Abstract
Objective: Describe the safety profile of bimatoprost 0.03% ophthalmic solution as once-daily topical treatment for idiopathic or chemotherapy-induced eyelash hypotrichosis. Design: Pooled data from six randomized, multicenter, double-masked, parallel-group clinical studies of at least three-months’ duration with at least one bimatoprost treatment group. Setting: Study sites in the United States, Canada, United Kingdom, and Japan from 2007 to 2012. Participants: Adults with eyelash hypotrichosis, defined as baseline Global Eyelash Assessment of minimal or moderate, who received bimatoprost 0.03% (n=680) or vehicle, with no prior exposure to bimatoprost (n=379). Measurements: Safety assessments included adverse events, vital sign measurements, and physical examinations. Common (≥2%) and treatment-related adverse events were analyzed at time points up to four months and through end of treatment, up to 12 months. Results: Similar overall adverse events incidence was reported in bimatoprost and vehicle groups for subjects with idiopathic hypotrichosis; a higher incidence in both groups was reported for postchemotherapy subjects. Common adverse events included conjunctival hyperemia, eyelid pruritus, blepharal pigmentation, nasopharyngitis, eyelid erythema, and punctate keratitis. Most adverse events occurred early in treatment, were mild in intensity, localized to treatment site, and reversible with treatment cessation. Discontinuations due to adverse events were low (3.2% for bimatoprost and 2.4% for vehicle). Conclusion: Adverse events were consistent with the known pharmacologic mechanism of bimatoprost. The safety profile was similar across the studies and no new safety signals were observed. Once-daily bimatoprost 0.03% for treatment of eyelid hypotrichosis has a favorable safety and tolerability profile when applied topically to the upper eyelid margin.
In 2001, the United States Food and Drug Administration (FDA) approved bimatoprost ophthalmic solution 0.03% (Lumigan®; Allergan, Inc., Irvine, California) as an eyedrop for the treatment of open-angle glaucoma and ocular hypertension.1 Enhanced eyelash prominence was observed in some subjects as an adverse event (AE) in clinical trials examining the ocular antihypertensive properties of bimatoprost.2-4 This finding led to evaluations of bimatoprost, a prostamide and synthetic structural analog of prostaglandin F2α,1 for the treatment of eyelash hypotrichosis, a condition characterized by reduced eyelash growth that can be caused by a number of factors, including aging, heredity, physical trauma, alopecia areata, and chemotherapy.5 Regardless of etiology, eyelash hypotrichosis may result in an increased risk of ocular injury, as eyelashes play a protective role in keeping small foreign bodies and irritants from the ocular surface.5-8 Moreover, eyelash hypotrichosis can negatively affect self-image by modifying integral facial features, which can lead to emotional distress and impairment in psychosocial function.8-10
Application of bimatoprost ophthalmic solution 0.03% to the upper eyelid margin once daily increases eyelash prominence, with correlating increases in eyelash length, thickness, and darkness (intensity) compared with vehicle in adults with idiopathic hypotrichosis and chemotherapy-induced hypotrichosis.11-13 The FDA approved bimatoprost ophthalmic 0.03% (Latisse®; Allergan, Inc., Irvine, California) in 2008 for the treatment of eyelash hypotrichosis.13 When used for glaucoma treatment, bimatoprost 0.03% has a favorable safety and tolerability profile, as summarized in a pooled analysis of six long-term studies.13 In a similar manner, safety data from six clinical studies conducted in adult subjects with either idiopathic or chemotherapy-induced eyelash hypotrichosis were pooled to demonstrate the safety of bimatoprost 0.03% as a once-daily topical treatment for eyelash hypotrichosis.
METHODS
Study population. Data for individual subjects with eyelash hypotrichosis in six clinical trials conducted between April 2007 and May 2012 were pooled for this analysis (see Table 1 for clinicaltrials.gov identifier numbers for each study). All six studies were randomized, multicenter, double-masked, parallel-group clinical trials that included at least one group treated with bimatoprost 0.03%, and all studies were from three months to up to 12 months in duration (Figure 1). Adult subjects randomized to the bimatoprost treatment arms in these trials applied bimatoprost 0.03% once daily to the bilateral upper eyelid margins using sterile, single-use-per-eye applicators. Subjects in the comparator groups applied either vehicle control or exploratory bimatoprost formulations; the latter subjects were not included in this pooled analysis and are not described further. Thus, the study population for this pooled analysis comprised all subjects who received bimatoprost 0.03% or those who received vehicle control with no prior exposure to bimatoprost. All studies based the safety analyses on a tabulation of AEs and serious AEs.
TABLE 1.
Summary of studies included in pooled analysis of bimatoprost 0.03% for eyelash hypotrichosis treatment
| STUDY NUMBER* AND ETIOLOGY | SUBJECTS ENROLLED IN EACH GROUP, N | STUDY TREATMENT DURATION† MONTHS | STUDY DESIGN | STUDY POPULATION | MEAN AGE, YEARS (RANGE) | ||
|---|---|---|---|---|---|---|---|
| BIM 0.03% | VEH | ||||||
| Study 111 | |||||||
|
NCT00693420 Idiopathic Postchemotherapy |
137 - |
141 - |
4 | Phase 3 R, DM, PG | General (US, Canada) | 49.8 (22-78) | |
| Study 212 NCT01391273 Idiopathic Postchemotherapy |
87 - |
85 - |
4 | Phase 3 R, DM, PG | Japanese (Japan) | 40.8 (20-68) | |
| Study 312 NCT01391286 Idiopathic Postchemotherapy |
- 18 |
- 18 |
4 | Phase 3 R, DM, PG | Japanese (Japan) | 50.6 (31-74) | |
| Study 4 NCT00958035 Idiopathic Postchemotherapy |
46 - |
43 - |
4 | Phase 4 R, DM, PG | African American (US) | 46.5 (19-75) | |
| Study 5 NCT01064882 Idiopathic Postchemotherapy |
34 - |
- - |
3 | Phase 2 R, DM, AC, PG | Caucasian (US) | 46.3 (31-55) | |
| BIM/BIM | BIM/VEH | VEH/BIM | STUDY treatment duration† MONTHS | STUDY DESIGN | STUDY POPULATION | MEAN AGE, YEARS (RANGE) | |
| Study 6[14] NCT00907426 Idiopathic Postchemotherapy |
118 96 |
60 - |
59 33 |
12‡ | Phase 3 R, DM, PG | General (US, Europe) | 49.8 (20-76) |
Both the study number and ClinicalTrials.gov registration number (available at www.clinicaltrials.gov) are provided.
