Abstract
A wide investigation was conducted into the main organic matter (OM) sources supporting coral reef trophic networks in the lagoon of New Caledonia. Sampling included different reef locations (fringing, intermediate and barrier reef), different associated ecosystems (mangroves and seagrass beds) and rivers. In total, 30 taxa of macrophytes, plus pools of particulate and sedimentary OM (POM and SOM) were sampled. Isotopic signatures (C and N) of each OM sources was characterized and the composition of OM pools assessed. In addition, spatial and seasonal variations of reef OM sources were examined. Mangroves isotopic signatures were the most C-depleted (-30.17 ± 0.41 ‰) and seagrass signatures were the most C-enriched (-4.36 ± 0.72 ‰). Trichodesmium spp. had the most N-depleted signatures (-0.14 ± 0.03 ‰) whereas mangroves had the most N-enriched signatures (6.47 ± 0.41 ‰). The composition of POM and SOM varied along a coast-to-barrier reef gradient. River POM and marine POM contributed equally to coastal POM, whereas marine POM represented 90% of the POM on barrier reefs, compared to 10% river POM. The relative importance of river POM, marine POM and mangroves to the SOM pool decreased from fringing to barrier reefs. Conversely, the relative importance of seagrass, Trichodesmium spp. and macroalgae increased along this gradient. Overall, spatial fluctuations in POM and SOM were much greater than in primary producers. Seasonal fluctuations were low for all OM sources. Our results demonstrated that a large variety of OM sources sustain coral reefs, varying in their origin, composition and role and suggest that δ13C was a more useful fingerprint than δ15N in this endeavour. This study also suggested substantial OM exchanges and trophic connections between coral reefs and surrounding ecosystems. Finally, the importance of accounting for environmental characteristics at small temporal and spatial scales before drawing general patterns is highlighted.
Introduction
Marine primary producers are the basis of the functioning of coastal and pelagic ecosystems [1]. At the ocean scale phytoplanktonic production is largely responsible for organic carbon input into the food webs [2], whereas at the scale of a coastal ecosystem macrophyte algae (sensu lato) play a much greater role in the organic carbon input [3]. Tropical coral reefs are complex, highly diversified ecosystems with numerous potential sources of organic matter (OM), and the major primary producers on reefs are benthic macroalgae, including turf algae. Primary producers of reef-associated ecosystems such as mangroves and seagrass beds also provide a significant amount of organic carbon and other elements such as nitrogen. For instance, 1–100% of leaves may be exported from seagrass beds as organic matter, with an average of about 25% [4]. Similarly, approximately 30–50% of leaves may be exported from mangroves [5].
The major organic sources in an ecosystem are generally mixed together into global pools of OM [6]. These highly heterogeneous pools are distributed in the water and in the sediments and contain both living and dead organic materials from various origins; this is especially true for coastal zones. The particulate organic matter (POM) in water is a mixture of phytoplankton, bacteria, invertebrates and fish fecal pellets, and detrital particles [7]. Mainly detrital, the sedimentary organic matter (SOM) contains all of the above components plus the micro-phytobenthos and the meiofauna [8]. In some coastal zones SOM may also contain substantial continental inputs from river sediment [9]. These complex pools contain organic material produced by very different organisms using various photosynthetic processes. Teasing apart their respective contributions to ecosystem functioning remains difficult.
A solution to this problem may be provided by the use of isotopic signatures, as they allow discrimination of the various OM sources [10]. For example, terrestrial or marine OM origins can be identified by stable isotopes (specifically δ13C and δ15N), from which the relative OM contributions to SOM and/or POM pools can also be assessed [11]. This approach has been widely applied in temperate coastal zones [12–14], but has rarely been used in coral reefs [9]. Consequently, information about the functioning of highly diversified ecosystems such as coral reefs remains fragmentary.
Although partitioning the different OM sources among primary consumers largely underpins the transit of OM through food webs, studies that have investigated spatial and temporal fluctuations of OM sources using isotopic signatures have mostly focused on only one or a few primary producers [15, 16]. However, it is well known that numerous species feed on macroalgae, turf algae and seagrass leaves [17, 18]. These plants represent the main source of energy for shallow coastal ecosystems and play an important role in benthic nutrient recycling. POM and SOM also influence ecosystem functioning as they transit through trophic networks from the moment they are consumed by primary producers [19]. To properly assess OM origin and flow across coral reefs trophic networks it is necessary to simultaneously study the respective isotopic signatures of POM, SOM, macroalgae and seagrass.
Our present study aimed at characterizing the isotopic signatures (δ13C and δ15N) of several potential OM sources in coral reef ecosystems. The wide, diversified and complex coral reef lagoon of New Caledonia, south-west Pacific, was used as a study case. The first objective was to assess the diversity of potential OM sources and their relative contributions to the pools of OM (POM and SOM). The second objective was to examine the spatio-temporal variations of isotopes δ13C and δ15N for the most common reef OM sources in selected lagoonal locations along two coast-to-ocean gradients and over two seasons.
Material and Methods
Study sites and data sampling / collection
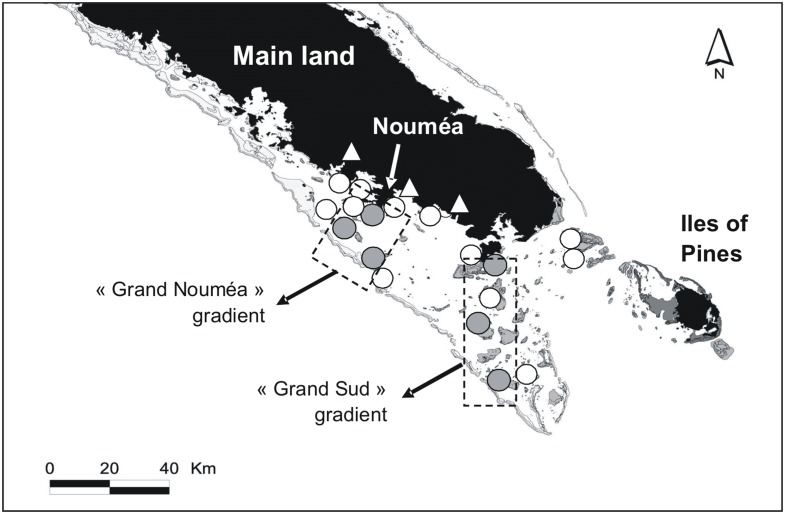
The study site is situated in the southern part of the New Caledonian lagoon (SW Pacific Ocean) (Fig 1). Habitats encompassing various marine landscapes were sampled: mangroves, seagrass beds, coastal soft-bottoms (i.e. without any rocky or coral structure) and coral reefs. The latter were represented by fringing reefs (i.e. close to the shoreline), intermediate reefs (i.e. around islets located in the middle of the lagoon) and barrier reefs separating the lagoon from the open ocean.
Fig 1. Study sites locations in the southwest lagoon of New Caledonia, SW Pacific Ocean.
White marks: OM sources sampled for the first objective in rivers (triangles) and lagoon sites (circles). Grey circles: OM sources sampled in lagoon sites along the two coast-to-ocean gradients for the second objective.
In March and April 2010, OM sources from each marine habitat were sampled at various locations in shallow waters, from 0.5 to 5 m deep, and with a mean water temperature of 26°C (Fig 1). A total of 605 samples of OM sources were collected; 27 different macroalgae (including turf algae; see below) and seagrass species, two mangrove species, plus POM and SOM from the superficial subsurface layer of water (< 10 cm) and sediment (< 3 cm) respectively. Subsurface freshwater samples from there small rivers were also collected, approximately 5–10 km from the river mouth and at low tide to avoid sampling any marine water. Marine water POM was collected near passes whereas coral reef POM was collected on fringing, intermediate and barrier reefs. No specific permissions were required for these locations for such kind of samples that did not involve endangered or protected species.
In summer 2011, hurricane Vania (14th January) and the moderate depression Zellia (17th January) hit New Caledonia. This generated strong rainfall, causing unusually large ground erosion and large amounts of freshwater POM flowed into the lagoon. Freshwater samples were collected just after the major flooding event in order to assess the possible influence of freshwater POM on isotopic (C-N) signatures. In March 2011, a large Trichodesmium spp. bloom occurring in lagoon waters was also sampled. These algae are known to play important role in nitrogen fixation [20]. Flood and bloomevents offered excellent opportunities to examine the impact of unusual events on the isotopic (C-N) signatures of the studied ecosystem (e.g. subsurface seawaters δ15N signature).
In February-April (summer) and August-September (winter) 2011 some common OM sources were sampled again along two coast-to-ocean gradients in the lagoon (i.e. fringing, intermediate and barrier reefs on the “Grand Nouméa” and “Grand Sud” gradients, Fig 1); Nineteen different macroalgae and seagrass species were collected, plus POM and SOM. Turf algae were accounted for as part of the macrophytes sources, as they are one of the major sources of OM on coral reefs [9, 21]. Turf algae will be hereafter referred as algal “turfs”. Algal turfs correspond here to the complex algal species assemblage dominated by Ceramials and usually found in pomacentrid’ territories [22, 23]. The most dominant species of macroalgae and seagrass were sampled when they were accessible. Dominance is here defined in terms of abundance or percentage of substrate cover. Subsurface seawater was collected at each site at mid-tides in order to capture the averaged signal of POM and to avoid a biased signal (at high tides the marine influence is high, whereas at low tides the terrigeneous signal is potentially stronger).
