C) Whole-cell voltage-clamp recording in a layer IV pyramidal cell in the presence of TTX and 4-AP during 5-Hz LED stimulation of axon fibers in the visual neocortex from an HDC-ChR mouse. There was an increase in Gtonic but in this experiment little change in sPSC rate.
(D) Scatterplot of the change in synaptic drive (Hz) onto layer IV visual cortex pyramidal cells for the four main experiments: stimulation of HDC-ChR axons, stimulation of HDC-ChR axons in the presence of TTX/4-AP, stimulation of HDC-ChR axons in the presence of H1 and H2 receptor antagonists, and stimulation of TMN-Δvgat/HDC-ChR axons. In HDC-ChR mice the sPSC frequency significantly increased from 6.4 ± 1.7 Hz (n = 9) to 9.5 ± 2.2 Hz (paired t test, p < 0.05). In TMN-Δvgat/HDC mice, a similar increase in sPSC frequency was observed from 8.7 ± 2.8 Hz (n = 9) to 15.6 ± 3.3 Hz (paired t test, p < 0.05). With the H1 receptor antagonist pyrilamine (20 μM) and the H2 receptor antagonist ranitidine (5 μM), the ChR-stimulated increase in the rate of asynchronous sPSCs was 23.9 ± 6.0 Hz at the start and 21.9 ± 5.4 Hz at the end of stimulation (n = 11, paired t test, p = 0.3).
Discussion
Histamine neurons form a globally projecting hub that integrates brain functions associated with wakefulness (Wada et al., 1991). Starting in the TMN, histaminergic axons course large distances throughout the brain, generating extrasynaptic pools of transmitter. We showed that selective pharmacogenetic stimulation of these histamine neurons in vivo behaviorally excites the animals, as would be expected (Haas et al., 2008; Lin et al., 1988; Monnier et al., 1967; Nicholson et al., 1991). But we also established that histaminergic neurons use vGAT to release GABA. Similar to histamine, the GABA released from histaminergic neurons functions in a paracrine manner, which would make these neurons an unexpected source of GABAergic volume transmission in the neocortex and striatum. As part of their integrative role in specifying behavioral state, we expect that these hypothalamic TMN “GABA-histamine” neurons will contribute to tonic inhibition of many neural circuits simultaneously. Mice whose GABA-histamine neurons cannot release GABA are hyperactive, and they sleep less and do not catch up on this lost sleep. The “sleepless phenotype” manifests during the night. Their behavior might be described as a form of mania (Kirshenbaum et al., 2011), which in humans would be considered as part of a bipolar disorder.
Neurons often corelease small molecules and peptides (Adamantidis, 2015; Klausberger and Somogyi, 2008; Schöne and Burdakov, 2012; Tong et al., 2008; van den Pol, 2012). “Orexinergic” neurons, for example, in the lateral hypothalamus corelease glutamate and orexin onto histaminergic neurons in the TMN (Schöne et al., 2014). The different time course of the excitatory actions of the two transmitters (glutamate, fast synaptic excitation; orexin, slow and sustained excitation) allows them to signal different aspects of “metabolic integration” (Schöne et al., 2014). In addition to peptides, some neurons release several small molecule transmitters simultaneously, sometimes of the same functional type, e.g., GABA-glycine (Jonas et al., 1998), but often of functionally antagonist pairings, for example, GABA-glutamate, or GABA-acetylcholine or GABA-dopamine (Hnasko and Edwards, 2012; Vaaga et al., 2014). In contrast to our findings on GABA-histamine neurons, other investigations into GABA cotransmission or corelease, including cotransmission with peptides, found only fast monosynaptic responses generated by the GABA component (Jego et al., 2013; Root et al., 2014; Shabel et al., 2014; Tong et al., 2008; Tritsch et al., 2012). It might be expected that midbrain dopamine and histamine networks function similarly with respect to GABA release, but they do not. GABA is not synthesized in the dopamine neurons themselves—unlike the histamine neurons, they do not express the gad1 or gad2 genes—but GABA is instead scavenged into dopaminergic terminals by the transporters mGAT1 and mGAT4; it is hypothesized that GABA is then transported into dopamine vesicles by the monoamine transporter VMAT2 (Tritsch et al., 2012, 2014). For histamine neurons, vgat knockout removes GABA release; the VMAT2 in histamine neurons does not substitute for vGAT. In cultured TMN neurons from embryos, GABA is in putative vesicles distinct from histaminergic ones, suggesting cotransmission rather than corelease (Kukko-Lukjanov and Panula, 2003).