Time of active study treatment (does not include post-treatment periods).
Study 6 was a 12-month study comprising two six-month treatment periods. For the first six months, subjects were randomized to treatment with either bimatoprost 0.03% or vehicle (treatment period 1). From Month 6 to Month 12 (treatment period 2), all subjects who received vehicle during treatment period 1 were switched to bimatoprost, whereas subjects who received bimatoprost during treatment period 1 were randomized to either continue receiving bimatoprost or switch to vehicle.
AC=active controlled; BIM=bimatoprost; DM=double masked; PG=parallel group; R=randomized; US=United States; VEH=vehicle
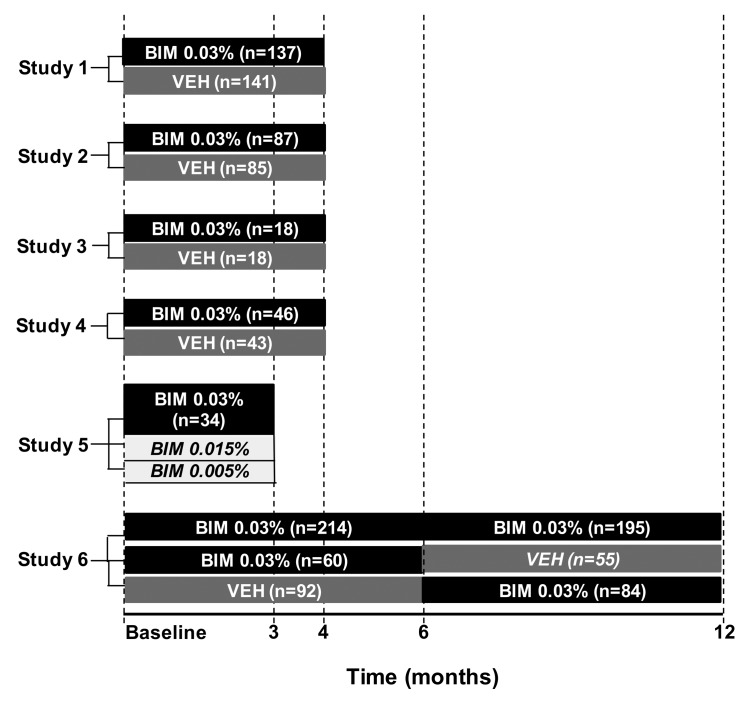
Figure 1.
Study timelines and treatment groups for the six studies pooled for the Month 4 and end-of-treatment analyses. Italics indicate data not included in the pooled safety analyses. BIM=bimatoprost; VEH=vehicle
Summary of individual studies. The study design and duration, number of randomized subjects by treatment group, and subject age range for each of the six trials included in the pooled analysis are summarized in Table 1. Studies 1, 2, and 3 were double-masked, four-month, Phase 3 studies in which subjects were randomized (1:1) to receive either bimatoprost 0.03% or vehicle. Study 1 was conducted in the United States and enrolled subjects with idiopathic eyelash hypotrichosis.11 Two of the studies were conducted in Japan: Study 2 enrolled subjects with idiopathic eyelash hypotrichosis, and Study 3 enrolled subjects with chemotherapy-induced eyelash hypotrichosis.12 Study 4 was a double-masked, four-month, Phase 4 study wherein self-identified black subjects with idiopathic eyelash hypotrichosis received bimatoprost 0.03% or vehicle. In Study 5, an active-controlled, three-month, Phase 2 study, Caucasian females with idiopathic eyelash hypotrichosis received bimatoprost 0.03% and exploratory formulations of 0.015% or 0.005%. Study 6 was a double-masked, 12-month, Phase 3 study conducted in the United States and United Kingdom in subjects with either idiopathic or chemotherapy-induced eyelash hypotrichosis.14 This long-term study comprised two six-month treatment periods. For the first six months (treatment period 1), subjects received either bimatoprost 0.03% or vehicle. From Month 6 to Month 12 (treatment period 2), all subjects who received vehicle during treatment period 1 were switched to bimatoprost, whereas subjects who received bimatoprost during treatment period 1 were randomized to either continue receiving bimatoprost or switch to vehicle. Therefore, the vehicle-controlled period did not exceed six months for any group.
All studies were conducted in compliance with Good Clinical Practice guidelines and in accordance with applicable institutional review board regulations. All subjects provided written informed consent prior to enrollment in each study. Primary and secondary endpoints for the six studies were prospectively defined.
