Abstract
Background
Diagnosis of pancreatic cystic neoplasms remains problematic. We hypothesize that inflammatory mediator proteins in pancreatic cyst fluid can differentiate branch duct intraductal papillary mucinous neoplasms (BD-IPMNs) and pancreatic inflammatory cysts. We aim to 1) detect inflammatory mediator proteins (IMPs) using a multiplexed IMP-targeted microarray in pancreatic cyst fluid obtained during endoscopic ultrasound fine needle aspiration (EUS-FNA) and 2) compare IMP profiles in pancreatic cyst fluid from BD-IPMNs and inflammatory cysts. Pancreatic cyst fluid from ten patients (5 BD-IPMN and 5 inflammatory cysts) was obtained by EUS-FNA and analyzed directly with a multiplexed microarray assay to determine concentrations of 89 IMPs. Statistical analysis was performed using non-parametric methods.
Results
Eighty-three of the 89 assayed IMPs were detected in at least one of the 10 patient samples. Seven IMPs were detected in BD-IPMN, but not inflammatory cysts, while eleven IMPs were identified in inflammatory cysts, but not BD-IPMN. Notably, granulocyte-macrophage colony-stimulating factor (GM-CSF) expression was present in all five inflammatory cyst samples. Hepatocyte growth factor (HGF) was present in significantly higher concentrations in inflammatory cysts compared to BD-IPMN.
Conclusion
Our exploratory analysis reveals that GM-CSF and HGF in EUS-FNA-collected pancreatic cyst fluid can distinguish between BD-IPMN and inflammatory cyst. Coupling microarray molecular techniques to EUS-FNA may represent a major step forward to our understanding complex pancreatic disease.
Keywords: Endoscopic ultrasound fine needle aspiration, pancreas cyst, biomarker, cytokine, pseudocyst, IPMN
Graphical Abstract
1. Introduction
Pancreatic cystic lesions present a clinical challenge to the primary care physician, gastroenterologist, and surgeon (Pitman et al.; Khalid and Brugge, 2007). As a result of improvements in, and utilization of radiologic imaging, these lesions are being identified with ever increasing frequency (Berland et al.; Brugge, 2008). Unlike most hepatic and renal cysts, pancreatic cystic lesions raise clinical concern because of the potential for malignant transformation associated with specific types, including branch duct intraductal papillary mucinous neoplasm (BD-IPMN) (Pitman et al.). Current tools used to classify lesions rely mainly on analysis of cyst fluid obtained during endoscopic ultrasound fine needle aspiration (EUS-FNA) (Brugge, 2009; Khalid et al., 2009). Although this technique is safe (Lee et al., 2005), it is limited by the quantity of fluid aspirated from small cysts and the accuracy of the markers assayed from the cyst fluid, including carcinoembryonic antigen (CEA), cytology, and most recently, DNA markers. Better diagnostic markers for pancreatic cystic lesions that require smaller volumes of fluid are needed, particularly because small cysts often yield insufficient fluid for currently available biochemical analyses.
Inflammatory mediator proteins (IMPs), which include cytokines and chemokines, are commonly associated with acute and chronic disease states. Their expression may offer insights into differentiating inflammatory cysts, such as pancreatic pseudocysts, from premalignant lesions including BD-IPMN. Cytokines are regulatory proteins involved in intercellular signaling that interact with specific cell-surface receptors, leading to signaling cascades modulating inflammatory and immune responses. These low molecular weight proteins, critical to the development and function of the immune response cascade, are often secreted under cellular stress. Cytokines are generally released in picomolar amounts; however, their concentration can increase over 1000-fold under physiological stress, such as trauma or infection (Cannon, 2000). This class of proteins includes interleukins (IL) and cell signal molecules, such as growth factors (GF), tumor necrosis factor (TNF), and interferons (INF), which trigger inflammation and respond to infections.
Chemokines are a superfamily of small cytokines (8-10 kDa) that are chemoattractants guiding the migration of cells via corresponding chemokine receptors (Fernandez and Lolis, 2002). These proteins attract leukocytes, neutrophils, monocytes, and other effector cells from the blood to sites of infection or tissue damage (Rottman, 1999). As with cytokines, many chemokines are pro-inflammatory, while others are homeostatic and have roles in controlling the migration of cells during normal tissue maintenance or development (Fernandez and Lolis, 2002). Chemokines include eotaxin, fractalkine, growth-regulated oncogene (GRO), interferon-inducible protein (IP), monocyte chemoattractant protein (MCP), myeloma cell metalloproteinase (MDC), macrophage inflammatory protein (MIP), and rantes.
We analyzed EUS-FNA aspirates with a suspension microarray assay. This assay uses capture antibodies coupled to color-coded microspheres, allowing the simultaneous concentration measurement of numerous IMPs in a single experiment. The advantages of such a multiplexed immunoassay are high specificity, small sample volume requirements, and cost-effectiveness. As such, it follows that the IMP microarray is both sensitive to the low concentration of cytokines and amenable to high-throughput analysis (Fitzgerald et al., 2008). Although in clinical settings such technology is primarily used for analysis of urine and blood, our group has recently shown that pancreatic fluid analysis also benefits from microarray-based approaches (Paulo et al., 2011).
The primary objectives of our exploratory investigation are to
-
1)
detect IMPs using a multiplexed IMP-targeted microarray in pancreatic cyst fluid obtained during EUS-FNA and
-
2)
compare IMP profiles in the pancreatic cyst fluid of patients with BD-IPMNs and inflammatory cysts.