There is an intriguing potential for synergy directly at the receptor level between histamine and GABA. At (high) concentrations of around 1 mM, histamine is an effective positive allosteric modulator of α4 subunit-containing GABAA receptors (Bianchi et al., 2011), which are the type of GABAA receptors often associated with extrasynaptic activation (Brickley and Mody, 2012). However, when we removed vgat from histamine (HDC-ChR) axons, this abolished the light-evoked increase in tonic inhibition on layer IV pyramidal neurons, suggesting that the histamine released was not sufficient to activate any α4 subunit-containing GABAA receptors that might have been present. Similarly, the vgat deletion experiment also ruled out that the increase in Gtonic was due to activating (speculative) histamine-gated chloride channels (Fleck et al., 2012). In the absence of vGAT, light triggered a general increase in synaptic drive onto pyramidal neurons, which was blocked by H1 and H2 receptor antagonists. This effect of histamine is likely to reflect the engagement of multiple changes in the inputs impinging onto pyramidal neurons, as described for striatal neurons (Ellender et al., 2011). As the histamine system innervates most of the brain, further studies will be required to see if GABA is coreleased in other areas. In the ventral lateral preoptic hypothalamus, histamine axons do not release GABA when stimulated optogentically, but instead activate GABA interneurons by histamine release (Williams et al., 2014). By contrast, our experiments using TTX in the neocortex suggest that in this brain region no intermediary interneurons convey TMN axon-evoked GABA release. About 20% of histamine neurons in the TMN only contain histamine (Figure 2A), and these could be the ones that innervate VLPO.
The “GABA-histamine” TMN neurons only fire during wakefulness, so GABA will be released from their axons into the neocortex and striatum during the wake state. The paracrine nature of the GABA signal explains the lack of any phasic synaptic inhibition associated with GABA release from the histamine axons. Why would GABA-histamine neurons use contradictory “stop-go” signals? One reason could be to stop networks getting too excited by histamine: the HDC-vgat KD and TMN-Δvgat mice are more active and sleep less because the histaminergic system is overactive. The function of sleep is unknown, but an overactive histamine system, resulting in less sleep, may damage health and cause mania. GABA release from histamine neurons could keep the animal in the “optimal arousal zone.”
A second reason could be related to the fact that in the awake neocortex, inhibition enhances processing (Haider et al., 2013). Indeed, the wake-active GABAergic volume transmission generated by TMN neurons may sharpen cognitive responses, and so GABA could work together with histamine to enhance wakefulness. Increased ambient GABA levels will generate greater Gtonic, which by speeding up the membrane time constant will increase the requirement for EPSP-spike precision, and constrain coincidence detection so that only closely timed inputs trigger action potentials (Brickley and Mody, 2012; Bright et al., 2007; Duguid et al., 2012; Hamann et al., 2002; Wlodarczyk et al., 2013). The long distances traversed by the GABA-histamine axons mean that extrasynaptic inhibition could be coordinated by the TMN over large cortical areas.
In conclusion, we think it is no longer correct to refer to TMN neurons as “histaminergic”: the GABA arm of their activity is so striking that they might be referred to as “GABA-histamine” neurons. We have demonstrated that the wide-ranging GABA-histamine axons of the hypothalamic TMN broadcast dual inhibitory-excitatory signals in the neocortex: an increased Gtonic generated by GABA release, and an increased synaptic drive generated by histamine. The two components may work together to regulate the amount of wakefulness. If this balance of GABA-histamine cotransmission changes unfavorably, mania-like behaviors could emerge.