The studies had similar key inclusion and exclusion criteria. The entry criterion of eyelash hypotrichosis was uniformly defined across all of the studies as a baseline Global Eyelash Assessment (GEA) score of 1 (minimal) or 2 (moderate) as assessed by certified, trained clinicians. Subjects were also required to have a best-corrected visual acuity score equivalent to a Snellen acuity of 20/100 or better in each eye, as well as intraocular pressure (IOP) of 20mmhg or lower in each eye. Study 5 was an exception to this as those enrolling were not assessed for IOP. Postchemotherapy subjects had to have an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1 and have completed a course of chemotherapy of at least four weeks, but no more than 24 weeks, prior to baseline. Subjects were excluded if they had significant asymmetry of right and left eyelashes, active ocular disease, uncontrolled systemic disease, or suspected trichotillomania disorder. Subjects who had any ocular surgery, including filtering, laser, or refractive surgery, within the previous three months; were receiving concurrent ocular or systemic prostaglandin or prostamide treatment; who used eyelash or hair growth products within the previous six months; or who had a contraindication to study medication were also excluded. Women who were pregnant, nursing, or of childbearing potential who were not using reliable birth control were excluded.
Safety analyses. The analysis of safety data was performed on the safety population, which included all subjects who received at least one dose of bimatoprost 0.03% or one dose of vehicle that was not preceded by bimatoprost treatment. Safety assessments collected in each study included AEs, ophthalmic examinations, vital sign measurements, and physical examinations. Analyses were performed on observed cases without data imputation. The IOP measurement, best-corrected visual acuity, biomicroscopy, and ophthalmoscopy were collected in all studies except Study 5. Iris color assessments were collected only for Studies 1, 4, and 6.
Pooled analyses were performed on data up to four months and on data at the end of treatment of each study (up to 12 months) from the safety populations of included studies. For the pooled safety analyses through Month 4, data were analyzed for the entire four-month treatment periods of Studies 1,2,3,4, and up to the Month 4 time point of Study 6 (Figure 1). For this four-month analysis, all subjects in the safety population in Study 6 received at least one treatment with bimatoprost or vehicle only (i.e., no prior treatment). Data from Study 5 were not considered for the Month 4 analysis because the treatment period was only three months and the study did not include a vehicle comparator group.
An integrated analysis of data collected through the end of treatment (i.e., up to 12 months) in each study was conducted to present all bimatoprost and all vehicle (with no prior bimatoprost) treatment periods across all six studies. This analysis through the end of treatment in the individual studies included data from the entire three-month treatment period of Study 5, the entire four-month treatment periods of Studies 1, 2, 3, and 4, and through the Month 12 treatment period of Study 6 (Figure 1). Inclusion of Study 6 allowed analysis of the long-term safety profile through 12 months of treatment for subjects receiving bimatoprost and through six months of treatment for subjects receiving vehicle comparator.
For the pooled analyses, terms used for the AEs in the individual studies were coded or recoded to terminology from the Medical Dictionary for Regulatory Activities (MedDRA) version 16.1. Within each MedDRA-preferred term, multiple episodes of an event were counted only once per subject for the period in which it was first reported. The incidence of each AE was tabulated, regardless of causality or relationship to study treatment. Frequency distribution analyses were performed for AEs occurring up to four months of treatment and through end of treatment up to 12 months for commonly reported AEs, defined as those with an incidence of at least two percent for bimatoprost 0.03%, and for treatment-related AEs in subjects receiving bimatoprost 0.03% or vehicle. Because of differences in the duration of treatment exposure across the studies, between-group comparisons through the end of treatment up to 12 months were not assessed for the pooled safety analyses. The incidence of AEs was also tabulated by demographic subgroups.
RESULTS
Demographics and treatment exposure. In total, 680 subjects received at least one dose of bimatoprost 0.03% and 379 subjects received at least one dose of vehicle that had not been preceded by bimatoprost treatment across the six pooled studies (Table 2). The demographics were generally comparable across the treatment groups. In the overall pooled population, the mean age was 47.9 years, the majority of subjects were Caucasian (57.8%) and female (96.8%), and all were between the ages of 19 and 78 years. All subjects had either minimal or moderate eyelash prominence at baseline, as indicated by GEA scores of 1 (32%) or 2 (68%). Subjects with chemotherapy-induced eyelash hypotrichosis were nearly all female, had more profound eyelash hypotrichosis at baseline, and were slightly older compared with subjects who presented with idiopathic hypotrichosis. The median duration of exposure to bimatoprost 0.03% and vehicle was 133.5 and 118.0 days for the overall population, respectively. For the 214 subjects from Study 6 who received up to 12 months of bimatoprost 0.03%, the median treatment exposure was 364.0 days.
TABLE 2.