Stable isotope preparation
All subsurface fresh- and seawater samples were filtered on pre-weighted Whatman GF/F filters (porosity 0.7 μm) pre-combusted for 4 h at 450°C. The 63–200 μm-sized fraction was considered to be the best proxy for analyzing the main phytoplankton components of the community [24, 25]. The present study focused on obtaining broad isotopic signatures of fresh- and seawater POM, rather than analyzing the various fractions of phytoplankton. For instance, the smallest components of phytoplankton, namely pico- and nanoplankton, were not taken into account in this study. However, the largest particles and detritus were removed in order to avoid bias in isotopic values.
Seawater and freshwater POM samples collected on GF/F filters were freeze-dried and cut into small pieces. Vegetal and sediment samples were freeze-dried and ground into a fine powder (< 6 μm) using a mortar and pestle. Calcareous macrophytes, marine and coral reef POM and SOM were divided into two sub-samples each. One sub-sample was allocated to the carbon isotope analysis; it was acidified with 1% HCl solution to remove carbonates, rinsed with distilled water and oven-dried at 40°C for 24 h. This protocol is in agreement with the carbonates’ higher δ13C in comparison to organic carbon [26]. The other sample was allocated to the nitrogen isotope analysis; it was not acidified to prevent undesirable enrichment in 15N [27]. Samples from non-calcareous macrophytes and from freshwater POM were analysed without any pre-treatment. Considering the thickness of sediments collected (superficial subsurface layer), we assumed that isotopic values of SOM samples were not biased by any partial remineralisation through bacterial activity.
The 13C:12C and 15N:14N ratios were analyzed by continuous-flow isotope-ratio mass spectrometry. The spectrometer (Delta V Plus stable-isotope analyzer coupled with a Falsh EA 2000 analyzer; Thermo Scientific, Bremen, Germany) was operated in dual isotope mode. The analytical precision was <0.15% for both N and C, estimated from standards analyzed along with the samples. Internal standards were 1 mg leucine calibrated against ‘Europa flour’ and IAEA standards N1 and N2. Isotope ratios were expressed as parts per 1000 (‰) differences from a standard reference material:
δX = [(Rsample x R-1 standard) − 1] x 103; where X is 13C or 15N, R is the corresponding ratio (13C:12C or 15N:14N) and δ is the measure of heavy to light isotope in the sample. The international standard references are Vienna Pee Dee Belemnite (vPDB) for carbon and atmospheric N2 for nitrogen.
Data analyses
Comparison tests
The Levene test was run on the variances of OM sources (i.e. POM, SOM and macrophytes) to assess their homogeneity prior their analysis, and consequently t-tests were performed to compare among means [28]. In the event of heterogeneous variances, non-parametric Kruskall–Wallis tests were run for the analyses. When a source had been sampled during only one season, only spatial analyses were conducted and ANOVAs tests or non-parametric Kruskall-Wallis tests were used for this purpose.
Assessments of sources of potential contributions to POM and SOM pools
Different models can be used to evaluate the contribution of various sources to an organic pool, including Bayesian mixing-models [29]. Irrespective of the method used, a pool isotopic signature is considered as the mean of the signatures of the various constitutive or incorporated sources [30]. For instance, in a pool made of three potential sources, where each source has specific δ13C and δ15N signature, the resulting signature of the pool is expressed as follow [31]:
where δ13Ci and δ15Ni are the isotopic signatures for sources 1 to 3 and f is the relative proportion of the contribution of a source to the pool.
The relative contributions of various OM sources to POM and SOM pools in different habitats were here assessed with Bayesian mixing-models (R software and SIAR package [32]). Firstly, the relative contribution of the river POM, marine POM, and Trichodesmium spp. blooms to the particulate OM from lagoon waters at fringing, intermediate and barrier reef sites was examined. Secondly, the relative contribution of mangrove leaves, seagrass, macroalgae (algal turf, calcareous Chlorophytae, Phaeophycae and Rhodophytae), Trichodesmium spp., river POM and marine POM on the sedimentary OM from all habitats (mangroves, coastal soft-bottoms, fringing reefs, intermediate reefs and barrier reefs) was determinded.
These models calculated the most feasible solutions to explain isotopic ratios measured for POM or SOM and they also allowed integrating all uncertainties related to the OM sources. A major issue with the use of mixing models lies in the choice of trophic enrichment factors (TEFs), which strongly influence the models outputs [33]. TEF was set to null as no consumption process was involved and only the mix of several potential sources of OM was considered.
Results
Diversity of organic matter sources
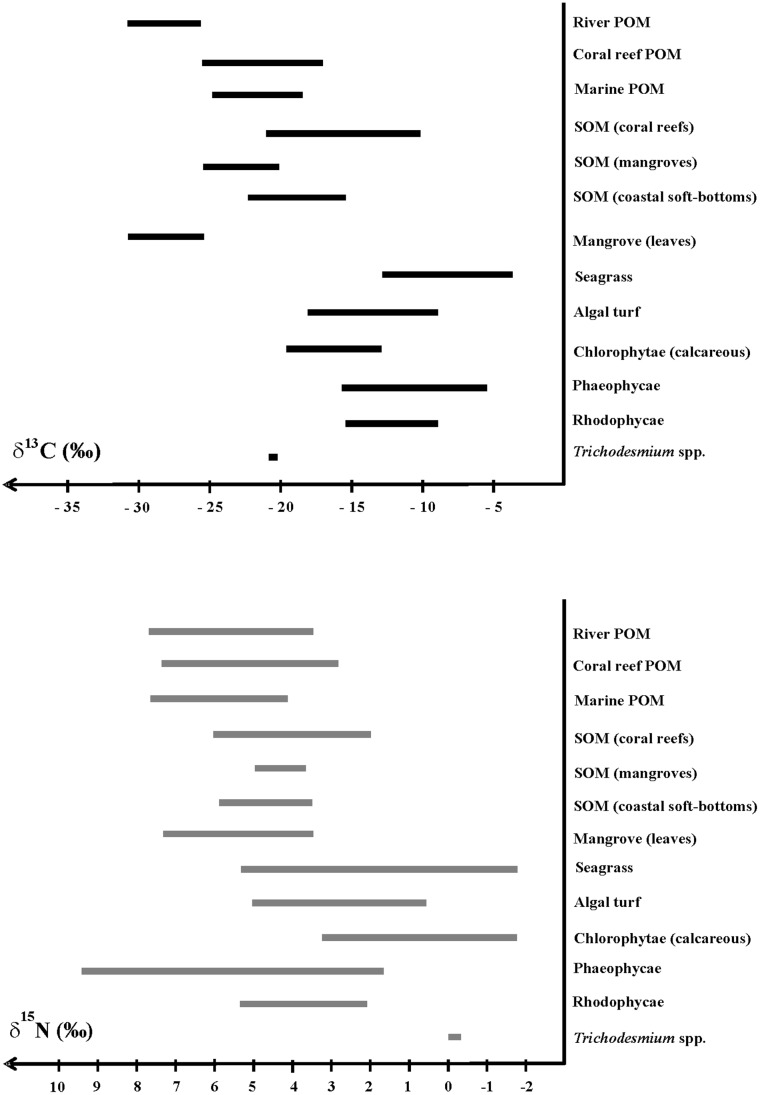
River POM was the most 13C-depleted pool (δ13C from -30.78 to -26.67 ‰, Fig 2). Pre- and post-hurricane δ13C and δ15N signatures were not significantly different (p> 0.75 in both cases) and therefore these data were pooled. Coral reef SOM (fringing, intermediate and barrier reefs) was the most 13C-enriched pool (δ13C from -22.16 to -10.28 ‰). Marine and coral reef POM, mangrove SOM and soft-bottom SOM pools varied at intermediate levels between these extremes (Fig 2). River POM, marine and coral reef POM and coral reef SOM showed relatively similar ranges of δ15N signatures, but with minimal and maximal isotopic signatures being lower for the latter (Fig 2). Mangrove SOM and soft-bottom SOM δ15N signatures overlapped in value, with the range of the former being narrower.
Fig 2. Ranges of isotopic signature values (top: δ13C; bottom: δ15N) for the major OM sources in the SW lagoon of New Caledonia.
All the data sampled were pooled into one single data set.
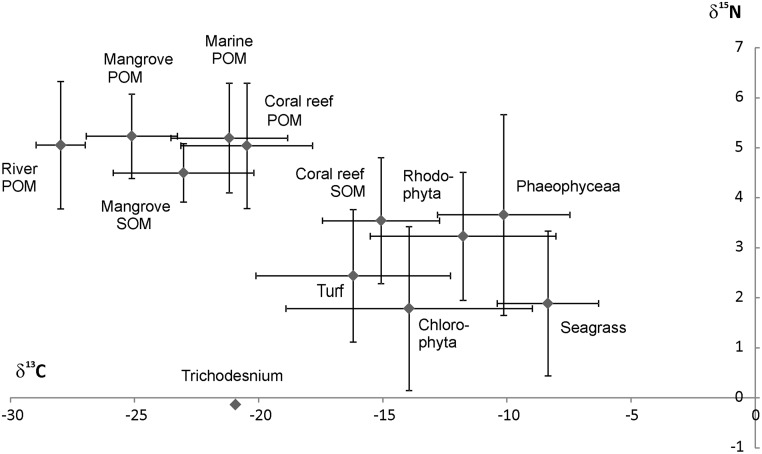
Among primary producers, mangrove was the most 13C-depleted (from -30.95 to -25.91 ‰) and seagrass was the most 13C-enriched (from -13.21 to -3.50 ‰). Algal turfs (from -17.87 to -7.54 ‰), Chlorophytea (from -20.79 to -4.73 ‰), Phaeophycea (from -16.47 to -4.26 ‰), and Rhodophytea (from -13.21 to -5.83 ‰) displayed intermediate values (Fig 2). Trichodesmium spp. signatures were in narrow ranges for both δ13C (from -21.03 to -20.78 ‰) and δ15N (from -0.36 to 0.02 ‰). Overall, the primary producers’ δ15N signatures typically overlapped. This overlap was due to strong inter-species variations in each primary producer category (Table 1, Fig 3). For instance, within the seagrass group, Synrigodium iseotifolium displayed a significantly higher δ13C value and Cymodocea rotondata presented a significantly lower δ15N value compared to the other seagrass species (Table 1). Similarly, in the Chlorophytae group, Halimeda cylindracea δ15N value was significantly lower than the H. discoïdea signature (Table 1).