2. Materials and Methods
2.1 Study population
The study design was an IMP analysis of EUS-FNA-collected pancreatic cyst fluid using a multiplexed suspension microarray assay in an academic center. The Institutional Review Board at Brigham and Women's Hospital approved this protocol. The study population included adult patients referred to the Center for Pancreatic Diseases at Brigham and Women's Hospital for further evaluation of their pancreatic cystic lesions. All subjects underwent the following: 1) comprehensive history and physical examination, 2) review of radiologic and endoscopic data, and 3) EUS-FNA.
Only patients with a diagnosis of BD-IPMN and pancreatic inflammatory cyst were included. Definitive diagnosis was obtained from a combination of methodologies: physician review of patient medical history (LSL, PAB, DLC), radiologic imaging (NS), and/or surgical pathology (AMB). A single abdominal radiologist who was blinded to the official read of the radiology studies reviewed the images. A macrocystic or mixed macrocystic-microcystic loculated lesion with lobulated or smooth margins, with or without presence of septal or wall calcification demonstrating definite communication with a nondilated main pancreatic duct was diagnosed as a BD-IPMN. Cystic lesions developing in a background of acute or chronic pancreatitis with rounded configuration, thin or thick wall, with or without communication with the pancreatic duct were diagnosed as inflammatory cysts.. Inflammatory cysts incorporate thick walled pancreatic pseudocysts in chronic pancreatitis and walled-off pancreatic necrosis (WOPN) in acute pancreatitis. All these lesions were confirmed as inflammatory cysts on cystgastrostomy or necrosectomy.
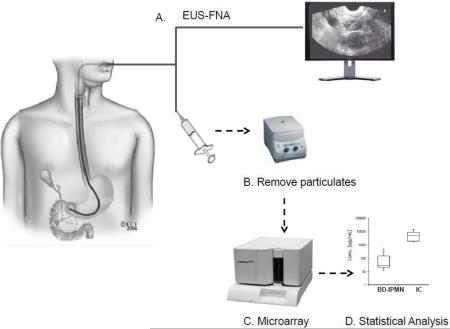
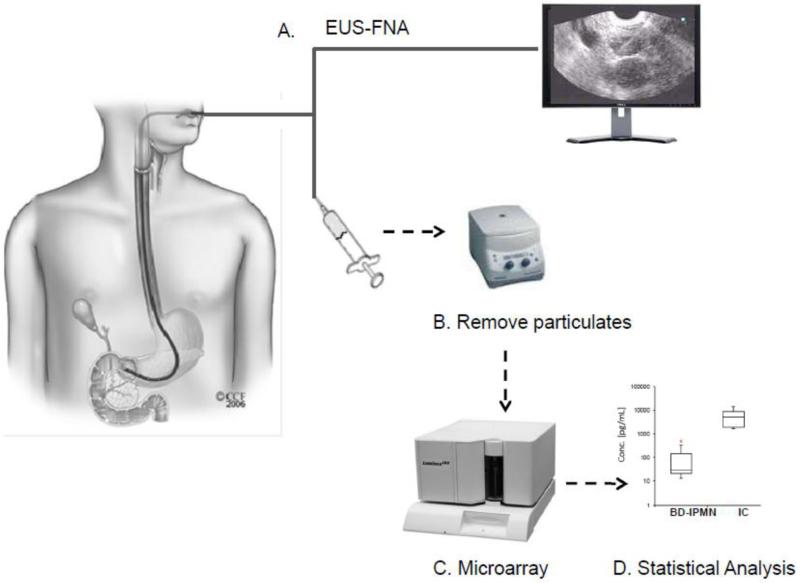
2.2 Experimental workflow (Figure 1)
Figure 1. Methodology overview.
A) Cyst fluid was collected via EUS-FNA. B) Particulates were removed via centrifugation. C) Fluid was directly assayed for IMP via a suspension-based microarray assay. D) Data was analyzed in SAS to determine IMPs with statistically significant differences.
The overall analysis proceeded as follows: A) EUS-FNA, B) particulate removal via centrifugation, C) microarray assays, and D) statistical analysis of the resulting profiles.
2.3 Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA)
Endosonography was performed using a curvilinear echoendoscope (Olympus GF-UC(T)140P-OL5; Olympus America Inc., Center Valley, PA). The processors used included Aloka SSD-Alpha 5 and Alpha 10 (Olympus America Inc., Center Valley, PA). Linear echoendoscopes are modified oblique forward-viewing instruments with curved linear ultrasound transducers that provide real-time visualization of the aspiration needle. In brief, after informed consent, the patient was placed in the left lateral position, the patient's throat sprayed with local anesthetic, and intravenous conscious sedation administered. The echoendoscope was advanced into the upper gastrointestinal tract and after localizing the target lesion, FNA of the cyst fluid was performed using 19 and 22 gauge adjustable needles (Echotip 19 gauge, Cook, Winston-Salem, NC; EZ Shot, Olympus, Center Valley, PA). Aspirates were divided into three aliquots for: 1) biochemical analysis for CEA and amylase levels, 2) IMP assay, and 3) cytologic evaluation of fluid placed into Cytolyt preservative (Cytyc, Boxborough, MA). Samples were stored at −80°C prior to IMP analysis. Antibiotic prophylaxis was administered during the procedure and for 3 days following the procedure.