Subject demographics in the six pooled eyelash hypotrichosis studies
| CHARACTERISTIC | OVERALL | IDIOPATHIC | POSTCHEMOTHERAPY | |||
|---|---|---|---|---|---|---|
| BIM 0.03% (n=680) | VEH (n=379) | BIM 0.03% (n=534) | VEH (n=328) | BIM 0.03% (n=146) | VEH (n=51) | |
| Age, years Mean (SD) Median Min, max |
48.2 (11.09) 49.0 20, 77 |
47.8 (12.5) 48.0 19, 78 |
47.7 (11.49) 48.0 20, 77 |
47.0 (12.17) 47.0 19, 78 |
50.1 (9.22) 50.5 26, 76 |
52.9 (9.89) 51.0 26, 74 |
| Age distribution, n (%) <45 years 45-65 years ≥65 years |
241 (35.4) 401 (59.0) 38 (5.6) |
141 (37.2) 214 (56.5) 24 (6.3) |
203 (38.0) 299 (56.0) 32 (6.0) |
133 (40.5) 177 (54.0) 18 (5.5) |
38 (26.0) 102 (69.9) 6(4.1) |
8 (15.7) 37 (72.5) 6 (11.8) |
| Sex, n (%) Male Female |
16 (2.4) 664 (97.6) |
16 (4.2) 363 (95.8) |
15 (2.8) 519 (97.2) |
16 (4.9) 312 (95.1) |
1 (0.7) 145 (99.3) |
0 (0.0) 51 (100.0) |
| Race, n (%) Caucasian African American Asian Hispanic Other* |
444 (65.3) 75 (11.0) 135 (19.9) 21 (3.1) 5 (0.7) |
190 (50.1) 53 (14.0) 123 (32.5) 10 (2.6) 3 (0.8) |
341 (63.9) 62 (11.6) 113 (21.2) 13 (2.4) 5 (0.9) |
165 (50.3) 49 (14.9) 103 (31.4) 8 (2.4) 3 (0.9) |
103 (70.5) 13 (8.9) 22 (15.1) 8 (5.5) 0 (0.0) |
25 (49.0) 4 (7.8) 20 (39.2) 2 (3.9) 0 (0.0) |
| Iris color, n (%) Dark* Light‡ Unavailable |
230 (33.8) 311 (45.7) 139 (20.4) |
142 (37.5) 134 (35.4) 103 (27.2) |
173 (32.4) 240 (44.9) 121 (22.7) |
126 (38.4) 117 (35.7) 85 (25.9) |
57 (39.0) 71 (48.6) 18 (25.9) |
16 (31.4) 17 (33.3) 18 (35.3) |
| GEA score, n (%) 1 - Minimal 2 - Moderate |
236 (34.7) 444 (65.3) |
110 (29.0) 269 (71.0) |
132 (24.7) 402 (75.3) |
73 (22.3) 255 (77.7) |
104 (71.2) 42 (28.8) |
37 (72.5) 14 (27.5) |
Other race included American Indian, African American, and Caucasian, First Nation (Haida), half African American, and half Guamian, Indonesian, Middle Eastern American, Native Hawaiian Pacific Islander, and Pacific Islander.
Dark iris colors included brown and dark brown. If other category contained black, it was grouped as dark.
Light iris colors included blue, blue-gray, blue/gray-brown, gray, green, green-brown, hazel, and other.
Iris color was not collected as part of the study procedures in Studies 2, 3, and 5.
BIM=bimatoprost; GEA=Global Eyelash Assessment; SD=standard deviation; VEH=vehicle
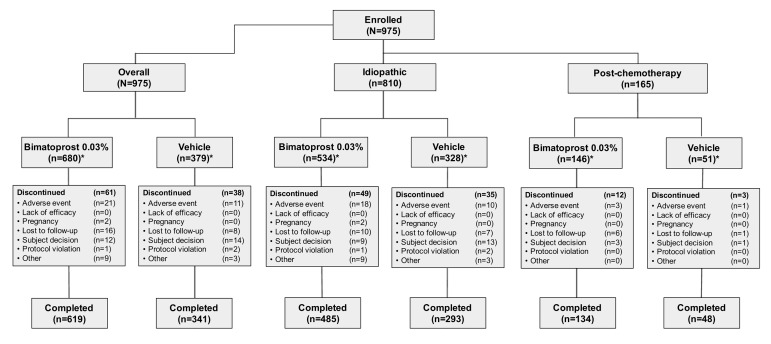
Study and treatment discontinuations. The end-of-treatment safety analysis comprised 975 subjects (680 subjects in the bimatoprost 0.03% group and 379 subjects in the vehicle group); the 84 subjects from Study 6 who received vehicle in treatment period 1 and bimatoprost 0.03% in treatment period 2 were included in the analysis for both groups. Of the subjects in the bimatoprost 0.03% group, 619 (91.0%) completed the study, as did 341 (90.0%) in the vehicle group (Figure 2). The reasons for study discontinuation were similar between treatment groups for both the overall population as well as for the idiopathic and postchemotherapy subpopulations. Of the 61 (9.0%) subjects in the overall population who discontinued bimatoprost treatment, the most common reasons given were AEs (n=21, 3.1%), lost to follow-up (n=16, 2.4%), and personal reasons (n=12, 1.8%). Of the 38 (10.0%) subjects who discontinued vehicle treatment, the most common reasons given were personal reasons (n=14, 3.7%), AEs (n=ll, 2.9%), and lost to follow-up (n=8, 2.1%).
Figure 2.
Subject disposition
*Number of subjects in bimatoprost and vehicle treatment groups includes some subjects from Study 6 who contributed data to both treatment groups.
Incidence of AEs by etiology. The overall incidence of AEs, regardless of causality, was slightly higher for subjects receiving up to 12 months of bimatoprost 0.03% compared with up to six months of vehicle (47.4% vs 34.3%, respectively; Table 3). For subjects with idiopathic eyelash hypotrichosis, the incidence of AEs was similar to that in the overall population, with 41.8 percent and 31.1 percent in the bimatoprost 0.03% and vehicle treatment groups, respectively. In contrast, subjects with chemotherapy-induced eyelash hypotrichosis experienced a higher incidence of AEs in both the bimatoprost 0.03% and vehicle treatment groups (67.8% vs. 54.9%, respectively).
TABLE 3.