Table 1. Mean δ13C and δ15N isotopic ratios (± sd) of primary producers sampled in the SW lagoon of New Caledonia (data from 2010 and 2011 pooled).
| Category | Species | N | δ13C (‰) | δ15N (‰) |
|---|---|---|---|---|
| Algal turf | 54 | -16.19 ± 2.92 | 2.44 ± 1.33 | |
| Chlorophytae | Halimeda borneensis | 14 | -14.87 ± 3.41 | 1.29 ± 1.52 |
| H. cylindracea | 39 | -11.80 ± 3.22 | 0.33 ± 1.75 | |
| H. discoïdea | 15 | -14.52 ± 3.02 | 3.62 ± 1.36 | |
| H. heteromorpha | 3 | -16.85 ± 0.63 | 1.51 ± 0.40 | |
| H. macroloba | 12 | -13.14 ± 0.98 | 0.89 ± 0.79 | |
| H. micronesica | 6 | -11.35 ± 2.97 | 2.22 ± 0.48 | |
| H. opuntia | 50 | -15.89 ± 4.53 | 2.35 ± 0.93 | |
| Phaeophycae | Cystoseira sp. | 6 | -12.16 ± 0.97 | 3.09 ± 1.16 |
| Padina australis | 20 | -8.07 ± 1.33 | 3.47 ± 1.82 | |
| Sargassum cristaefolium | 3 | -13.93 ± 0.92 | 2.36 ± 0.24 | |
| S. spinuligerum | 20 | -12.84 ± 2.11 | 5.15 ± 1.67 | |
| Sargassum sp. | 11 | -14.60 ± 1.19 | 3.91 ± 0.20 | |
| Turbinaria conoïdes | 14 | -9.35 ± 1.39 | 2.88 ± 0.90 | |
| T. ornata | 15 | -9.69 ± 1.20 | 3.43 ± 1.52 | |
| Turbinaria sp. | 3 | -7.44 ± 0.84 | 2.86 ± 0.31 | |
| Rhodophytae | Acanthophora spicifera | 3 | -11.71 ± 0.62 | 2.09 ± 0.22 |
| Digenea simplex | 3 | -14.76 ± 1.13 | 4.54 ± 0.14 | |
| Hormophysa cuneiformis | 6 | -12.90 ± 0.49 | 3.15 ± 0.59 | |
| Laurencia sp. | 15 | -13.38 ± 2.52 | 3.29 ± 1.57 | |
| Liagora sp.1 | 6 | -3.16 ± 1.20 | 2.75 ± 0.30 | |
| Liagora sp.2 | 6 | -6.29 ± 0.71 | 2.68 ± 0.24 | |
| Lobophora variegata | 3 | -12.56 ± 1.04 | 3.92 ± 0.07 | |
| Seagrass | Cymodocea rotundata | 6 | -7.24 ± 0.64 | 0.64 ± 1.54 |
| C. serrulata | 18 | -9.39 ± 1.60 | 2.97 ± 1.22 | |
| Halodule uninervis | 27 | -8.55 ± 1.24 | 1.66 ± 1.33 | |
| Halophila ovalis | 6 | -7.78 ± 1.95 | 1.20 ± 0.23 | |
| Synrogodium iseotifolium | 6 | -4.36 ± 0.72 | 1.57 ± 1.16 | |
| Mangrove tree | Avicenia marina | 3 | -27.26 ± 0.30 | 6.47 ± 0.41 |
| Rhizophora stylosa | 6 | -30.17 ± 0.41 | 3.72 ± 0.25 |
Fig 3. δ13C versus δ15N scatterplot of major OM sources in the SW lagoon of New Caledonia.
Relative contributions of organic matter sources to POM pool
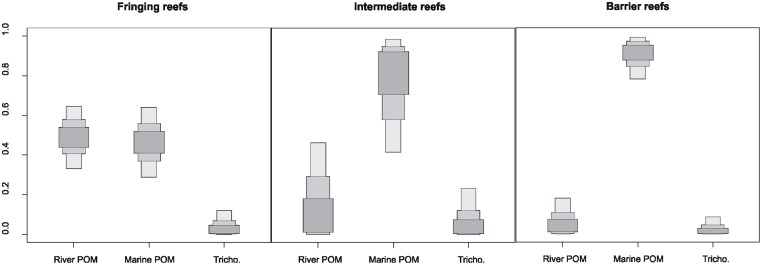
Following the coast to barrier reef gradient the influence of river POM clearly decreased whereas the influence of marine POM increased (Fig 4). Both the Grand Nouméa gradient and Grand Sud gradient displayed a similar pattern (S1 Fig). At coastal sites, the POM pool was 50% composed of river POM and 50% of marine POM; the Grand Nouméa gradient showed a slightly higher contribution of river POM than the Grand Sud gradient (55–60% compared to 40%). At barrier reef sites, the POM pool was dominated by marine POM (90% compared to 10% for river POM). The relative contribution of Trichodesmium spp. to POM was typically less than 10% (Fig 4), although on some of the Grand Sud gradient reefs it reached approximately 15% (S1 Fig).
Fig 4. Relative importance of river POM, marine POM and Trichodesmium spp. (Tricho.) in the isotopic composition of coral reef POM on fringing, intermediate, and barrier reefs.
Shaded boxes represent, from dark to light grey, 50%, 75%, and 95% Bayesian credibility intervals.
Some components of POM are missing in our approach, such as nano- and picophytoplankton because they cannot be collected on GF/F filters. Consequently, some potential sources of POM are missing from this study.
Relative contributions of organic matter sources to SOM pool
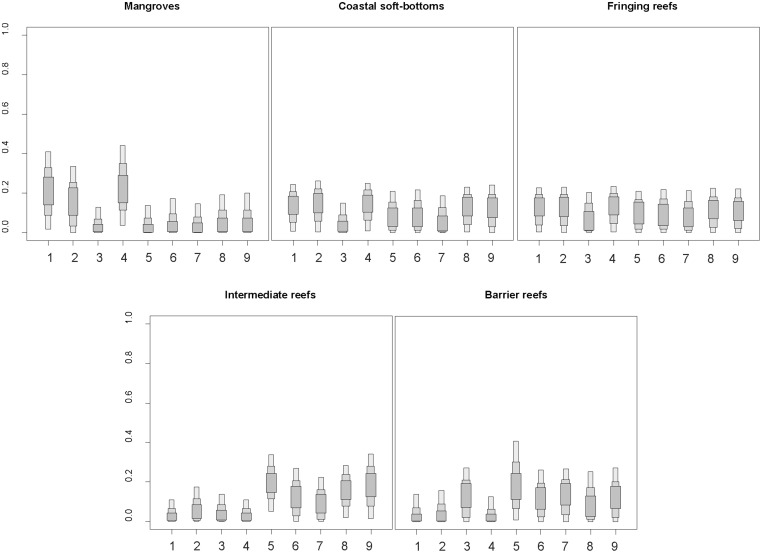
All OM sources contributed to the SOM pool, with variations among habitats and along the coast-to-ocean gradient (Fig 5). River POM, marine POM and mangroves’ relative contributions to SOM pool decreased from coast to barrier reef. Conversely, the relative importance of seagrass, Trichodesmium spp., and to a lesser extent macroalgae, progressively increased along this gradient (Fig 5). Approximately 60% of mangrove SOM derived from mangrove leaves (approximately 25%), river POM (approximately 20%) and marine POM (approximately 15%); the other sources contributed to less than 10%. Fringing reefs and coastal soft-bottom SOM isotopic signatures were relatively similar. Their compositions were diverse, with each source contributing 5% to 15% (Fig 5). Intermediate and barrier reefs SOM mainly derived from algal turf, seagrass and macroalgae, with proportions of each ranging from approximately 10% to over 20%. Trichodesmium spp. was also important on barrier reefs, where it contributed to approximately 15% of the SOM isotopic signature (Fig 5).
Fig 5. Relative importance of various OM sources in the isotopic composition of SOM in mangroves, coastal soft-bottoms, fringing, intermediate, and barrier reefs.
Sources codes: (1) river POM, (2) marine POM, (3) Trichodesmium spp., (4) mangroves leaves, (5) seagrass, (6) algal turf, (7) Chlorophytae (calcareous), (8) Phaeophycae, and (9) Rhodophytae. Shaded boxes represent 50%, 75% and 95% Bayesian credibility intervals from dark to light grey.