2.4 Pancreatic cyst fluid IMP microarray analysis
A suspension microarray assay was used to measure the concentration of 89 cytokines in the pancreatic cyst fluid samples from the 10 patients. We selected the 89-cytokine panel, which was the most comprehensive panel commercially available at the time of this study. Unlike mass spectrometry-based proteomic assays of pancreatic fluids (Paulo et al., 2010b; Paulo et al., 2010a; Paulo et al., 2010c), only limited sample preparation was necessary for this assay, as the sole prerequisite for performing the assay was the removal of minimal particulates via centrifugation. Immediately following fluid collection, samples were aliquoted into 1.5 mL microtubes and centrifuged on an Eppendorf Centrifuge 5415R at 4°C and 10,000×g to remove any particulates. The supernatant was transferred into a new tube and stored at −80°C prior to analysis.
Concentrations of each IMP in cyst fluid were determined using a microsphere-based suspension microarray technology (AssayGate, Ijamsville, MD) (Opalka et al., 2003). The assayed cytokines are listed in Supplemental Table 1. The microarray analysis was performed according to previously published methods (Carson and Vignali, 1999; Vignali, 2000; Sachdeva and Asthana, 2007). In brief, multiple analytes in a single aliquot (75 μL) of pancreatic fluid were simultaneously quantified with Bio-Plex 200 Bead Reader System (Bio-Rad, Hercules, CA). Microparticles were conjugated to differing concentrations of two fluorophores to generate distinct bead sets. Each bead set was coated with a capture antibody specific for one analyte. Captured analyte was detected using a biotinylated detection antibody and streptavidin-phycoerythrin. The bead analyzer was a dual laser, flow-based, sorting and detection platform. One laser was bead-specific and determined which analyte was being detected. The second laser determined the magnitude of phycoerythrin-derived signal, which was directly proportional to the amount of bound analyte. At most, 75μL of pancreatic cyst fluid was used for each assay, and each sample was tested in duplicate.
Immediately before the microarray analysis, the concentrations of known standards were determined by a five-parameter logistic regression algorithm with analysis of the median fluorescence intensity readings of an 8-point protein standard curve. This procedure ensured that the reading was within the linear range of the assay. Once a regression equation was derived, the fluorescence intensity values of the standards were treated as unknowns, and the concentration of each standard was calculated. A ratio of the calculated value to the expected value of this standard was determined. A ratio between 70 and 130% for each standard indicated a good fit. If fluorescence intensity values of samples reached a plateau or were outside the range of standard curves, a re-test with diluted samples was performed to ensure that the fluorescence intensity measurement of unknown samples falls inside the linear range of standard curves.
2.5 Statistical analysis
The IMP concentrations were expressed in picograms per milliliter (pg/mL) of pancreatic cyst fluid. Statistically significant differences in IMPs appearing in both cohorts were determined by the Wilcoxon rank-sum non-parametric test using SAS 9.2 (Cary, NC). Fisher Exact test was used (SAS 9.2) when zero values were present. P-value < 0.05 was considered statistically significant, while p-value < 0.1 was considered marginally statistically significant. We included marginally significant proteins to capture IMPs that may have demonstrated significance with increased sample size. Values that were not detected (N.D.) were treated as missing values in the median, interquartile range, and Wilcoxon calculations. For the Wilcoxon test, the Z-value included a continuity correction as assigned by SAS. The Bonferroni or Benjamini correction method is often used to account for multiple testing of the collected samples but was not used here as it is generally not required for exploratory data analysis (Bender and Lange, 2001). Box plots were constructed using a Microsoft Excel add-in (Vertex42, LLC).
3. Results
3.1 Patient characteristics
Table 1 displays the demographics and clinical characteristics of the 10 subjects in the study. Pancreatic cyst fluid was safely collected via EUS-FNA from all subjects. Five patients had asymptomatic BD-IPMN with the final diagnosis made by surgical pathology in 3 patients and radiology in 2 patients. The other five patients had thick walled inflammatory cysts with 3 resulting from antecedent attacks of acute pancreatitis (WOPN) and 2 from chronic calcific alcoholic pancreatitis (pseudocyst). All five inflammatory cyst patients had abdominal pain and underwent cystgastrostomy or necrosectomy. BD-IPMN subjects were older. Average cyst size was significantly larger in pseudocysts (72.6 mm) than BD-IPMN (17.4 mm). As expected, higher amylase concentrations were observed in the inflammatory cysts and higher CEA concentrations in the BD-IPMN.
Table 1.
Demographics and clinical data.
| BD-IPMN (n=5) | Inflammatory Cyst (n=5) | p-value | |
|---|---|---|---|
| Mean age (years) | 68.2 | 48.2 | 0.05 |
| Gender (female) | 4 | 3 | >0.50 |
| Symptoms | None | All abdominal pain | <0.01 |
| History of acute pancreatitis | 1 (distant) | 3 | >0.50 |
| History of chronic pancreatitis | 0 | 2 | >0.50 |
| Mean cyst size (mm) | 17.4 | 72.6 | <0.01 |
| Cytology | 3 non-diagnostic, 2 benign with no malignant cells | 3 non-diagnostic, 1 benign with no malignant cells, 1 cytology not performed | >0.50 |
| Median amylase (U/L) [range] | 86.7 [65, 40085] | 44590 [8480, 80700] | 0.15 |
| Median CEA (ng/mL) [range] | 2363 [87.4, 5980] | 13.7 [9.1, 18.2] | 0.18 |
3.2 Protein microarray assay detected IMPs directly from all pancreatic cyst fluid samples
Supplemental Table 2 displays the IMP concentrations in all study subjects and associated statistical significance values. The concentration of IMPs ranged from below the limit of detection by the instrument to greater than 50,000 pg/mL. Several proteins had medianIMP concentrations >1000 pg/mL. In the inflammatory cyst samples, HGF (hepatocyte growth factor), ICAM-I (intercellular adhesion molecule 1), IL-8 (interleukin-8), MCP-1 (monocyte chemotactic protein 1), and SCGF-b (stem cell growth factor-beta) were detected in concentrations greater than 2000 pg/mL. In the BD-IPMN samples, NAP-2 (nucleosome assembly protein 2) had median concentrations greater than 3800 pg/mL.