Experimental Procedures
Animals
All experiments were performed in accordance with the UK Home Office Animal Procedures Act (1986); all procedures were approved by the Imperial College Ethical Review Committee. The following strains of mice were used: HDC-Cre (129SVJ and C57BL/6J) (Zecharia et al., 2012) (JAX stock number 021198); Rosa26-loxP-Stop-loxP-YFP (C57BL/6J), kindly provided by F. Costantini (Srinivas et al., 2001); vgatlox/lox (C57BL/6J and 129S4), kindly provided by B.B. Lowell (Tong et al., 2008) (Jax stock number 012897); vGAT-Cre, kindly provided by B.B. Lowell (Vong et al., 2011); and Rosa26-loxP-Stop-loxP-tdTomato, kindly provided by H. Zeng (Madisen et al., 2010). See Supplemental Information for genotyping.
AAV Transgene Plasmids
Plasmid pAAV-iCre-2A-Venus was provided by Thomas Kuner (Abraham et al., 2010). Plasmid pAAV-GFP was a gift from John T. Gray (Addgene plasmid 32396). Plasmid pAAV-EF1α-flex-ChR2H134R-EYFP was a gift from Karl Deisseroth (Addgene plasmid 20298). The H134R mutation enhances light-evoked ChR currents and reduces spike-frequency adaptation (Nagel et al., 2005; Pulver et al., 2009). Plasmid pAAV-hSyn-flex-hM3Dq-mCherry was a gift from Bryan L. Roth (Addgene plasmid 44361) (Krashes et al., 2011). The pPRIME system, cloned into AAV transgenes, was used to generate shRNAs in vivo (Stegmeier et al., 2005). See Supplemental Information for shRNA sequences.
Adeno-Associated Virus Preparation and Stereotaxic Injections
To produce adeno-associated virus (AAV), the adenovirus helper plasmid pFΔ6, the AAV helper plasmids pH21 (AAV1) and pRVI (AAV2), and the pAAV transgene plasmids were cotransfected into HEK293 cells and the subsequent AAV particles harvested on heparin columns, as described previously (Klugmann et al., 2005). To deliver the AAV into the brain, stereotaxic injections were performed using an Angle Two apparatus (Leica) linked to a digital brain atlas (Leica Biosystems Richmond, Inc.). Before injection, 1 μl AAV virus was mixed with 1 μl 20% mannitol (MERCK K93152782 111). The virus and mannitol mixture were injected into a pulled-glass pipette (Warner Instruments; OD = 1.00 mm, ID = 0.78 mm, length = 7.5 cm). Virus was injected at a speed of 25 nl/min. Virus (1 μl) was bilaterally injected into the brain, 0.5 μl for each side. For TMN, the injection coordinates were as follows: ML (−0.92 mm), AP (−2.70 mm), DV (−5.34 mm); ML (0.92 mm), AP (−2.70 mm), and DV (−5.34 mm). For unilateral injections, the injection coordinates were as follows: CPu, ML (−2.10 mm), AP (0.50 mm), and DV (−3.66 mm).
Histamine Measurements
Neocortex or striatum was collected and homogenized with 10 μl of 0.2 M perchloric acid per mg tissue and centrifuged at 10,000 rpm for 5 min at 4°C. The supernatants were collected and neutralized with an equal volume of 1 M potassium borate buffer. Brain histamine levels were determined with an ELISA kit (Beckman Coulter Co. number IM2562).