Most common adverse events (≥2%) reported by etiology and length of bimatoprost 0.03% exposure in the six pooled studies
| ADVERSE EVENT | ETIOLOGY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall, n(%) | Idiopathic, n(%) | Postchemotherapy, n(%) | ||||||||||
| ≤4 Months* | ≤12 Months† | ≤4 Months* | ≤12 Months† | ≤4 Months* | ≤12 Months† | |||||||
| BIM (n=562) | VEH (n=379) | BIM (n=680) | VEH (n=379) | BIM (n=448) | VEH (n=328) | BIM (n=534) | VEH (n=328) | BIM (n=114) | VEH (n=51) | BIM (n=146) | VEH (n=51) | |
| Overall | 228 (40.6) | 116 (30.6) | 322 (47.4) | 130 (34.3) | 173 (38.6) | 94 (28.7) | 223 (41.8) | 102 (31.1) | 55 (48.2) | 22 (43.1) | 99 (67.8) | 28 (54.9) |
| Conjunctival hyperemia | 31 (5.5) | 5 (1.3) | 43 (6.3) | 5 (1.3) | 19 (4.2) | 4 (1.2) | 24 (4.5) | 4 (1.2) | 12 (10.5) | 1 (2.0) | 19 (13.0) | 1 (2.0) |
| Eyelids pruritus | 18 (3.2) | 8 (2.1) | 23 (3.4) | 8 (2.1) | 15 (3.3) | 7 (2.1) | 20 (3.7) | 7 (2.1) | 3 (2.6) | 1 (2.0) | 3 (2.1) | 1 (2.0) |
| Nasopharyngitis | 13 (2.3) | 10 (2.6) | 23 (3.4) | 10 (2.6) | 13 (2.9) | 6 (1.8) | 18 (3.4) | 6 (1.8) | 0 | 4 (7.8) | 5 (3.4) | 4 (7.8) |
| Blepharal pigmentation | 16 (2.8) | 1 (0.3) | 23 (3.4) | 2 (0.5) | 12 (2.7) | 0 | 16 (3.0) | 1 (0.3) | 4 (3.5) | 1 (2.0) | 7 (4.8) | 1 (2.0) |
| Erythema of eyelid | 11 (2.0) | 2 (0.5) | 22 (3.2) | 3 (0.8) | 9 (2.0) | 2 (0.6) | 17 (3.2) | 3 (0.9) | 2 (1.8) | 0 | 5 (3.4) | 0 |
| Punctate keratitis | 13 (2.3) | 3 (0.8) | 22 (3.2) | 6 (1.6) | 9 (2.0) | 3 (0.9) | 10 (1.9) | 4 (1.2) | 4 (3.5) | 0 | 12 (8.2) | 2 (3.9) |
| URT infection | 4 (0.7) | 6 (1.6) | 16 (2.4) | 6 (1.6) | 3 (0.7) | 5 (1.5) | 8 (1.5) | 5 (1.5) | 1 (0.9) | 1 (2.0) | 8 (5.5) | 1 (2.0) |
| Eye pruritus | 13 (2.3) | 3 (0.8) | 15 (2.2) | 3 (0.8) | 7 (1.6) | 2 (0.6) | 7 (1.3) | 2 (0.6) | 6 (5.3) | 1 (2.0) | 8 (5.5) | 1 (2.0) |
| Dry eye | 12 (2.1) | 3 (0.8) | 14 (2.1) | 3 (0.8) | 10 (2.2) | 1 (0.3) | 12 (2.2) | 1 (0.3) | 2 (1.8) | 2 (3.9) | 2 (1.4) | 2 (3.9) |
| Sinusitis | 4 (0.7) | 4 (1.1) | 9 (1.3) | 5 (1.3) | 3 (0.7) | 4 (1.2) | 4 (0.7) | 5 (1.5) | 1 (0.9) | 0 | 5 (3.4) | 0 |
| Radiation skin injury | 8 (1.4) | 2 (0.5) | 8 (1.2) | 2 (0.5) | 0 | 0 | 0 | 0 | 8 (7.0) | 2 (3.9) | 8 (5.5) | 2 (3.9) |
| Blepharitis | 4 (0.7) | 1 (0.3) | 8 (1.2) | 1 (0.3) | 3 (0.7) | 1 (0.3) | 4 (0.7) | 1 (0.3) | 1 (0.9) | 0 | 4 (2.7) | 0 |
| Nausea | 4 (0.7) | 2 (0.5) | 7 (1.0) | 2 (0.5) | 2 (0.4) | 2 (0.6) | 3 (0.6) | 2 (0.6) | 2 (1.8) | 0 | 4 (2.7) | 0 |
| Headache | 3 (0.5) | 4 (1.1) | 7 (1.0) | 4 (1.1) | 1 (0.2) | 4 (1.2) | 3 (0.6) | 4 (1.2) | 2 (1.8) | 0 | 4 (2.7) | 0 |
| Bronchitis | 4 (0.7) | 5 (1.3) | 6 (0.9) | 5 (1.3) | 2 (0.4) | 5 (1.5) | 2 (0.4) | 5 (1.5) | 2 (1.8) | 0 | 4 (2.7) | 0 |
| Procedural pain | 3 (0.5) | 3 (0.8) | 4 (0.6) | 3 (0.8) | 1 (0.2) | 2 (0.6) | 1 (0.2) | 2 (0.6) | 2 (1.8) | 1 (2.0) | 3 (2.1) | 1 (2.0) |
| Hypothyroidism | 1 (0.2) | 1 (0.3) | 4 (0.6) | 1 (0.3) | 0 | 1 (0.3) | 1 (0.2) | 1 (0.3) | 1 (0.9) | 0 | 3 (2.1) | 0 |
| Pyrexia | 2 (0.4) | 4 (1.1) | 3 (0.4) | 4 (1.1) | 0 | 2 (0.6) | 1 (0.2) | 2 (0.6) | 2 (1.8) | 2 (3.9) | 2 (1.4) | 2 (3.9) |
| Ligament sprain | 2 (0.4) | 2 (0.5) | 3 (0.4) | 2 (0.5) | 0 | 2 (0.6) | 0 | 2 (0.6) | 2 (1.8) | 0 | 3 (2.1) | 0 |
| Cough | 3 (0.5) | 3 (0.8) | 3 (0.4) | 3 (0.8) | 0 | 2 (0.6) | 0 | 2 (0.6) | 3 (2.6) | 1 (2.0) | 3 (2.1) | 1 (2.0) |
| Ovarian cyst‡ | 1 (0.2) | 0 | 3 (0.5) | 0 | 0 | 0 | 0 | 0 | 1 (0.9) | 0 | 3 (2.1) | 0 |
| Insomnia | 2 (0.4) | 2 (0.5) | 2 (0.3) | 2 (0.5) | 0 | 0 | 0 | 0 | 2 (1.8) | 2 (3.9) | 2 (1.4) | 2 (3.9) |
Subjects in Study 5 are not included in the analysis up to four months.