Figure 2 summarizes the proteins detected in the BD-IPMN and inflammatory cyst samples. Of the 89 IMPs assayed, 7 were detected in BD-IPMN and not inflammatory cysts, while 11 in inflammatory cysts but not BD-IPMN, and 65 in both types of cysts. Six IMPs were not identified in either type of cyst.
Figure 2. Venn diagram of cytokines identified in BD-IPMN and pancreatic inflammatory cyst (IC).
IMPs detected in a particular cyst type are listed adjacent to the diagram. Of the 89 cytokines assayed, six were not detected in either cyst type.
3.3 Seven IMPs were detected in BD-IPMN and not inflammatory cysts (Table 2)
Table 2.
Inflammatory mediator proteins detected in BD-IPMN, not in pancreatic inflammatory cysts.
| IMP | BD-IPMN |
|||||||
|---|---|---|---|---|---|---|---|---|
| Samples, concentration pg/mL |
Number of samples with IMP | Median | IQR | |||||
| BD1 | BD2 | BD3 | BD4 | BD5 | ||||
| 6Ckine | N.D. | N.D. | 104.4 | N.D. | N.D. | 1/5 (20%) | 14.4 | 0.0 |
| CTACK | N.D. | 1.0 | 2.7 | N.D. | N.D. | 2/5 (40%) | 1.9 | 0.9 |
| ENA-78 | N.D. | N.D. | 2566.7 | 139.5 | N.D. | 2/5 (40%) | 1353.1 | 1213.6 |
| IL-20 | N.D. | N.D. | 50.2 | N.D. | N.D. | 1/5 (20%) | 5.2 | 0.0 |
| IL-28A | 2.9 | N.D. | N.D. | N.D. | N.D. | 1/5 (20%) | 2.9 | 0.0 |
| MIP-3a | N.D. | N.D. | 47.3 | N.D. | N.D. | 1/5 (20%) | 47.3 | 0.0 |
| MIP-3b | N.D. | N.D. | 2.1 | N.D. | N.D. | 1/5 (20%) | 2.1 | 0.0 |
N.D., not detected; IQR, interquartile range
These IMPs were: 6Ckine (chemokine with 6 cysteines), CTACK (cutaneous T-cell-attracting chemokine), ENA-78 (epithelial-derived neutrophil-activating protein 78), IL-20, IL-28A, MIP-3a (macrophage inflammatory protein 3-alpha), and MIP-3b. The concentrations of IMPs in individual samples ranged from 1 to 2567 pg/mL. Each of these 7 proteins was detected in only 1 or 2 of the 5 BD-IPMN samples.
3.4 Eleven IMPs were detected in inflammatory cysts and not BD-IPMN (Table 3)
Table 3.
Inflammatory mediator proteins detected in pancreatic inflammatory cysts (IC), not in BD-IPMN.
| IMP | Pancreatic Inflammatory Cyst |
|||||||
|---|---|---|---|---|---|---|---|---|
| Samples, concentration pg/mL |
Number of samples with IMP | Median | IQR | |||||
| IC1 | IC2 | IC3 | IC4 | IC5 | ||||
| GM-CSF | 11.81 | 57.65 | 19.17 | 13.85 | 22.85 | 5/5 (100%) | 19.2 | 9.0 |
| I-309 | N.D. | 2.41 | 4.85 | N.D. | 0.34 | 3/5 (60%) | 2.4 | 2.3 |
| IL-17 | 0.29 | N.D. | 69.58 | N.D. | 0.9 | 2/5 (40%) | 0.9 | 34.6 |
| IL-5 | 0.67 | 2.59 | 1.75 | N.D. | 1.16 | 4/5 (80%) | 1.5 | 0.9 |
| IL-9 | N.D. | N.D. | N.D. | 2.07 | N.D. | 1/5 (20%) | 2.7 | 0.0 |
| TGF-b1 | N.D. | 251.39 | N.D. | N.D. | N.D. | 1/5 (20%) | 251.4 | 0.0 |
| TGF-b2 | N.D. | 339.11 | 323.79 | N.D. | N.D. | 2/5 (40%) | 331.5 | 7.7 |
| TGF-b3 | N.D. | 4.6 | N.D. | N.D. | N.D. | 1/5 (20%) | 4.6 | 0.0 |
| TNF-b | 1.91 | 4.41 | 4.14 | N.D. | 2.57 | 4/5 (80%) | 3.4 | 1.8 |
| TPO | 26.7 | N.D. | N.D. | N.D. | 20.61 | 2/5 (40%) | 14.7 | 12.0 |
| TSLP | 6.74 | N.D. | N.D. | N.D. | N.D. | 1/5 (20%) | 6.7 | 0.0 |
N.D., not detected; IQR, interquartile range.