qPCR on Tissue Punches and Single-Cell qPCR
Total RNA from tissue punches was extracted using Trizol (Invitrogen). Reverse transcription and PCR were performed using a TaqMan RNA-to-Ct 1-Step Kit (Life Technologies, 4392653) and an Applied Biosystems machine (Foster City, USA). Acute slices of the TMN area were made (see “Electrophysiology”). AAV-transduced neurons were identified by fluorescence. After patching and recording (see “Electrophysiology”), we used the Single-Cell-to-CT Kit (Ambion). The content of the neuron was aspirated into the recording pipette and expelled into cell lysis/DNase I solution. Reverse transcription and cDNA preamplification were performed according to the kit protocol. qPCR was performed using the TaqMan Gene Expression Assay system. The TaqMan assay probes were designed and purchased from Invitrogen (UK): mvgat, Mm00494138_m1; m18srRNA, Mm03928990_g1; and mhdc, Mm00456104_m1.
EEG Analysis and Sleep-Wake Behavior
EEG and EMG signals were recorded using Neurologger 2A devices (Anisimov et al., 2014; Yu et al., 2014). The sleep state (wake, W; non-rapid eye movement, NREM; rapid eye movement, REM; doubt, D) was scored manually. See Supplemental Information for details. The sleep deprivation procedure, with presentation of novel objects, was performed as described (Yu et al., 2014).
General Behavioral Activity: Open Field Assay
All experiments were performed during “lights off” (active phase). The locomotion activity was detected in an activity test chamber with infrared sensors (Med Associates, Inc). The “instantaneous speed” was calculated as the distance traveled divided by the time spent moving (but not including the time the mice spent in the stationary state). For the DREADD experiments, clozapine-N-oxide (C0832, Sigma-Aldrich, dissolved in saline, 5 mg/kg) or saline was administered i.p. 30 min before the start of the behavioral observations.
Electrophysiology
Acute brain slices were prepared as described in Supplemental Information. For optogenetic experiments, a 470 nm collimated LED (M470L3-C1, Thorlabs) was used to illuminate the slice through the objective lens, and current-clamp recordings were first made from histaminergic neurons to confirm ChR2 expression. Subsequent voltage-clamp experiments in neocortex and stratum were used for cross-correlation analysis of synaptic events using a 100 ms sliding window before and after each LED pulse. Changes in the tonic GABAA receptor-meditated conductance (Gtonic) were calculated from the average holding current.
Acknowledgments
This work was supported by the Medical Research Council, UK (G0901892, S.G.B., N.P.F., and W.W.; G0800399, W.W.), the BBSRC (G021691 and BB/K018159/1, S.G.B., N.P.F., and W.W.), the Wellcome Trust (WT094211MA, S.G.B., N.P.F., and W.W.), and a UK-China Scholarships for Excellence/China Scholarship scheme (X.Y. and Z.Y.).
Published: June 18, 2015
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article at http://dx.doi.org/10.1016/j.neuron.2015.06.003.
Contributor Information
Nicholas P. Franks, Email: n.franks@imperial.ac.uk.
Stephen G. Brickley, Email: s.brickley@imperial.ac.uk.
William Wisden, Email: w.wisden@imperial.ac.uk.