Up to six months for vehicle group.
Percentage was calculated based on the number of females in each group.
BIM=bimatoprost; URT=upper respiratory tract; VEH=vehicle
Commonly reported AEs and severity. Most AEs that occurred in the overall population were mild in severity, primarily localized to the site of treatment, and reversible with cessation of study treatment. Eye disorders were the predominant AEs reported. In the pooled bimatoprost 0.03% group, the most commonly reported AEs were conjunctival hyperemia (6.3%), eyelid pruritus (3.4%), blepharal pigmentation (3.4%), nasopharyngitis (3.4%), erythema of the eyelid (3.2%), and punctate keratitis (3.2%) (Table 3). Of these commonly reported, eye-related AEs, most occurred with bimatoprost 0.03% during the first four months of treatment (Table 4). Five such cases of eye-related AEs were reported as severe: one case each of allergic conjunctivitis, conjunctival hyperemia, eye irritation, eye pruritus, and eyelid disorder. No new safety signals were observed with treatment for up to 12 months.
TABLE 4.
Common eye-related adverse events (≥2% and by >1 subject in any treatment group), by severity and length of bimatoprost 0.03% exposure in the six pooled studies
| ADVERSE EVENT | SEVERITY OF ADVERSE EVENTS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild, n (%) | Moderate, n (%) | Severe,‡ n (%) | ||||||||||
| ≤4 Months* | ≤12 Months† | ≤4 Months* | ≤12 Months† | ≤4 Months* | ≤12 Months† | |||||||
| BIM (n=562) | VEH (n=379) | BIM (n=680) | VEH (n=379) | BIM (n=562) | VEH (n=379) | BIM (n=680) | VEH (n=379) | BIM (n=562) | VEH (n=379) | BIM (n=680) | VEH (n=379) | |
| Conjunctival hyperemia | 30 (5.3) | 5 (1.3) | 41 (6.0) | 5 (1.3) | 0 | 0 | 1 (0.1) | 0 | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Punctate keratitis | 13 (2.3) | 3 (0.8) | 22 (3.2) | 6 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blepharal pigmentation | 14 (2.5) | 1 (0.3) | 20 (2.9) | 2 (0.5) | 2 (0.4) | 0 | 3 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Eyelids-pruritus | 16 (2.8) | 8 (2.1) | 20 (2.9) | 8 (2.1) | 2 (0.4) | 0 | 3 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Erythema of eyelid | 8 (1.4) | 2 (0.5) | 17 (2.5) | 3 (0.8) | 3 (0.5) | 0 | 5 (0.7) | 0 | 0 | 0 | 0 | 0 |
| Eye pruritus | 12 (2.1) | 2 (0.5) | 13 (1.9) | 2 (0.5) | 0 | 1 (0.3) | 1 (0.1) | 1 (0.3) | 1 (0.2) | 0 | 1 (0.1) | 0 |
Subjects in Study 5 are not included in the analysis up to four months.
Up to six months for vehicle group.
2 additional severe eye-related AEs did not meet cutoff criteria for inclusion in this table: one case each of allergic conjunctivitis and eye irritation
BIM=bimatoprost; VEH=vehicle
Treatment-related AEs. Almost all treatment-related AEs overall and in the idiopathic and postchemotherapy subpopulations were eye disorders, which occurred more frequently in bimatoprost-treated subjects than in vehicle-treated subjects, through up to 12 months (Table 5). Of the 680 subjects treated with bimatoprost 0.03% for up to 12 months, 143 (21.0%) experienced a treatment-related AE; of these, 126 subjects (18.5%) reported eye disorders. Thirty of the 379 subjects (7.9%) treated for up to six months with vehicle reported treatment-related AEs, of which 27 (7.1%) were eye disorders. Subjects in the postchemotherapy subpopulation who were treated with bimatoprost 0.03% for up to 12 months had a higher incidence of treatment-related AEs compared with the idiopathic subpopulation (25.3% vs. 19.9%, respectively). Most of the treatment-related AEs were mild in severity.
TABLE 5.