Variations of δ13C and δ15N isotopic signatures among the main habitats
Mangroves and seagrass OM displayed significantly different δ13C and δ15N values (p < 0.001; Tables 1 and 2). On coral reefs, algal turf and macroalgae primary producers’ δ13C isotopic signatures were statistically similar (p > 0.05). However, they were significantly different to seagrass and mangroves signatures (p < 0.05; Table 2). δ15N signatures alone were not sufficient to discriminate the various OM sources (Table 2). δ13C and δ15N Trichodesmium spp. signatures were significantly different from all the other primary producers (p < 0.005; Table 2).
Table 2. Mean isotopic signatures (± sd) in carbon (δ13C) and nitrogen (δ15N) for the major sources of organic matter in different habitats of the SW lagoon of New Caledonia.
| Source | Habitat | δ13C (‰) | δ15N (‰) | N |
|---|---|---|---|---|
| POM | River | -27.98 ± 0.99 | 5.05 ± 1.27 | 15 |
| Mangrove | -25.11 ± 1.83 | 5.23 ± 0.58 | 6 | |
| Coastal soft bottom | -22.57 ± 1.46 | 5.22 ± 0.53 | 9 | |
| Marine | -21.10 ± 2.45 | 5.34 ± 0.98 | 6 | |
| Coral reefs | -20.58 ± 2.65 | 5.04 ± 1.26 | 60 | |
| SOM | Mangrove | -23.02 ± 2.83 | 4.50 ± 0.58 | 6 |
| Coastal soft bottom | -17.88 ± 3.61 | 3.87 ± 0.52 | 9 | |
| Coral reefs | -15.70 ± 2.35 | 3.54 ± 1.26 | 60 | |
| Mangrove’ leaves | Mangrove | -28.22 ± 1.68 | 5.36 ± 1.31 | 9 |
| Seagrass | Mangrove | -11.42 ± 0.92 | 2.49 ± 1.94 | 6 |
| Seagrass | Coral reefs | -8.35 ± 2.04 | 1.88 ± 1.45 | 63 |
| Algal turf | Coral reefs | -16.19 ± 3.92 | 2.44 ± 1.33 | 54 |
| Chlorophytae | Coral reefs | -13.94 ± 3.69 | 1.78 ± 1.84 | 148 |
| Phaephocycea | Coral reefs | -10.13 ± 2.67 | 3.66 ± 2.01 | 92 |
| Rhodophytae | Coral reefs | -11.99 ± 2.69 | 3.28 ± 1.31 | 43 |
| Rhodophytae | Mangrove | -14.64 ± 1.91 | 3.85 ± 0.86 | 6 |
| Trichodesmium spp. | Lagoon waters | -20.94 ± 0.02 | -0.14 ± 0.03 | 6 |
POM = particulate organic matter, SOM = sedimentary organic matter (data from 2010 and 2011 pooled).
POM and SOM pools displayed significantly different δ13C signatures among habitats. River POM was significantly 13C-depleted compared to coral reef POM and to coral reef SOM (p < 0.001; Table 2). δ15N signatures were similar between coral reef POM and river POM, but both differed significantly from coral reef SOM (p < 0.001). POM and SOM pools displayed lower δ13C values and higher δ15N values than most primary producers (Table 2). River POM displayed similar C and N isotopic signatures to those of mangrove leaves (p > 0.05). The coral reef POM δ13C signature was significantly different from those of all primary producers (p < 0.05), except Trichodesmium spp. Coral reef POM δ15N value did not differ statistically from those of mangrove leaves and Chlorophytae (p > 0.05). Coral reef SOM δ13C and δ15N signatures significantly differed from those of mangrove leaves, mangrove SOM, and seagrass (p < 0.05 for all cases).
Spatial patterns of δ13C and δ15N isotopic signatures
Isotopic signatures of some OM sources significantly varied along the coast-to-ocean gradient (Table 3). POM and SOM pools fluctuate spatially more than primary producers; this was mainly for δ13C signatures, and the δ15N algal turf signature. POM δ13C signatures were significantly lower on barrier reefs than on fringing and intermediate reefs, whereas POM δ15N signatures were significantly higher on barrier reefs (Tables 3 and 4). These trends were found on Grand Nouméa and Grand Sud, despite some specific differences (S1 Table). SOM δ13C signatures were, overall, significantly lower on fringing reefs and significantly higher on intermediate reefs, whereas SOM δ15N signatures were significantly higher on barrier reefs (Tables 3 and 4). However, this general trend on both coast-to-ocean gradients displayed noticeable differences for intermediate (δ13C) or barrier reefs (δ15N) (S2 Table).
Table 3. Mean isotopic signatures (± sd) in carbon (δ13C) and nitrogen (δ15N) for the different sources of organic matter sampled along coast-to-ocean gradients, both zones and seasons pooled, in the SW lagoon of New Caledonia in 2011.
Only sources present on at least two reef types were shown.
| Category | Species | Site | δ13C (‰) | δ15N (‰) | N |
|---|---|---|---|---|---|
| POM | FR | -18.90 ± 1.70 | 4.85 ± 0.71 | 14 | |
| IR | -18.98 ± 1.33 | 3.79 ± 0.59 | 12 | ||
| BR | -20.82 ± 1.91 | 5.62 ± 0.75 | 12 | ||
| SOM | FR | -15.73 ± 0.72 | 3.59 ± 0.56 | 13 | |
| IR | -13.33 ± 0.85 | 2.30 ± 0.25 | 14 | ||
| BR | -14.77 ± 3.35 | 4.45 ± 2.17 | 14 | ||
| Algal turf | FR | -18.41 ± 2.15 | 2.67 ± 0.27 | 6 | |
| IR | -18.49 ± 2.33 | 1.94 ± 0.39 | 12 | ||
| BR | -19.48 ± 2.06 | 3.05 ± 0.99 | 12 | ||
| Chlorophytae | Halimeda borneensis | FR | -12.99 ± 0.16 | 1.93 ± 0.13 | 3 |
| IR | -13.33 ± 1.52 | 0.16 ± 1.13 | 9 | ||
| BR | -18.76 ± 0.19 | 3.34 ± 0.15 | 3 | ||
| H. cylindracea | FR | -16.05 ± 2.13 | 0.91 ± 0.17 | 6 | |
| IR | -12.14 ± 1.31 | 0.52 ± 1.59 | 9 | ||
| BR | -14.23 ± 0.50 | 0.82 ± 3.52 | 6 | ||
| H. discoïdea | IR | -13.95 ± 2.68 | 2.33 ± 1.29 | 10 | |
| BR | -15.37 ± 1.56 | 5.77 ± 1.21 | 6 | ||
| H. opuntia | FR | -18.75 ± 1.00 | 2.18 ± 0.30 | 9 | |
| IR | -18.05 ± 2.63 | 1.72 ± 0.63 | 12 | ||
| BR | -19.38 ± 0.90 | 3.07 ± 1.56 | 9 | ||
| Phaeophycae | Padina australis | FR | -7.31 ± 0.94 | 2.08 ± 0.15 | 6 |
| IR | -7.61 ± 0.77 | 2.11 ± 0.54 | 8 | ||
| Sargassum spinuligerum | FR | -14.00 ± 2.00 | 3.17 ± 0.13 | 3 | |
| IR | -12.46 ± 2.25 | 5.89 ± 1.29 | 9 | ||
| Turbinaria conoïdes | IR | -8.71 ± 1.23 | 2.84 ± 1.10 | 8 | |
| BR | -10.91 ± 0.80 | 1.78 ± 0.21 | 3 | ||
| T. ornata | IR | -10.56 ± 1.96 | 2.25 ± 0.52 | 6 | |
| BR | -9.47 ± 0.93 | 3.73 ± 1.55 | 12 | ||
| Seagrass | Cymodocea serrulata | FR | -9.51 ± 0.35 | 2.64 ± 0.86 | 6 |
| IR | -8.32 ± 0.14 | 3.04 ± 0.56 | 6 | ||
| Halodule uninervis | IR | -8.01 ± 0.67 | 2.58 ± 0.91 | 6 | |
| BR | -8.79 ± 0.96 | 1.06 ± 0.50 | 6 |
POM = particulate organic matter, SOM = sedimentary organic matter, FR = fringing reef, IR = intermediate reef and BR = barrier reef.
Table 4. Summary of the significant spatial variability of isotopic signatures (δ13C and δ15N) of the sources of organic matter in the SW lagoon of New Caledonia in 2011.
| Sources | Factors | δ13C | δ15N |
|---|---|---|---|
| POM | Site | *** | *** |
| zone x site | *** | * | |
| season | *** | ns | |
| zone x site x season | *** | * | |
| SOM | Site | *** | *** |
| zone x site | *** | *** | |
| season | *** | ns | |
| site x season | *** | * | |
| Algal turf | site | ns | *** |
| zone x site | ns | *** | |
| season | ** | *** | |
| site x season | *** | *** | |
| zone x site x season | * | *** | |
| Halimeda borneensis | site | *** | *** |
| season | ns | * | |
| H. cylindracea | site | *** | ns |
| zone x site | ** | *** | |
| season | ** | ** | |
| site x season | *** | ns | |
| H. opuntia | site | * | *** |
| zone x site | ns | *** | |
| season | * | ns | |
| site x season | *** | ** | |
| H. discoïdea | site | *** | *** |
| season | ** | *** | |
| Sargassum spinuligerum | site | ns | * |
| Turbinaria conoides | site | ns | ** |
| T. ornata | site | * | ns |
| Cymodocea serrulata | site | ** | ns |
| Halodule uninervis | site | ns | *** |
| season | * | ** |
Analyses were run with three-way ANOVAs or Kruskal-Wallis tests: zone (i.e. Grand Nouméa versus Grand Sud) x site (fringing versus intermediate versus barrier reef) x season (summer versus winter).
ns = p> 0.05
* p< 0.05
** p< 0.01
*** p< 0.001.
POM = particulate organic matter, SOM = sedimentary organic matter.