Supplemental Information
References
- Abraham N.M., Egger V., Shimshek D.R., Renden R., Fukunaga I., Sprengel R., Seeburg P.H., Klugmann M., Margrie T.W., Schaefer A.T., Kuner T. Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron. 2010;65:399–411. doi: 10.1016/j.neuron.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis A.R. Sleep: the sound of a local alarm clock. Curr. Biol. 2015;25:49–50. doi: 10.1016/j.cub.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Airaksinen M.S., Alanen S., Szabat E., Visser T.J., Panula P. Multiple neurotransmitters in the tuberomammillary nucleus: comparison of rat, mouse, and guinea pig. J. Comp. Neurol. 1992;323:103–116. doi: 10.1002/cne.903230109. [DOI] [PubMed] [Google Scholar]
- Alexander G.M., Rogan S.C., Abbas A.I., Armbruster B.N., Pei Y., Allen J.A., Nonneman R.J., Hartmann J., Moy S.S., Nicolelis M.A. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaclet C., Parmentier R., Ouk K., Guidon G., Buda C., Sastre J.P., Akaoka H., Sergeeva O.A., Yanagisawa M., Ohtsu H. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J. Neurosci. 2009;29:14423–14438. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov V.N., Herbst J.A., Abramchuk A.N., Latanov A.V., Hahnloser R.H., Vyssotski A.L. Reconstruction of vocal interactions in a group of small songbirds. Nat. Methods. 2014;11:1135–1137. doi: 10.1038/nmeth.3114. [DOI] [PubMed] [Google Scholar]
- Atasoy D., Aponte Y., Su H.H., Sternson S.M. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori M., Lau D., Tansey E.P., Chow A., Ozaita A., Rudy B., McBain C.J. H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking. Nat. Neurosci. 2000;3:791–798. doi: 10.1038/77693. [DOI] [PubMed] [Google Scholar]
- Bayliss D.A., Wang Y.M., Zahnow C.A., Joseph D.R., Millhorn D.E. Localization of histidine decarboxylase mRNA in rat brain. Mol. Cell. Neurosci. 1990;1:3–9. doi: 10.1016/1044-7431(90)90036-4. [DOI] [PubMed] [Google Scholar]
- Bianchi M.T., Clark A.G., Fisher J.L. The wake-promoting transmitter histamine preferentially enhances α-4 subunit-containing GABAA receptors. Neuropharmacology. 2011;61:747–752. doi: 10.1016/j.neuropharm.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley S.G., Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley S.G., Cull-Candy S.G., Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright D.P., Aller M.I., Brickley S.G. Synaptic release generates a tonic GABA(A) receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J. Neurosci. 2007;27:2560–2569. doi: 10.1523/JNEUROSCI.5100-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid I., Branco T., London M., Chadderton P., Häusser M. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J. Neurosci. 2012;32:11132–11143. doi: 10.1523/JNEUROSCI.0460-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellender T.J., Huerta-Ocampo I., Deisseroth K., Capogna M., Bolam J.P. Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J. Neurosci. 2011;31:15340–15351. doi: 10.1523/JNEUROSCI.3144-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck M.W., Thomson J.L., Hough L.B. Histamine-gated ion channels in mammals? Biochem. Pharmacol. 2012;83:1127–1135. doi: 10.1016/j.bcp.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Haas H.L., Sergeeva O.A., Selbach O. Histamine in the nervous system. Physiol. Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Haider B., Häusser M., Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M., Rossi D.J., Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hnasko T.S., Edwards R.H. Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S., Glasgow S.D., Herrera C.G., Ekstrand M., Reed S.J., Boyce R., Friedman J., Burdakov D., Adamantidis A.R. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Bischofberger J., Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum G.S., Clapcote S.J., Duffy S., Burgess C.R., Petersen J., Jarowek K.J., Yücel Y.H., Cortez M.A., Snead O.C., 3rd, Vilsen B. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase α3 sodium pump. Proc. Natl. Acad. Sci. USA. 2011;108:18144–18149. doi: 10.1073/pnas.1108416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T., Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M., Symes C.W., Leichtlein C.B., Klaussner B.K., Dunning J., Fong D., Young D., During M.J. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol. Cell. Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Krashes M.J., Koda S., Ye C., Rogan S.C., Adams A.C., Cusher D.S., Maratos-Flier E., Roth B.L., Lowell B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukko-Lukjanov T.K., Panula P. Subcellular distribution of histamine, GABA and galanin in tuberomamillary neurons in vitro. J. Chem. Neuroanat. 2003;25:279–292. doi: 10.1016/s0891-0618(03)00043-7. [DOI] [PubMed] [Google Scholar]