These IMPs were: GM-CSF, I-309 (T lymphocyte-secreted protein), IL-17, IL-5, IL-9, TGF-b1 (transforming growth factor beta-1), TGF-b2, TGF-b3, TNF-b, TPO (thyroid peroxidase), and TSLP (thymic stromal lymphopoietin). The concentrations of IMPs in individual samples ranged from 0.29 to 339 pg/mL. Although several of these IMPs were detected in only 1 or 2 samples, the majority were identified in at least 3 inflammatory cyst samples. Most impressive was GM-CSF (in bold typeface in Table 3), which was detected in all five inflammatory cyst samples at a median concentration of 19.2 pg/mL and in no BD-IPMN samples (Fisher's exact p = 0.0079). There were no differences in concentrations of IMPs between the 2 pseudocyst and 3 WOPN samples.
3.5 Higher concentrations of Hepatocyte Growth Factor (HGF) and Eotaxin were identified in inflammatory cysts (Table 4)
Table 4.
Differentially expressed inflammatory mediator proteins present in both BD-IPMN and inflammatory cyst (IC).
| Cytokine | BD-IPMN |
Inflammatory cyst |
p-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration, pg/ml |
Concentration, pg/ml |
||||||||||||||
| Samples |
Median | IQR | Samples |
Median | IQR | ||||||||||
| BD1 | BD2 | BD3 | BD4 | BD5 | IC1 | IC2 | IC3 | IC4 | IC5 | ||||||
| Eotaxin | 2.79 | 18.59 | 18.16 | 8.63 | N.D. | 13.40 | 11.10 | 26.73 | N.D. | 38.31 | 20.99 | 268.77 | 32.52 | 70.63 | 0.067 ** |
| HGF | 27.81 | 144.11 | 474.27 | 20.32 | 12.61 | 27.81 | 123.79 | 14657.23 | 5189.26 | 8639.81 | 1918.98 | 1613.07 | 5189.26 | 6720.83 | 0.034 * |
Marginally statistically significant difference in median concentration of cytokine in BD-IPMN vs. inflammatory cyst (p < 0.1).
Strongly statistically significant difference in median concentration of cytokine in BD-IPMN vs. inflammatory cyst (p < 0.05).
†: Fisher's exact test (p<0.05).
N.D., not detected.
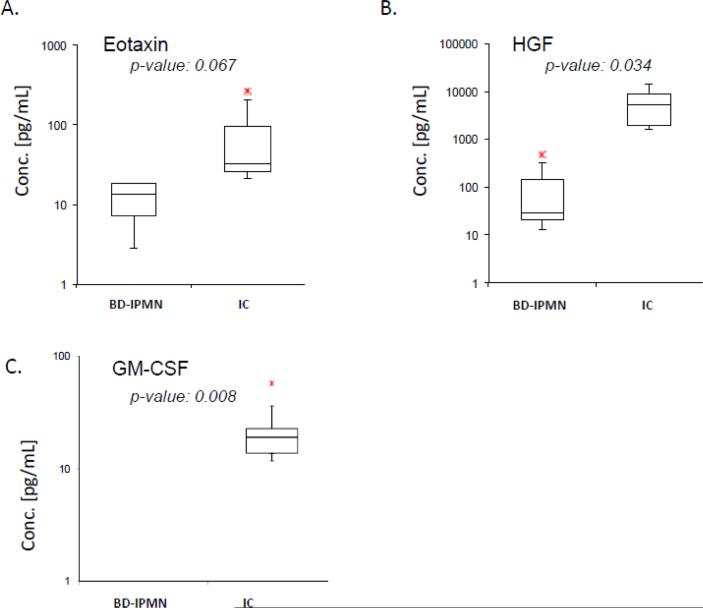
Most IMPs were detected in both BD-IPMN and inflammatory cyst samples. Supplemental Table 2 lists the 65 IMPs identified in both cyst types. Among these IMPs, HGF was detected in significantly greater concentrations (p=0.0335) in inflammatory cysts. Additionally, Eotaxin was detected in marginally greater concentrations (p=0.0671) in inflammatory cysts. Box and whisker plots for Eotaxin and HGF as well as GM-CSF (detected in inflammatory cysts) are illustrated in Figure 3.
Figure 3. Box and whisker plots of IMPs, which are potential biomarkers.
A) Eotaxin shows marginally significant difference, p-value <0.1.B) HGF shows a strong significant difference, p-value <0.05. C) GM-CSF appears in none of the BD-IPMN samples, but all 5 inflammatory cyst samples. The bottom and top edges of the box are located at the 25th and 75th percentiles, respectively. The horizontal line within the box marks the 50th percentile (median). Whiskers extend from the box as far as the data extend, at most 1.5 interquartile ranges.