Spatial fluctuations of isotopic signatures were heterogeneous among primary producers. δ13C signatures were generally significantly higher on intermediate reefs than on other reefs (H. borneensis, H. cylindracea, H. discoïdea, Turbinaria conoïdes, Cymodocea serrulata). This general pattern also applied to the Grand Sud gradient; no clear pattern was found on the Grand Nouméa gradient (S3 Table). δ15N signatures were often significantly lower on intermediate reefs than on other reefs (algal turf, Halimeda borneensis, H. discoïdea, H. opuntia, Turbinaria ornata) (Tables 3 and 4). Once again, this general pattern also applied to some extent to the Grand Sud gradient, whereas on the Grand Nouméa gradient δ15N signatures were significantly lower on barrier reefs (algal turf, H. cylindracea, H. opuntia, Halodule uninervis) (S3 Table). δ13C and δ15N signatures on intermediate reefs were significantly different from one coast-to-ocean gradient to the other (S3 Table): on the Grand Sud gradient, H. cylindracea δ13C signatures were lower whereas H. borneensis and H. discoïdea δ13C signatures were higher, and algal turf H. borneensis, H. cylindracea and H. opuntia δ15N signatures were lower.
Seasonal fluctuations of δ13C and δ15N isotopic signatures
The isotopic signatures of OM pools were relatively stable over time (Table 4). POM remained similar among the reef types across seasons, whereas SOM δ13C signatures on barrier reefs were significantly lower in summer (-15.83 ± 2.49 ‰) than in winter (-12.86 ± 2.23 ‰). δ13C signatures significantly varied spatially and across seasons, being systematically lower in summer than in winter on Grand Nouméa gradient for fringing reefs, and all reef types of the Grand Sud gradient (S4 Table). Primary producers also displayed a few significant seasonal variations (16 cases among 98 tests; S4 Table). In most cases, isotopic signatures were lower in summer than in winter, apart from the δ13C signatures of algal turfs on fringing and intermediate reefs on the Grand Nouméa gradient and the intermediate reef on the Grand Sud gradient.
Discussion
Diversity of organic matter sources on coral reefs
The isotopic signatures of OM sources obtained in this study generally fit within typical ranges [34] despite some marginal values (Table 5). For example, the maximal values of δ13C signatures for benthic macrophytes and seagrass were above documented ranges, and the minimal values for mangrove δ15N signatures were below documented ranges. This concurs with the previously established high variability of benthic primary producers’ δ13C signature [15, 27], and significantly help discriminate the OM sources from δ13C signatures.
Table 5. Ranges of isotopic signatures (δ13C and δ15N) of major primary producers on aquatic ecosystems following Ostrom and Fry (1993) (a), and comparison with the present study results (b).
| Primary producer | δ13C (‰) | δ15N (‰) | ||
|---|---|---|---|---|
| Marine phytoplankton | -24 to -18 (a) | -30.8 to -16.8 (b) | -2 to 12 (a) | -0.4 to 7.9 (b) |
| Estuarine phytoplankton | -30 to -15 (a) | 2 to 19 (a) | ||
| Benthic macrophytes | -27 to -10 (a) | -22.9 to -2.1 (b) | -1 to 10 (a) | -2.5 to 10.1 (b) |
| Seagrass | -16 to -4 (a) | -13.2 to -3.5 (b) | 0 to 6 (a) | -2.2 to 5.5 (b) |
| Mangrove trees | -29 to -25 (a) | -31.0 to -25.9 (b) | 6 to 7 (a) | 3.4 to 7.1 (b) |
Despite the high variability of isotopic signatures observed within the main groups, both at the genus and species levels, our results clearly confirm that δ13C signature is a efficient and reliable tool to discriminate OM sources in a highly diversified and complex coral reef lagoon as shown in less diversified ecosystems [8, 35]. A wide range of various isotopic signatures among benthic primary producers allows discrimination of the different contributory sources in trophic networks and hence track OM flows. For instance, δ13C signatures of coral reef primary producers (algal turf and macroalgae) were clearly distinct from those of associated ecosystems (mangrove and seagrass). Similarly, δ13C signatures of the main OM sources were clearly distinct from one another: namely terrestrial (river POM), coastal mangrove and marine (coral reef SOM, algal turf and macroalgae) (Fig 2).
Origin of OM pools’ isotopic composition
Isotopic signatures may provide useful insights on the relative importance of the various sources on a OM pool’s signature [36]. The Bayesian model showed that POM and SOM pools were influenced by various inputs, different in nature and / or amplitude, and that their relative contributions varied along the coast-to-ocean gradient.
POM, a pool largely under marine influence. Links between river inputs and coastal marine POM have been shown on large rivers with strong mean annual flows such as the Rhône river in the Mediterranean [13, 37], and in small rivers influencing fringing reefs in French Polynesia [9]. To our knowledge this paper is the first for coral reef ecosystem.
The influence of river inputs on POM depends on the site location along the coast-to-ocean gradient (Fig 3). Coastal sites are under greater river influence than other sites with a contribution of 50% to reef POM compared to 10% otherwise. Conversely, marine POM contributed to 90%- 95% to POM at other sites. We cannot exclude the possibility that other compounds may influence POM in coastal sites. For instance, degradation of algal and seagrass fragments emerging during low tides or washed onto beaches close to fringing reefs (these cases do not occur on intermediate and barrier reefs) might produce particular compounds that we were not able to take into account. The differences observed between the two gradients could be related to differences in river flows. The Grand Nouméa gradient is under the influence of a river with a mean flow of 11.5 m3.s-1, against a 3.2 m3.s-1 mean flow for the river influencing the Grand Sud gradient [38]. The estimated 80 000 m3.s-1 of marine water entering the SW lagoon of New Caledonia [39] is likely to explain why marine POM was such a strong contributor to coral reef POM. The results did not show any clear influence of Trichodesmium spp. blooms on coral reef POM. However, this kind of OM source is difficult to assess as blooms are usually localized in time and space. However, the intensity of the blooms means that at finer temporal and spatial scales, blooms may generate a significant contribution to POM isotopic signatures.
SOM, a pool from multiple origins
Influences on SOM also varied along the coast-to-ocean gradient, with a higher contribution of river and mangrove inputs at coastal sites and a higher contribution of seagrass, macroalgae and even Trichodesmium spp. at barrier reefs sites (Fig 4). The relative contribution of seagrass to SOM has been demonstrated in other ecosystems [8, 16], and is attributed to compounds of high molecular weight and low degradation rates which are usually trapped in sediments for long periods [40]. Coral reef POM is also known as a significant contributor to SOM, through sedimentation of dead phytoplankton and particulate matter [8, 9]. In the SW lagoon of New Caledonia, the residence time of marine waters ranges from 1 day (barrier reefs) to 3 months (coastal sites) [39]. This may explain the relative importance of marine POM in SOM isotopic composition.
On coastal systems under strong riverine influence the dominant contribution of terrestrial inputs to SOM generates a marked decrease in SOM δ13C signature value, which may be statistically undistinguishable to those of river POM [41]. In this study, POM and SOM isotopic compositions were distinct along each site of the coast-to-ocean gradient (Tables 2 and 3). This highlights the limited contribution of riverine POM to SOM, apart from its 15%- 20% contribution to mangrove SOM and coastal SOM. Mangrove leaves clearly contributed to coastal SOM, with up to a 10%- 15% contribution to fringing reefs SOM (compared to negligible contributions for intermediate and barrier reefs). This result highlights transfers of mangrove leaf-derived OM through various coastal ecosystems. Other OM sources, particularly the ones from benthic origins (seagrass, algal turf, and macroalgae) contributed to SOM isotopic signatures, probably through incorporation of their detritus or particular compounds. This contribution was particularly apparent on intermediate and barrier reefs.
Origin of macrophytes’ isotopic signatures
The ranges of isotopic values of POM and SOM pools and algal turf were relatively narrow compared to the other primary producers (Table 1). Macroalgae and seagrass usually display a high interspecific variability in δ13C and a low δ15N [8, 42]. This variability in δ13C and/or δ15N signatures can be high between species belonging to the same genus (Halimeda spp.) or remain low (Turbinaria spp.), and most likely reflected the macrophyte interspecific functional diversity (sensu metabolic / physiological). It remains challenging to better understand the factors driving these contrasting trophic roles.
The isotopic signatures of macrophytes are directly related to their phylogeny, to the biochemical process(es) involved for dissolved inorganic carbon (DIC) uptake during photosynthesis and to environmental characteristics [43]. Phylogeny is generally the main source of variability in δ13C signatures among primary producers [44, 45]. However in this study, coral reef macrophytes were hardly distinguishable; Phaeophycea, Rhodophytea and seagrass could not be discriminated by their δ13C signatures. Conversely, Chlorophytea appeared distinguishable possibly because Halimeda spp. were all calcareous. Only distinct groups (Chlorophytea and Phaeophycea) could be discriminated based on their δ15N signatures. This highlights the necessity to investigate phylogeny further to understand what differentiates genera from species. Extra complexity is added when concomitantly examining the photosynthetic processes involved in each species and their sensitivity to environmental conditions.