- Lee V., Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front. Neural Circuits. 2014;8:3. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.S., Sakai K., Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27:111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- Lin J.S., Sakai K., Vanni-Mercier G., Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier M., Fallert M., Battacharya I.C. The waking action of histamine. Experientia. 1967;23:21–22. doi: 10.1007/BF02142244. [DOI] [PubMed] [Google Scholar]
- Nagel G., Brauner M., Liewald J.F., Adeishvili N., Bamberg E., Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Nicholson A.N., Pascoe P.A., Turner C., Ganellin C.R., Greengrass P.M., Casy A.F., Mercer A.D. Sedation and histamine H1-receptor antagonism: studies in man with the enantiomers of chlorpheniramine and dimethindene. Br. J. Pharmacol. 1991;104:270–276. doi: 10.1111/j.1476-5381.1991.tb12418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P., Yang H.Y., Costa E. Histamine-containing neurons in the rat hypothalamus. Proc. Natl. Acad. Sci. USA. 1984;81:2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P., Pirvola U., Auvinen S., Airaksinen M.S. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Parmentier R., Ohtsu H., Djebbara-Hannas Z., Valatx J.L., Watanabe T., Lin J.S. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier R., Kolbaev S., Klyuch B.P., Vandael D., Lin J.S., Selbach O., Haas H.L., Sergeeva O.A. Excitation of histaminergic tuberomamillary neurons by thyrotropin-releasing hormone. J. Neurosci. 2009;29:4471–4483. doi: 10.1523/JNEUROSCI.2976-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L., Huber D., Sobczyk A., Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Pulver S.R., Pashkovski S.L., Hornstein N.J., Garrity P.A., Griffith L.C. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J. Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E., Sherman M.A. GABA--the quintessential neurotransmitter: electroneutrality, fidelity, specificity, and a model for the ligand binding site of GABAA receptors. Neurochem. Res. 1993;18:365–376. doi: 10.1007/BF00967239. [DOI] [PubMed] [Google Scholar]
- Root D.H., Mejias-Aponte C.A., Zhang S., Wang H.L., Hoffman A.F., Lupica C.R., Morales M. Single rodent mesohabenular axons release glutamate and GABA. Nat. Neurosci. 2014;17:1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Takahashi K., Anaclet C., Lin J.S. Sleep-waking discharge of ventral tuberomammillary neurons in wild-type and histidine decarboxylase knock-out mice. Front. Behav. Neurosci. 2010;4:53. doi: 10.3389/fnbeh.2010.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C.B., Fuller P.M., Pedersen N.P., Lu J., Scammell T.E. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C., Burdakov D. Glutamate and GABA as rapid effectors of hypothalamic “peptidergic” neurons. Front. Behav. Neurosci. 2012;6:81. doi: 10.3389/fnbeh.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C., Apergis-Schoute J., Sakurai T., Adamantidis A., Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7:697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senba E., Daddona P.E., Watanabe T., Wu J.Y., Nagy J.I. Coexistence of adenosine deaminase, histidine decarboxylase, and glutamate decarboxylase in hypothalamic neurons of the rat. J. Neurosci. 1985;5:3393–3402. doi: 10.1523/JNEUROSCI.05-12-03393.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel S.J., Proulx C.D., Piriz J., Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F., Hu G., Rickles R.J., Hannon G.J., Elledge S.J. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA. 2005;102:13212–13217. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundvik M., Panula P. Organization of the histaminergic system in adult zebrafish (Danio rerio) brain: neuron number, location, and cotransmitters. J. Comp. Neurol. 2012;520:3827–3845. doi: 10.1002/cne.23126. [DOI] [PubMed] [Google Scholar]