4. Discussion
We present, to our knowledge, the first multiplex cytokine microarray-based characterization of pancreatic cystic neoplasms sampled by EUS-FNA. In this exploratory analysis, we successfully identified IMPs in all EUS-FNA-collected pancreatic cyst fluid samples using a microsphere-based suspension protein microarray assay. A previous study examined cytokines from pancreatic cyst fluid, however these samples were obtained from resected specimens and were assayed using a different IMP panel (Allen et al., 2009). Our analysis revealed differences in the IMP profile of pancreatic cyst fluid from BD-IPMN compared to inflammatory cysts. Across both categories of pancreatic cysts, we identified a total of 20 IMPs among the 89 tested that were either only present in BD-IPMN (n=7) or inflammatory cyst (n=11) fluid, or present in statistically higher concentrations (n=2) in a particular type of pancreatic cyst. GM-CSF is of particular interest as it appeared in all five inflammatory cyst fluid samples and in none of the BD-IPMN samples. In addition, two IMPs (Eotaxin and HGF) were detected with higher concentrations in inflammatory cysts compared to BD-IPMN cysts. These proteins may serve as diagnostic biomarkers and provide insights into the malignant potential of pancreatic cystic neoplasms.
Differentiating among the types of pancreatic cystic lesions can be difficult. Currently available biomarkers are imperfect and include CEA, amylase, cytology, and DNA markers (Brugge et al., 2004; Maker et al., 2008; Khalid et al., 2009). While small volumes of cyst fluid are sufficient DNA analysis, larger volumes (approximately 0.5-1 mL) are required for CEA and amylase. Therefore, biomarkers with improved sensitivity, requiring only small quantities of cyst fluid are clearly needed. Multiplex microarray analysis allows the evaluation of a large number of proteins with a very small sample quantity and is thus ideally suited for use with pancreatic cyst fluid obtained by EUS-FNA.
IMPs functioning alone or in concert with others to induce a physiological response may be detected in pancreatic fluid and provide insights into pancreatic cystic neoplasms. The immune system has increasingly been recognized as an important factor in pancreatic cancer pathogenesis (Shimosegawa and Gorelick, 2009). In pancreatic cancer, pancreatic stellate cells activated by cytokines, chemokines, and growth factors (such as VEGF, PDGF-BB, TGF-b, TNF-a, IL-1, and IL-6), induce pancreatic fibrosis (Masamune et al., 2009). In addition, the desmoplasia associated with pancreatic ductal adenocarcinoma is tightly linked to factors secreted by pancreatic stellate cells, which have been activated by the aforementioned IMPs (Pandol et al., 2009). As such, we expect certain IMPs to be differentially expressed in fluid from the various types of premalignant and benign pancreatic cysts.
Several IMPs in our study were detected in only one particular pancreatic cyst type. Seven IMPs were detected only in BD-IPMN fluid samples, potentially representing diagnostic markers enabling more accurate diagnosis of these premalignant cysts. Several of these IMPs are functionally linked, particularly MIP-3b and 6Ckine. Both MIP-3b and 6Ckine play important roles in T-cell trafficking in the thymus, T-cell and B-cell migration to secondary lymphoid organs, and both share a common lymphocyte receptor, CCR7 (Campbell et al., 1998). Similarly, certain IMPs (including TGF b 1, 2, and 3 and IL-17) were detected only in in inflammatory cyst fluid samples. Interestingly, increased expression of TGF- b isoforms in the human pancreas may be involved in the tissue reparative process following necrotizing pancreatitis (Friess et al., 1998). IL-17A is produced by helper T cells and induces stromal cells to secrete pro-inflammatory and hematopoietic cytokines; it has been shown to affect regulation of the cytokine thymic stromal lymphopoietin (TSLP) (Xu et al., 2010).
Pancreatic inflammatory cysts develop in the setting of acute or chronic pancreatitis and are the most common type of pancreatic cyst with no malignant potential (Kim and Kim, 2012). Although history assists in diagnosing these inflammatory cysts, clinically it may be difficult to differentiate inflammatory cysts and BD-IPMN, particularly if the patient has not undergone abdominal imaging. Accurate diagnosis of the pancreatic cyst is critical to management of the patient as asymptomatic inflammatory cysts do not require further therapy, while BD-IPMN requires resection if it caused the attack of acute pancreatitis, based on Sendai guidelines.
GM-CSF is the most promising biomarker, as this IMP was detected in all five inflammatory cyst samples and none of the BD-IPMN samples. This protein, secreted by macrophages, T cells, mast cells, endothelial cells, and fibroblasts, functions to stimulate the growth and differentiation of hematopoietic precursor cells (Martinez-Moczygemba and Huston, 2003). In the pancreas, GM-CSF has been shown to recruit immune cells (Krakowski et al., 2002). In addition, evidence suggests that GM-CSF is a mediator in acute pancreatitis-associated lung injury (Frossard et al., 2002).
The IMPs Eotaxin and HGF were common to both cyst types, but demonstrated significantly higher concentrations in the inflammatory cyst samples. Eotaxin is a chemokine that directly promotes the accumulation of eosinophils, a prominent feature of allergic inflammatory reactions. In studies of the endocrine pancreas, Eotaxin has been implicated in type 1 diabetes pathogenesis (Hessner et al., 2004). HGF is secreted by mesenchymal cells and acts primarily on epithelial, endothelial cells, and hematopoietic progenitor cells (Comoglio, 1993). Moreover, HGF functions with GM-CSF to stimulate colony formation of hematopoietic progenitor cells (Kmiecik et al., 1992) and is up-regulated in the blood of individuals with acute pancreatitis (Ueda et al., 1996).
Due to the exploratory nature of this study, its main limitation is sample size. GM-CSF, in addition to Eotaxin and HGF, has the potential to be a strong biomarker differentiating BD-IPMN and inflammatory cysts. Greater sample size will be necessary to validate these IMPs as potential biomarkers. Additionally, we will expand future analyses of IMPs to other clinically relevant pancreatic cystic lesions, including main duct IPMN and mucinous cystadenomas.