δ13C, a fingerprint under multiple influences
Macrophytes use different metabolic pathways for DIC uptake in photosynthesis at family, genus, and species levels. Although general trends exist, those of marine primary producers are still subject to clarification and discussion [45]. Two groups are usually identified among Chlorophytea: i) species associated with active CO2 uptake and sometimes to Carbon Concentrating Mechanisms “CCM” (δ13C signatures comprised between -21 ‰ and -8 ‰), and ii) species associated with CO2 diffusion (δ13C signatures comprised between -32 ‰ and -25 ‰ [45–47]. Identifying species using HCO3 - or CO2 within Phaeophycea remains challenging as their δ13C signatures do not vary much. Rhodophytea δ13C signatures are reported to be more heterogeneous [48]; yet caution is necessary, as the origins of the carbon used by species with intermediate δ13C signatures remain dubious. One exception is seagrass: their δ13C signatures are higher than those of macroalgae highlighting the involvement of a C3 photosynthetic process or possibly the preferential use of HCO3 - [45].
The range of δ13C signatures obtained in this study indicates that the macrophytes (including seagrass) may base their metabolism on an active DIC uptake via the use of CCM during photosynthesis [43]. As carbonic anhydrase activity is negatively correlated to δ13C values, the more dependent a species is on this metabolic pathway, the higher its δ13C signatures [49]. The groups’ averaged δ13C signatures suggest that seagrass use these active mechanisms more than Rhodophytea, which use them more than Phaeophycea, which in turn use them more than Chlorophytea.
δ13C signatures are usually higher for Chlorophytea, intermediate for Phaeophycea and lower for Rhodophytea [49, 50]. The opposite pattern was obtained in our study and can be explained by several hypotheses: i) the samples composition of genera is likely a key driving-factor of the δ13C signatures trends for the main groups of macrophytes. A low group diversity of genera as obtained here, particularly for Chlorophytae with only one genus (Halimeda) [49, 50], is known to impact the signature pattern (e.g. Halimeda vs. Codium or Ulva and Acanthophora or Liagora vs. Laurencia or Hypnea; [48–50]); ii) the tropical location of our study site can also be proposed as an explanatory factor. Most macroalgae and seagrass of New Caledonia displayed higher mean δ13C signatures than other regions (S5 Table). The CO2 diffusion process is negatively correlated with temperature, and surface seawaters in temperate regions generally have higher CO2 concentrations and lower δ13C values than in seawater from tropical regions [51]. Thus, a photosynthetic organism using DIC via a metabolism based on CO2 diffusion process will have a lower δ13C value in temperate regions than in tropical ones [44, 45, 50]; iii) habitat type and light intensity likely influence the δ13C signatures of macrophytes. Environmental parameters such as light intensity are known to influence DIC uptake during photosynthesis and thus impact δ13C values [43, 50]. The species with the most 13C-depleted signatures (Halimeda opuntia and H. heteromorpha) were mostly encountered on hard substrates, in very shallow waters that are subject to high light intensities. Conversely the species with the most 13C-enriched signatures (Padina australis, Liagora spp., Turbinaria spp. and seagrass) were mostly encountered on sandy/detrital substrates, in deeper waters subject to lower light intensities.
δ15N, a fingerprint mostly under local environmental influences
The main taxa were hardly discriminable from their δ15N signatures. Chlorophytae still displayed a slightly lower mean δ15N signature (1.19 ± 1.34 ‰) than segrass (1.78 ± 1.34 ‰), Rhodophytea (2.51 ± 0.39 ‰), and Phaeophycea (2.93 ± 1.40 ‰). This supports the hypothesis that phylogeny is not a key factor to explain differences in nitrogen uptake processes [52]. In addition, due to a relatively poor knowledge of the enrichment factors associated with inorganic nitrogen uptake it remains more challenging to relate δ15N signature to nitrogen inputs in a given site than δ13C signatures to DIC [50]. The δ15N values of primary producers seem to be related to local environmental characteristics, such as depth [44, 45], anthropogenic activities [53] and river inputs [9].
Spatial fluctuations of OM sources
Variations in light intensity, temperature or nutriment concentrations may change productivity rates of primary producers and, therefore, their δ13C signatures. Similarly, fluctuations in ammonium and nitrate concentrations in seawater can modify δ15N signatures of OM sources [15]. These different parameters can vary strongly in coastal shallow ecosystems, potentially causing variability in OM sources δ13C and δ15N signatures [54].
Our results highlighted a strong spatial variability in the isotopic signatures of OM sources between sites (Table 4). POM isotopic signatures usually closely reflect spatial variations of phytoplankton [55]. This was most likely the case in our study, as POM was strongly influenced by marine inputs and generally coastal / river inputs had a limited influence, except in some coastal sites. The influence of site is more difficult to interpret for primary producers due to their high inter-specific variability in isotopic signatures. The spatial variability of primary producers signatures depends on both the organisms present and the environmental characteristics, such as hydrodynamic conditions or nutrient availability [54, 56].
The signatures of POM and SOM pools, and most macrophytes, were generally 13C-depleted and 15N-enriched on barrier reefs compared to the other sites. The coast-to-ocean differences in nitrogen-based nutrients concentrations drive patterns of planktonic biomass and generate important modifications in planktonic community structures [57]. This possibly explains the POM variations obtained in our study, where phytoplankton biomasses varied little along the coast-to-ocean gradient [58]. Yet the isotopic patterns remain unexplained. Conversely, clear composition differences across the gradient possibly explain the isotopic patterns observed: micro phytoplankton dominating coastal sites and picoplankton dominating the rest of the lagoon [57]. In addition, POM δ13C signatures at coastal and river sites suggest an impact of terrestrial runoff [9]. Nutrient inputs from terrestrial origin only significantly influenced coastal sites [58]. In turn, modification of phytoplanktonic community structure influenced the nutrient cycle [59] and the structure of other food web compartments, such as SOM and benthic primary producers [60]. POM, SOM and primary producers consequently display similar patterns of spatial variability.
Spatial variability observed between the Grand Nouméa and Grand Sud gradients highlighted local characteristics. δ13C signatures of fringing reefs POM were higher for Grand Nouméa than Grand Sud. This difference is possibly related to the anthropogenic activities in that zone, such as wastes from a hotel complex and industrial activities in the neighboring bay. SOM and primary producers’ signatures followed a typical pattern along the Grand Sud, but not the Grand Nouméa. On both gradients, SOM and primary producers δ13C signatures were low and δ15N signatures were high on coastal sites, suggesting an influence of anthropogenic activities and/or terrestrial inputs. Unusually high δ15N and low δ13C signatures on the Grand Sud barrier reef remain difficult to explain. Despite the absence of data clearly supporting this hypothesis, we suggest that the pattern results from particular hydrologic and/or sedimentary conditions. Examination of spatial variability at such a small scale showed the role of local characteristics, but also demonstrated the necessity to be cautious when attempting to extrapolate local results of δ13C and δ15N signatures to a wider scale and when interpreting the role of site-dependent environmental factors to assess the transfer of OM from sources to higher trophic levels [56].
Temporal variations of OM sources
Primary producers’ isotopic signatures are known to fluctuate over time in various ecosystems [12]. Temperature, light intensity, water chemical characteristics, or river runoffs are among the environmental factors that can be involved in such fluctuations [15, 61]. However in our study, temporal variability remained remarkably low over seasons, and mainly concerned δ13C signatures with a general trend of lower values during winter. Biogeochemical models indicate that seasonal variations of phytoplankton abundance in the SW lagoon of New Caledonia are mainly explained by nutrients inputs related to rainfall events in the wet season (summer) [58]. In addition, modifications of the phytoplankton community occurred between summer and winter. This implied a change in trophic conditions from mesotrophic to oligotrophic waters [57]. Sporadic events such as hurricanes impacted the amount of nutrients inputs to the lagoon, mostly in coastal zones, but did not alter their isotopic signatures (non-significant differences). Overall, the low freshwater flows and the strong hydrodynamic conditions in the lagoon rapidly homogenized the water composition [58]. SOM and primary producers displayed lower seasonal variations than POM, although δ13C and/or δ15N seasonal fluctuations have been reported for benthic algae and seagrass [12, 54]. At the seasonal scale of our study, this suggests that variations in temperature or light intensity have a much lower influence on the δ13C and/or δ15N signatures of primary producers than the homogenization of environmental parameters by hydrodynamic conditions.
Integration of OM within trophic networks
Most of the studies conducted to assess the relative importance of algae and vascular plants through isotopic analyses highlight algae as the main source of OM for consumers [14, 62]. Their low nutritive value and high lignocellulose concentration make seagrass a second-choice source of food for many herbivorous animals [63]. A high C/N ratio is usually considered as a good proxy for low nutritive value. With a mean C/N ratio of 17.5 (result not shown) seagrass likely constituted an indirect source of OM in coral reef lagoon networks, through detrital processes, accumulation or decomposition within sediments [64, 65]. Conversely, macroalgae and particularly algal turf represent important sources of carbon for some fish [9, 66]. Finally, coral reef POM constitutes a source of OM for planktonic invertebrates and planktonophagous fish.
Supporting Information
Shaded boxes represent, from dark to light grey, 50%, 75%, and 95% Bayesian credibility intervals.
(TIF)
Numbers of samples (N) and significance of differences (p) between sites are given. FR = fringing reefs; IR = intermediate reefs; BR = barrier reefs; ns = p> 0.05; * p<0.05; ** p<0.01; *** p< 0.001.