Common treatment-related adverse events (≥2% and >1 subject in either group in a subpopulation), reported by etiology and length of bimatoprost 0.03% exposure in the six pooled studies
| ANY TREATMENT-RELATED AE | Overall, n (%) | Idiopathic, n (%) | Postchemotherapy, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤4 Months* | ≤12 Months† | ≤4 Months* | ≤12 Months† | ≤4 Months* | ≤12 Months† | |||||||
| BIM (n=562) | VEH (n=379) | BIM (n=680) | VEH (n=379) | BIM (n=448) | VEH (n=328) | BIM (n=534) | VEH (n=328) | BIM (n=114) | VEH (n=51) | BIM (n=146) | VEH (n=51) | |
| 111 (19.8) | 25 (6.6) | 143 (21.0) | 30 (7.9) | 86 (19.2) | 21 (6.4) | 106 (19.9) | 25 (7.6) | 25 (21.9) | 4 (7.8) | 37 (25.3) | 5 (9.8) | |
| EYE DISORDERS | ||||||||||||
| Overall | 94 (16.7) | 22 (5.8) | 126 (18.5) | 27 (7.1) | 71 (15.8) | 19 (5.8) | 90 (16.9) | 23 (7.0) | 23 (20.2) | 3 (5.9) | 36 (24.7) | 4 (7.8) |
| Conjunctival hyperemia | 28 (5.0) | 2 (0.5) | 36 (5.3) | 2 (0.5) | 17 (3.8) | 2 (0.6) | 22 (4.1) | 2 (0.6) | 11 (9.6) | 0 | 14 (9.6) | 0 |
| Blepharal pigmentation | 15 (2.7) | 1 (0.3) | 22 (3.2) | 2 (0.5) | 11 (2.5) | 0 | 15 (2.8) | 1 (0.3) | 4 (3.5) | 1 (2.0) | 7 (4.8) | 1 (2.0) |
| Eyelids pruritus | 16 (2.8) | 7 (1.8) | 21 (3.1) | 7 (1.8) | 13 (2.9) | 6 (1.8) | 18 (3.4) | 6 (1.8) | 3 (2.6) | 1 (2.0) | 3 (2.1) | 1 (2.0) |
| Erythema of eyelid | 10 (1.8) | 2 (0.5) | 20 (2.9) | 3 (0.8) | 8 (1.8) | 2 (0.6) | 16 (3.0) | 3 (0.9) | 2 (1.8) | 0 | 4 (2.7) | 0 |
| Punctate keratitis | 11 (2.0) | 1 (0.3) | 16 (2.4) | 3 (0.8) | 8 (1.8) | 1 (0.3) | 8 (1.5) | 2 (0.6) | 3 (2.6) | 0 | 8 (5.5) | 1 (2.0) |
| Eye pruritus | 9 (1.6) | 2 (0.5) | 10 (1.5) | 2 (0.5) | 6 (1.3) | 1 (0.3) | 6 (1.1) | 1 (0.3) | 3 (2.6) | 1 (2.0) | 4 (2.7) | 1 (2.0) |
Subjects in Study 5 are not included in the analysis up to four months.
Up to six months for vehicle group.
BIM=bimatoprost; VEH=vehicle
AEs resulting in study treatment discontinuation.
Twenty-two subjects (3.2%) in the bimatoprost 0.03% group and nine subjects (2.4%) in the vehicle group discontinued study treatment due to an AE. The primary type of AE leading to the discontinuation of bimatoprost 0.03% treatment was eye disorders, for which three subjects discontinued because of conjunctival hyperemia, three of erythema of the eyelid, three of dry eye, and two of eye irritation (Table 6). Most AEs resulting in study discontinuation occurred within the first four months of study treatment. Subjects with idiopathic hypotrichosis experienced a greater proportion of discontinuations attributable to bimatoprost 0.03% than did those with postchemotherapy hypotrichosis (3.4% vs. 2.7%, respectively).
TABLE 6.
Adverse events leading to study discontinuations, reported by length of exposure in the six pooled studies
| ADVERSE EVENT | DISCONTINUATIONS, N (%) | |||
|---|---|---|---|---|
| ≤4 Months† | ≤12 Months‡ | |||
| BIM 0.03% (n=562) | VEH (n=379) | BIM 0.03% (n=680) | VEH (n=379) | |
| Overall subject discontinuations* | 17 (3.0) | 7 (1.8) | 22 (3.2) | 9 (2.4) |
| Erythema of eyelid | 1 (0.2) | 1 (0.3) | 3 (0.4) | 1 (0.3) |
| Conjunctival hyperemia | 3 (0.5) | 0 | 3 (0.4) | 0 |
| Dry eye | 3 (0.5) | 0 | 3 (0.4) | 0 |
| Eye irritation | 2 (0.4) | 0 | 2 (0.3) | 0 |
| Contact dermatitis | 2 (0.4) | 0 | 2 (0.3) | 0 |
| Metastatic breast cancer | 1 (0.2) | 0 | 2 (0.3) | 0 |
| IOP decreased | 1 (0.2) | 1 (0.3) | 1 (0.1) | 1 (0.3) |
| Conjunctivitis allergic | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Enophthalmos | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Eye pruritus | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Eye inflammation | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Eyelid margin crusting | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Increased lacrimation | 0 | 0 | 1 (0.1) | 0 |
| Eyelid exfoliation | 0 | 0 | 1 (0.1) | 0 |
| Eyelid edema | 0 | 0 | 1 (0.1) | 0 |
| Eyelids pruritus | 0 | 0 | 1 (0.1) | 0 |
| Conjunctival hemorrhage | 0 | 1 (0.3) | 0 | 1 (0.3) |
| Breast cancer | 0 | 1 (0.3) | 1 (0.1) | 1 (0.3) |
| Cardiac arrest | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Vertigo | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Ovarian cancer§ | 1 (0.2) | 0 | 1 (0.2) | 0 |
| Facial pain | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Pulmonary embolism | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Eczema | 1 (0.2) | 0 | 1 (0.1) | 0 |
| Dry mouth | 0 | 1 (0.3) | 0 | 1 (0.3) |
| Hordeolum | 0 | 1 (0.3) | 0 | 1 (0.3) |
| Dissociative disorder | 0 | 1 (0.3) | 0 | 1 (0.3) |
| Type 2 diabetes mellitus | 0 | 0 | 0 | 1 (0.3) |
| Lymphoma | 0 | 0 | 0 | 1 (0.3) |
Some subjects reported more than one AE leading to discontinuation
Subjects in Study 5 are not included in the analysis up to four months.