In conclusion, we have successfully identified IMPs in pancreatic cyst fluid using EUS-FNA in tandem with cytokine microarray technology. The advantages of this targeted investigation of pancreatic fluid include high specificity, small sample volume requirement, cost-effectiveness, and complementarity to other detection methods, such as mass spectrometry and Western blotting (Pollard et al., 2007). The application of this approach to the various pancreatic cystic neoplasms and clinical stages of pancreatic dysfunction may lead to major insights into cytokine-mediated mechanisms of inflammation and pathogenesis of pancreatic cancer.
Supplementary Material
HIGHLIGHTS.
EUS-FNA-sampled pancreatic cystic neoplasms are analyzed using a cytokine microarray
Seven IMPs were detected in BD-IPMN, but not in inflammatory cysts
Eleven IMPs were detected in inflammatory cysts, but not in BD-IMPNs
GM-CSF and HGF in pancreatic cyst fluid distinguishes BD-IPMN and inflammatory cysts
Coupling microarrays to EUS-FNA is a major step in understanding pancreatic disease
ACKNOWLEDGMENTS
Funds were provided by the American College of Gastroenterology Clinical Research Award (LL, 2011) and the NIH NIDDK NRSA Fellowship (JP, NIH NIDDK 1 F32 DK085835-01A1) and Harvard Digestive Diseases Center (DC, NIH 5 P30 DK034854-24) and NIH NIDDK (DC, 1R21 DK081703-01A2).
LIST OF ABBREVIATIONS
- BD-IPMN
branch duct intraductal papillary mucinous neoplasm
- EUS-FNA
endoscopic ultrasound fine needle aspiration
- IMP
inflammatory mediator proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
DC conceived the study. LL collected the pancreas cyst fluid specimens. JP carried out the laboratory experiments. JP and LL drafted the original manuscript. JP, LL, and DC participated in its design and coordination. All authors helped to draft the manuscript and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
Contributor Information
Linda S. Lee, Center for Pancreatic Disease, Division of Gastroenterology Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston, MA.
Peter A. Banks, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston, MA.
Andrew M. Bellizzi, Department of Pathology, University of Iowa Hospitals and Clinics, Iowa City, IA.
Nisha I. Sainani, Department of Radiology, Brigham and Women's Hospital, Boston, MA.
Vivek Kadiyala, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston, MA.
Shadeah Suleiman, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston, MA.
Darwin L. Conwell, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston, MA.
Joao A. Paulo, Department of Pathology, Children's Hospital Boston, Boston, MA Proteomics Center at Children's Hospital Boston, Boston, MA Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston, MA.
References
- Allen PJ, Qin LX, Tang L, Klimstra D, Brennan MF, Lokshin A. Pancreatic cyst fluid protein expression profiling for discriminating between serous cystadenoma and intraductal papillary mucinous neoplasm. Ann Surg. 2009;250:754–60. doi: 10.1097/SLA.0b013e3181bd7f20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54:343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, Brink JA, Baker ME, Federle MP, Foley WD, Francis IR, Herts BR, Israel GM, Krinsky G, Platt JF, Shuman WP, Taylor AJ. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 7:754–73. doi: 10.1016/j.jacr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Brugge WR. The incidental pancreatic cyst on abdominal computerized tomography imaging: diagnosis and management. Clin Gastroenterol Hepatol. 2008;6:140–4. doi: 10.1016/j.cgh.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Brugge WR. The use of EUS to diagnose cystic neoplasms of the pancreas. Gastrointestinal endoscopy. 2009;69:S203–9. doi: 10.1016/j.gie.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Bowman EP, Murphy K, Youngman KR, Siani MA, Thompson DA, Wu L, Zlotnik A, Butcher EC. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J Cell Biol. 1998;141:1053–9. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG. Inflammatory Cytokines in Nonpathological States. News Physiol Sci. 2000;15:298–303. doi: 10.1152/physiologyonline.2000.15.6.298. [DOI] [PubMed] [Google Scholar]
- Carson RT, Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- Comoglio PM. Structure, biosynthesis and biochemical properties of the HGF receptor in normal and malignant cells. EXS. 1993;65:131–65. [PubMed] [Google Scholar]
- Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–99. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- Fitzgerald SP, McConnell RI, Huxley A. Simultaneous analysis of circulating human cytokines using a high-sensitivity cytokine biochip array. Journal of proteome research. 2008;7:450–5. doi: 10.1021/pr070409o. [DOI] [PubMed] [Google Scholar]
- Friess H, Lu Z, Riesle E, Uhl W, Brundler AM, Horvath L, Gold LI, Korc M, Buchler MW. Enhanced expression of TGF-betas and their receptors in human acute pancreatitis. Ann Surg. 1998;227:95–104. doi: 10.1097/00000658-199801000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard JL, Saluja AK, Mach N, Lee HS, Bhagat L, Hadenque A, Rubbia-Brandt L, Dranoff G, Steer ML. In vivo evidence for the role of GM-CSF as a mediator in acute pancreatitis-associated lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L541–8. doi: 10.1152/ajplung.00413.2001. [DOI] [PubMed] [Google Scholar]