(DOCX)
Numbers of samples (N) and significance of differences (p) between sites are given. FR = fringing reefs; IR = intermediate reefs; BR = barrier reefs; * p<0.05; ** p<0.01; *** p< 0.001.
(DOCX)
Differences between sites and significance (p) are given. FR = fringing reefs; IR = intermediate reefs; BR = barrier reefs; ns = p> 0.05; * p<0.05; ** p<0.01; *** p< 0.001;-: not tested.
(DOCX)
Differences between sites and significance (p) are given. WI = winter; SU = summer; FR = fringing reefs; IR = intermediate reefs; BR = barrier reefs; ns = p> 0.05; * p<0.05; ** p<0.01; *** p< 0.001;-: not tested.
(DOCX)
See Table 1 for comparison with our results in New Caledonia.
(DOCX)
Acknowledgments
This work is a part of the first author’s PhD thesis, granted by the École Doctorale du Pacifique (ED 469) and by the Fondation Total. We are grateful to Professor C. Payri for her valuable help in determining algal and seagrass species, to T. Fauvel and A. Riou for their help during field sampling, to the DENV (Province Sud) and E. Potut (Scaphca) for logistic support during fieldwork, to C. Pigot for his help in preparing the samples. We would like to thank J. Scopelitis for proof-reading and A. Harborne for improving the English version of the manuscript, and to the anonymous reviewers for their valuable suggestions and criticisms that improved the paper. The study was carried out under permit 930-2011/ARR/DENV, Province Sud, New Caledonia.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is a part of the first author’s PhD thesis, granted by the École Doctorale du Pacifique (ED 469) and was supported by the Fondation Total (http://fondation.total.com/), no grant number attributed. Funds were received by YL via the Université de la Nouvelle Calédonie. Funds were used to buy materials required and to pay stable isotope analysis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Falkowski P, Scholes RJ, Boyle EEA, Canadell J, Canfield D, Elser J, et al. The global carbon cycle: a test of our knowledge of earth as a system. Science. 2000; 290: 291–296. [DOI] [PubMed] [Google Scholar]
- 2. Uitz J, Claustre H, Gentili B, Stramski D. Phytoplankton class-specific primary production in the world's oceans: Seasonal and interannual variability from satellite observations. Glob Biogeoch Cycles. 2010; 24: 1944–9224. [Google Scholar]
- 3. Charpy-Roubaud C, Sournia A. The comparative estimation of phytoplanktonic, microphytobenthic and macrophytobenthic primary production in the oceans. Mar Microb Food Webs. 1990; 4: 31–57. [Google Scholar]
- 4. Duarte CM, Cebrián J. The fate of marine autotrophic production. Limnol Oceanogr. 1996; 41: 1758–1766. [Google Scholar]
- 5. Jennerjahn TC, Ittekkot V. Relevance of mangroves for the production and deposition of organic matter along tropical continental margins. Naturwissenschaften. 2002; 89: 23–30. [DOI] [PubMed] [Google Scholar]
- 6. Knox GA. Estuarine ecosystems: A systems approach. Boca Raton, Florida, CRC Press; 1986. [Google Scholar]
- 7. Volkman JK, Tanoue E. Chemical and biological studies of particulate organic matter in the ocean. J Oceanogr. 2002; 58: 265–279. [Google Scholar]
- 8. Cresson P, Ruitton S, Fontaine MF, Harmelin-Vivien ML. Spatio-temporal variation of suspended and sedimentary organic matter quality in the Bay of Marseilles (NW Mediterranean) assessed by biochemical and isotopic analyses. Mar Poll Bul. 2012; 64: 1112–1121. [DOI] [PubMed] [Google Scholar]
- 9. Letourneur Y, de Loma TL, Richard P, Harmelin-Vivien ML, Cresson P, Banaru D, et al. Identifying carbon sources and trophic position of coral reef fishes using diet and stable isotope (δ15N and δ13C) analyses in two contrasted bays in Moorea, French Polynesia. Coral Reefs. 2013; 32: 1091–1102. [Google Scholar]
- 10. Vander Zanden MJ, Rasmussen JB. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr. 2001; 46: 2061–2066. [Google Scholar]
- 11. Tesi T, Miserocchi S, Goni MA, Langone L, Boldrin A, Turchetto M. Organic matter origin and distribution in suspended particulate materials and surficial sediments from the Western Adriatic Sea (Italy). Est Coast Shelf Sci. 2007; 73: 431–446. [Google Scholar]
- 12. Vizzini S, Mazzola A. Seasonal variations in the stable carbon and nitrogen isotope ratios (13C/12C and 15N/14N) of primary producers and consumers in a western Mediterranean coastal lagoon. Mar Biol. 2003; 142: 1009–1018. [Google Scholar]
- 13. Bautista-Vega AA, Letourneur Y, Harmelin-Vivien ML, Salen-Picard C. Difference in diet and size-related trophic level in two sympatric fish species, the red mullets Mullus barbatus and Mullus surmuletus, in the Gulf of Lions (north-west Mediterranean Sea). J Fish Biol. 2008; 73: 2402–2420. [Google Scholar]
- 14. Cresson P, Ruitton S, Harmelin-Vivien ML. Artificial reefs do increase secondary biomass production: mechanisms evidenced by stable isotopes. Mar Ecol Progr Ser. 2014; 509: 15–26. [Google Scholar]
- 15. Hemminga MA, Mateo MA. Stable carbon isotopes in seagrasses: variability in ratios and use in ecological studies. Mar Ecol Progr Ser. 1996; 140: 285–298. [Google Scholar]
- 16. Papadimitriou S, Kennedy H, Kennedy DP, Duarte CM, Marbá N. Sources of organic matter in seagrass-colonized sediments: A stable isotope study of the silt and clay fraction from Posidonia oceanica meadows in the western Mediterranean. Org Geochem. 2005; 36: 949–961. [Google Scholar]
- 17. Polunin NVC, Klumpp DW. Algal food supply and grazer demand in a very productive coral-reef zone. J Exper Mar Biol Ecol. 1992; 164: 1–15. [Google Scholar]
- 18. Choat JH, Clements KD. Vertebrate herbivores in marine and terrestrial environments: a nutritional ecology perspective. Ann Rev Ecol System 1998; 29: 375–403. [Google Scholar]
- 19. Martineau C, Vincent WF, Frenette JJ, Dodson JJ. Primary consumers and particulate organic matter: isotopic evidence of strong selectivity in the estuarine transition zone. Limnol Oceanogr. 2004; 49: 1679–1686. [Google Scholar]
- 20. Rodier M, Le Borgne R. Population dynamics and environmental conditions affecting Trichodesmium spp. (filamentous cyanobacteria) blooms in the south–west lagoon of New Caledonia. J Exp Mar Biol Ecol. 2008; 358: 20–32. [Google Scholar]
- 21. Vermeij MJ, van Moorselaar I, Engelhard S, Hörnlein C, Vonk SM, Visser PM. The effects of nutrient enrichment and herbivore abundance on the ability of turf algae to overgrow coral in the Caribbean. PLoS One. 2010; 5: e14312 10.1371/journal.pone.0014312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Letourneur Y. Spatial and temporal variability in territoriality of a tropical benthic damselfish on a coral reef (Réunion Island). Env Biol Fish. 2000; 57: 377–391. [Google Scholar]
- 23. Hata H, Watanabe K, Kato M. Geographic variation in the damselfish-red alga cultivation mutualism in the Indo-West Pacific. Evol Biol. 2010; 10: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rau GH, Ainley DG, Bengtson JL, Torres JJ, Hopkins TL. 15N/14N and 13C/12C in Weddell sea birds, seals, and fish: implications for diet and trophic structure. Mar Ecol Progr Ser. 1992; 84: 1–8. [Google Scholar]
- 25. Rolff C, Elmgren R. Use of riverine organic matter in plankton food webs of the Baltic Sea. Mar Ecol Prog Ser. 2000; 197: 81–101. [Google Scholar]
- 26. DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978; 42: 495–506. [Google Scholar]
- 27. Pinnegar JK, Polunin NVC. Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Funct Ecol. 1999; 13: 225–231. [Google Scholar]
- 28. Underwood AJ. Environmental decision-making and the precautionary principle: what does this principle mean in environmental sampling practice? Lands Urban Plan. 1997; 37: 137–146. [Google Scholar]
- 29. Phillips DL, Gregg JW. Source partitioning using stable isotopes: coping with too many sources. Oecologia. 2003; 136: 261–269. [DOI] [PubMed] [Google Scholar]
- 30. Phillips DL. Mixing models in analyses of diet using multiple stable isotopes: a critique. Oecologia. 2001;, 127: 166–170. 10.1007/s004420000571 [DOI] [PubMed] [Google Scholar]
- 31. Fry B. Stable isotope ecology. Baton Rouge, Louisiana: Springer; (Third Edition); 2008. [Google Scholar]
- 32. Parnell AC, Inger R, Bearhop S, Jackson AL. Source partitioning using stable isotopes: coping with too much variation. Plos One. 2010; 5: e9672 10.1371/journal.pone.0009672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bond AL, Diamond AW. Recent Bayesian stable-isotope mixing models are highly sensitive to variation in discrimination factors. Ecol Appl. 2011; 21: 1017–1023. [DOI] [PubMed] [Google Scholar]