- Takagi H., Morishima Y., Matsuyama T., Hayashi H., Watanabe T., Wada H. Histaminergic axons in the neostriatum and cerebral cortex of the rat: a correlated light and electron microscopic immunocytochemical study using histidine decarboxylase as a marker. Brain Res. 1986;364:114–123. doi: 10.1016/0006-8993(86)90992-3. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Lin J.S., Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J. Neurosci. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N., Inagaki S., Shiosaka S., Taguchi Y., Oertel W.H., Tohyama M., Watanabe T., Wada H. Immunohistochemical evidence for the coexistence of histidine decarboxylase-like and glutamate decarboxylase-like immunoreactivities in nerve cells of the magnocellular nucleus of the posterior hypothalamus of rats. Proc. Natl. Acad. Sci. USA. 1984;81:7647–7650. doi: 10.1073/pnas.81.23.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q., Ye C.P., Jones J.E., Elmquist J.K., Lowell B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F., Riveros M.E., Contreras M., Valdes J.L. Histamine and motivation. Front. Syst. Neurosci. 2012;6:51. doi: 10.3389/fnsys.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch N.X., Ding J.B., Sabatini B.L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch N.X., Oh W.J., Gu C., Sabatini B.L. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. eLife. 2014;3:e01936. doi: 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier S., Chotard C., Traiffort E., Unmehopa U., Fisser B., Swaab D.F., Schwartz J.C. Co-localization of histamine with GABA but not with galanin in the human tuberomamillary nucleus. Brain Res. 2002;939:52–64. doi: 10.1016/s0006-8993(02)02546-5. [DOI] [PubMed] [Google Scholar]
- Tye K.M., Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaga C.E., Borisovska M., Westbrook G.L. Dual-transmitter neurons: functional implications of co-release and co-transmission. Curr. Opin. Neurobiol. 2014;29:25–32. doi: 10.1016/j.conb.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A.N. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S.R., Hökfelt T., Skirboll L.R., Wu J.Y. Hypothalamic gamma-aminobutyric acid neurons project to the neocortex. Science. 1983;220:1309–1311. doi: 10.1126/science.6857253. [DOI] [PubMed] [Google Scholar]
- Vong L., Ye C., Yang Z., Choi B., Chua S., Jr., Lowell B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H., Inagaki N., Yamatodani A., Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci. 1991;14:415–418. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Taguchi Y., Hayashi H., Tanaka J., Shiosaka S., Tohyama M., Kubota H., Terano Y., Wada H. Evidence for the presence of a histaminergic neuron system in the rat brain: an immunohistochemical analysis. Neurosci. Lett. 1983;39:249–254. doi: 10.1016/0304-3940(83)90308-7. [DOI] [PubMed] [Google Scholar]
- Williams R.H., Chee M.J., Kroeger D., Ferrari L.L., Maratos-Flier E., Scammell T.E., Arrigoni E. Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J. Neurosci. 2014;34:6023–6029. doi: 10.1523/JNEUROSCI.4838-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Cope D., Klausberger T., Hauer B., Sinkkonen S.T., Tretter V., Lujan R., Jones A., Korpi E.R., Mody I. Ectopic expression of the GABA(A) receptor alpha6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology. 2002;43:530–549. doi: 10.1016/s0028-3908(02)00151-x. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk A.I., Xu C., Song I., Doronin M., Wu Y.W., Walker M.C., Semyanov A. Tonic GABAA conductance decreases membrane time constant and increases EPSP-spike precision in hippocampal pyramidal neurons. Front. Neural Circuits. 2013;7:205. doi: 10.3389/fncir.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik S.M., Katsurabayashi S., Guillemin I., Friauf E., Rosenmund C., Brose N., Rhee J.S. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Yu X., Zecharia A., Zhang Z., Yang Q., Yustos R., Jager P., Vyssotski A.L., Maywood E.S., Chesham J.E., Ma Y. Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr. Biol. 2014;24:2838–2844. doi: 10.1016/j.cub.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecharia A.Y., Yu X., Götz T., Ye Z., Carr D.R., Wulff P., Bettler B., Vyssotski A.L., Brickley S.G., Franks N.P., Wisden W. GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness. J. Neurosci. 2012;32:13062–13075. doi: 10.1523/JNEUROSCI.2931-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.