- Hessner MJ, Wang X, Meyer L, Geoffrey R, Jia S, Fuller J, Lernmark A, Ghosh S. Involvement of eotaxin, eosinophils, and pancreatic predisposition in development of type 1 diabetes mellitus in the BioBreeding rat. J Immunol. 2004;173:6993–7002. doi: 10.4049/jimmunol.173.11.6993. [DOI] [PubMed] [Google Scholar]
- Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. The American journal of gastroenterology. 2007;102:2339–49. doi: 10.1111/j.1572-0241.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointestinal endoscopy. 2009;69:1095–102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Kim KO, Kim TN. Acute Pancreatic Pseudocyst: Incidence, Risk Factors, and Clinical Outcomes. Pancreas. 2012 doi: 10.1097/MPA.0b013e3182374def. [DOI] [PubMed] [Google Scholar]
- Kmiecik TE, Keller JR, Rosen E, Vande Woude GF. Hepatocyte growth factor is a synergistic factor for the growth of hematopoietic progenitor cells. Blood. 1992;80:2454–7. [PubMed] [Google Scholar]
- Krakowski M, Abdelmalik R, Mocnik L, Krahl T, Sarvetnick N. Granulocyte macrophage-colony stimulating factor (GM-CSF) recruits immune cells to the pancreas and delays STZ-induced diabetes. J Pathol. 2002;196:103–12. doi: 10.1002/path.1013. [DOI] [PubMed] [Google Scholar]
- Lee LS, Saltzman JR, Bounds BC, Poneros JM, Brugge WR, Thompson CC. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231–6. doi: 10.1016/s1542-3565(04)00618-4. [DOI] [PubMed] [Google Scholar]
- Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. 2008;15:3187–92. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Huston DP. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112:653–65. doi: 10.1016/S0091. quiz 666. [DOI] [PubMed] [Google Scholar]
- Masamune A, Watanabe T, Kikuta K, Shimosegawa T. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7:S48–54. doi: 10.1016/j.cgh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10:108–15. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol S, Edderkaoui M, Gukovsky I, Lugea A, Gukovskaya A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009;7:S44–7. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo JA, Lee LS, Wu B, Banks PA, Steen H, Conwell DL. Cytokine profiling of pancreatic fluid using the ePFT collection method in tandem with a multiplexed microarray assay. J Immunol Methods. 2011;369:98–107. doi: 10.1016/j.jim.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo JA, Lee LS, Wu B, Repas K, Banks PA, Conwell DL, Steen H. Optimized sample preparation of endoscopic collected pancreatic fluid for SDS-PAGE analysis. Electrophoresis. 2010a;31:2377–87. doi: 10.1002/elps.200900762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo JA, Lee LS, Wu B, Repas K, Banks PA, Conwell DL, Steen H. Proteomic analysis of endoscopically (endoscopic pancreatic function test) collected gastroduodenal fluid using in-gel tryptic digestion followed by LC MS/MS. Proteomics Clin Appl. 2010b;4:715–25. doi: 10.1002/prca.201000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo JA, Lee LS, Wu B, Repas K, Mortele KJ, Banks PA, Steen H, Conwell DL. Identification of pancreas-specific proteins in endoscopically (endoscopic pancreatic function test) collected pancreatic fluid with liquid chromatography--tandem mass spectrometry. Pancreas. 2010c;39:889–96. doi: 10.1097/MPA.0b013e3181cf16f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman MB, Genevay M, Yaeger K, Chebib I, Turner BG, Mino-Kenudson M, Brugge WR. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than “positive” cytology. Cancer Cytopathol. 118:434–40. doi: 10.1002/cncy.20118. [DOI] [PubMed] [Google Scholar]
- Pitman MB, Lewandrowski K, Shen J, Sahani D, Brugge W, Fernandez-del Castillo C. Pancreatic cysts: preoperative diagnosis and clinical management. Cancer Cytopathol. 118:1–13. doi: 10.1002/cncy.20059. [DOI] [PubMed] [Google Scholar]
- Pollard HB, Srivastava M, Eidelman O, Jozwik C, Rothwell SW, Mueller GR, Jacobowitz DM, Darling T, Guggino WB, Wright J, Zeitlin PL, Paweletz CP. Protein microarray platforms for clinical proteomics. Proteomics Clinical Applications. 2007;1:934–952. doi: 10.1002/prca.200700154. [DOI] [PubMed] [Google Scholar]
- Rottman JB. Key role of chemokines and chemokine receptors in inflammation, immunity, neoplasia, and infectious disease. Vet Pathol. 1999;36:357–67. doi: 10.1354/vp.36-5-357. [DOI] [PubMed] [Google Scholar]
- Sachdeva N, Asthana D. Cytokine quantitation: technologies and applications. Front Biosci. 2007;12:4682–95. doi: 10.2741/2418. [DOI] [PubMed] [Google Scholar]
- Shimosegawa T, Gorelick FS. Inflammation and carcinogenesis in the pancreas and biliary tract: mechanisms and practice. Clin Gastroenterol Hepatol. 2009;7:S1–2. doi: 10.1016/j.cgh.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Ueda T, Takeyama Y, Toyokawa A, Kishida S, Yamamoto M, Saitoh Y. Significant elevation of serum human hepatocyte growth factor levels in patients with acute pancreatitis. Pancreas. 1996;12:76–83. doi: 10.1097/00006676-199601000-00010. [DOI] [PubMed] [Google Scholar]
- Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–55. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhang L, Wang DY, Xu R, Liu Z, Han DM, Wang XD, Zuo KJ, Li HB. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy. 2010;65:581–9. doi: 10.1111/j.1398-9995.2009.02252.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.