- 34. Ostrom PH, Fry B. Sources and cycling of organic matter within modern and prehistoric food webs In: Engel MH, Macko SA, editors. Organic geochemistry. Springer US; 1993; pp. 785–798. [Google Scholar]
- 35. Fry B, Scalan RS, Parker PL. Stable carbon isotope evidence for two sources of organic matter in coastal sediments: seagrasses and plankton. Geochim Cosmochim Acta. 1977; 41: 1875–1877. [Google Scholar]
- 36. Kennedy H, Gacia E, Kennedy DP, Papadimitriou S, Duarte CM. Organic carbon sources to SE Asian coastal sediments. Est Coast Shelf Sci. 2004; 60: 59–68. [Google Scholar]
- 37. Harmelin-Vivien ML, Loizeau VR, Mellon C, Beker B, Arlhac D, Bodiguel X, et al. Comparison of C and N stable isotope ratios between surface particulate organic matter and microphytoplankton in the Gulf of Lions (NW Mediterranean). Contin Shelf Res. 2008; 28: 1911–1919. [Google Scholar]
- 38. Ouillon S, Douillet P, Lefebvre JP, Le Gendre R, Jouon A, Bonneton P, et al. Circulation and suspended sediment transport in a coral reef lagoon: The south-west lagoon of New Caledonia. Mar Poll Bul. 2010; 61: 269–296. [DOI] [PubMed] [Google Scholar]
- 39. Jouon A, Douillet P, Ouillon S, Fraunié P. Calculations of hydrodynamic time parameters in a semi-opened coastal zone using a 3D hydrodynamic model. Contin Shelf Res. 2006; 26: 1395–1415. [Google Scholar]
- 40. Boudouresque CF, Mayot N, Pergent G. The outstanding traits of the functioning of the Posidonia oceanica seagrass ecosystem. Biol Mar Medit. 2006; 13: 109–113. [Google Scholar]
- 41. Banaru D, Harmelin-Vivien ML, Gomoiu MT, Onciu TM. Influence of the Danube River inputs on C and N stable isotope ratios of the Romanian coastal waters and sediment (Black Sea). Mar Poll Bul. 2007; 54: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 42. Vizzini S, Sarà G, Michener RH, Mazzola A. The role and contribution of Posidonia oceanica (L.) Delile organic matter for secondary consumers as revealed by carbon and nitrogen stable isotope analysis. Acta Oecol. 2002; 23: 277–285. [Google Scholar]
- 43. Maberly SC, Raven JA, Johnston AM. Discrimination between 12C and 13C by marine plants. Oecologia. 1992; 91: 481–492. [DOI] [PubMed] [Google Scholar]
- 44. Raven JA, Johnston AM, Kübler JE, Korb R, McInroy SG, Handley LL, et al. Seaweeds in cold seas: evolution and carbon acquisition. Ann Bot. 2002a; 90: 525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raven JA, Johnston AM, Kübler JE, Korb R, McInroy SG, Handley LL, et al. Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct Plant Biol. 2002b; 29: 355–378. [DOI] [PubMed] [Google Scholar]
- 46. Kevekordes K, Holland D, Häubner N, Jenkins S, Koss R, Roberts S, et al. Inorganic carbon acquisition by eight species of Caulerpa (Caulerpaceae, Chlorophyta). J Inform. 2006; 45: 442–449. [Google Scholar]
- 47. Raven JA, Cockell CS, De La Rocha CL. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Phil Trans Royal Soc B: Biol Sci. 2008; 363: 2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang WL, Yeh HW. δ13C values of marine macroalgae from Taiwan. Bot Bull Acad Sci. 2003; 44: 107–112. [Google Scholar]
- 49. Mercado JM, de los Santos CB, Lucas Pérez-Lloréns J, Vergara JJ. Carbon isotopic fractionation in macroalgae from Cádiz Bay (Southern Spain): Comparison with other bio-geographic regions. Est Coast Shelf Sci. 2009; 85: 449–458. [Google Scholar]
- 50. Marconi M, Giordano M, Raven JA. Impact of taxonomy, geography, and depth on δ13C and δ15N variation in a large collection of macroalgae. J Phycol. 2011; 47: 1023–1035. [DOI] [PubMed] [Google Scholar]
- 51. Zeebe RE, Wolf-Gladrow D. CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Amsterdam: Elsevier Oceanography Series; 2001. [Google Scholar]
- 52. Yoneyama T. Nitrogen metabolism and fractionation of nitrogen isotopes in plants In: Wada E, Yoneyama T, Minagawa M, Ando T, Fry BD, editors. Stable Isotopes in Biosphere. Kyoto University Press; 1995; pp. 92–102. [Google Scholar]
- 53. Lapointe BE, Barile PJ, Littler MM, Littler DS. Macroalgal blooms on southeast Florida coral reefs II. Crossshelf discrimination of nitrogen sources indicates widespread assimilation of sewage nitrogen. Harmful Algae. 2005; 4: 1106–1122. [Google Scholar]
- 54. Fourquerean JW, Marba N, Duarte CM, Diaz-Almela E, Ruiz-Halpern S. Spatial and temporal variation in the elemental and stable isotopic content of the seagrasses Posidonia oceanica and Cymodocea nodosa from the Iles Balears, Spain. Mar Biol. 2007; 151: 219–232. [Google Scholar]
- 55. Covazzi Harriague A, Albertelli G, Bonomi A, Fabiano M, Zunini-Sertorio T. Pelagic–benthic coupling in a subtidal system of the North-Western Mediterranean. J Chem Ecol. 2007; 23: 263–277. [Google Scholar]
- 56. Vizzini S. Mazzola A. Sources and transfer of organic matter in food webs of a Mediterranean coastal environment: Evidence for spatial variability. Est Coast Shelf Sci. 2006; 66: 459–467. [Google Scholar]
- 57. Jacquet S, Delesalle B, Torréton JP, Blanchot J. Response of phytoplankton communities to increased anthropogenic influences (southwestern lagoon, New Caledonia). Mar Ecol Progr Ser. 2006; 320: 65–78. [Google Scholar]
- 58. Bujan S, Grenz C, Fichez R, Douillet P. Evolution saisonnière du cycle biogéochimique dans le lagon sud-ouest de Nouvelle-Calédonie. Application d’un modèle compartimental. CR Acad Sci Paris, Ser III, Sci Vie. 2000; 323: 225–233. [DOI] [PubMed] [Google Scholar]
- 59. Sakka A, Legendre L, Gosselin M, Niquil N, Delesalle B. Carbon budget of the planktonic food web in an atoll lagoon (Takapoto, French Polynesia). J Plankt Res. 2002; 24: 301–320. [Google Scholar]
- 60. Olsen Y, Reinertsen H, Vadstein O, Andersen T, Gismervikb I, Duartec C, et al. Comparative analysis of food webs based on flow networks: effects of nutrient supply on structure and function of coastal plankton communities. Contin Shelf Res. 2001; 21: 2043–2053. [Google Scholar]
- 61. Stephenson RL, Tan FC, Mann KH. Stable isotope variability in marine macrophytes and its implications for food web studies. Mar Biol. 1984; 81: 223–230. [Google Scholar]
- 62. Lepoint G, Nyssen F, Gobert S, Dauby P, Bouquegneau JM. Relative impact of a seagrass bed and its adjacent epilithic algal community in consumer diets. Mar Biol. 2000; 136: 513–518. [Google Scholar]
- 63. Cebrián J, Duarte CM. The dependence of herbivory on growth rate in natural plant communities. Funct Ecol. 1994; 8: 518–525. [Google Scholar]
- 64. Mann KH. Production and use of detritus in various freshwater, estuarine and coastal marine ecosystems. Limnol Oceanogr. 1988; 33: 910–930. [Google Scholar]
- 65. Cebrián J, Duarte CM, Marbà N, Enriquez S. Magnitude and fate of the production of four co-occurring western Mediterranean seagrass species. Mar Ecol Progr Ser. 1997; 155: 29–44. [Google Scholar]
- 66. Dromard CR. Bouchon-Navaro Y, Cordonnier S, Fontaine MF, Verlaque M, Harmelin-Vivien ML, et al. Resource use of two damselfishes, Stegastes planifrons and Stegastes asustus, on Guadeloupean reefs (Lesser Antilles): Inference from stomach content and stable isotope analysis. J Exp Mar Biol Ecol. 2013; 440: 116–125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shaded boxes represent, from dark to light grey, 50%, 75%, and 95% Bayesian credibility intervals.
(TIF)
Numbers of samples (N) and significance of differences (p) between sites are given. FR = fringing reefs; IR = intermediate reefs; BR = barrier reefs; ns = p> 0.05; * p<0.05; ** p<0.01; *** p< 0.001.
(DOCX)
Numbers of samples (N) and significance of differences (p) between sites are given. FR = fringing reefs; IR = intermediate reefs; BR = barrier reefs; * p<0.05; ** p<0.01; *** p< 0.001.
(DOCX)
Differences between sites and significance (p) are given. FR = fringing reefs; IR = intermediate reefs; BR = barrier reefs; ns = p> 0.05; * p<0.05; ** p<0.01; *** p< 0.001;-: not tested.
(DOCX)
Differences between sites and significance (p) are given. WI = winter; SU = summer; FR = fringing reefs; IR = intermediate reefs; BR = barrier reefs; ns = p> 0.05; * p<0.05; ** p<0.01; *** p< 0.001;-: not tested.
(DOCX)
See Table 1 for comparison with our results in New Caledonia.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.