Up to six months for vehicle group.
Percentage was calculated based on the number of females in each group.
BIM=bimatoprost; IOP=intraocular pressure; VEH=vehicle
Adverse events of special interest were consistent with the known pharmacologic mechanisms of bimatoprost. Two subjects discontinued study treatment because of low IOP (≤5mmHg), one each from the bimatoprost and vehicle groups. No associated changes in visual acuity or other biomicroscopy or ophthalmoscopy findings were observed in either of these subjects. The incidence of low IOP measurements and variability in IOP were similar between the two treatment groups. Across all post-baseline time points through four months, the minimum and maximum IOP measurements were comparable for both treatment groups, 4.8 and 21.5mmHg in bimatoprost 0.03%-treated groups compared with 5.0 and 20.8mmHg in vehicletreated groups, respectively. Additionally, the minimum and maximum mean changes from baseline in IOP across studies were similar for both treatment groups, -9.0mmHg and 6.0mmHg in the bimatoprost 0.03% treatment groups and -9.25 and 7.3mmHg in the vehicle treatment groups, respectively. Periods of continually low IOP were not observed in any subject in any of the studies. Iris hyperpigmentation was reported as an AE in two subjects with idiopathic hypotrichosis, one in Study 1 and one in Study 6. Both cases were described as mild. Photographs of these subjects’ eyes before and after treatment are shown in Figure 3. For the subject in Study 1, assessment of iris color by the investigator was recorded as blue/gray-brown at screening, as hazel at Month 3, and blue/gray-brown upon study exit at Month 5. This event was reported to have resolved prior to the end of the study. For the subject in Study 6, a one-category change on a 10-category scale, from blue/gray to blue/gray-brown, was recorded two months after completion of bimatoprost treatment (treatment period 1) while the subject was receiving vehicle (treatment period 2). In the overall pooled population receiving bimatoprost 0.03% or vehicle, blepharal hyperpigmentation occurred in 23 (3.4%) and two (0.5%) subjects, respectively, and skin hyperpigmentation occurred in four (0.6%) and zero subjects, respectively. Photographs of skin hyperpigmentation in the periorbital area in Asian (Study 2) and African-American (Study 4) subjects are shown in Figure 4. Enophthalmos, or deepening of the upper eyelid sulcus, was reported in one subject in the bimatoprost 0.03% group from Study 6. This AE was first reported 58 days after initiation of treatment and was ongoing five months after treatment discontinuation; photographs are shown in Figure 5. Madarosis, or loss of eyelashes, was reported in six bimatoprost-treated subjects (0.9%) across the six trials. Subject photographs are shown in Figure 6.
Figure 3.
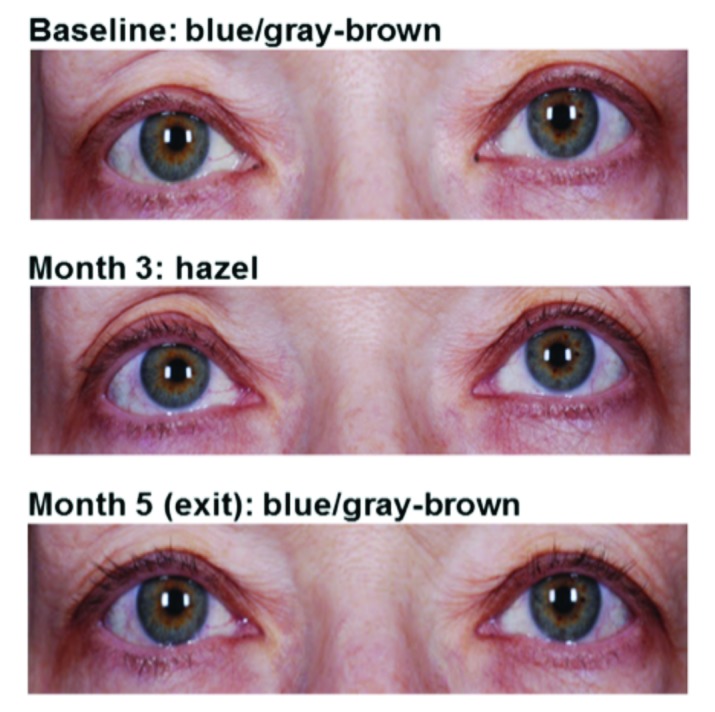
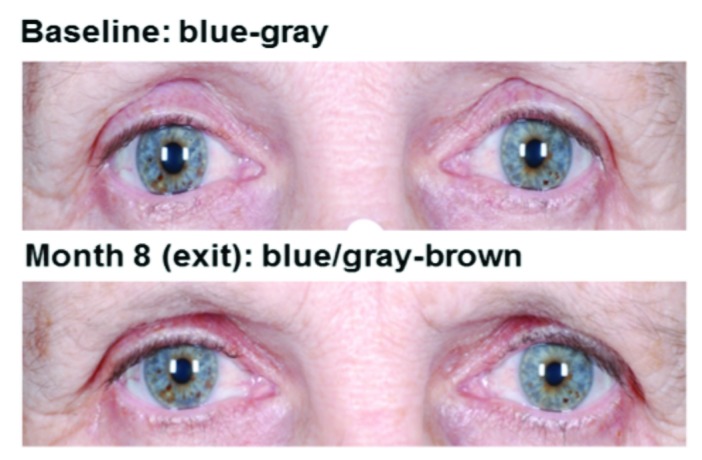
Example of iris hyperpigmentation reported for two subjects who received bimatoprost 0.03% in Study 1 (A) or in Study 6 (B). In Study 1, the AE was reported to have resolved by the end of the study.
Figure 4.
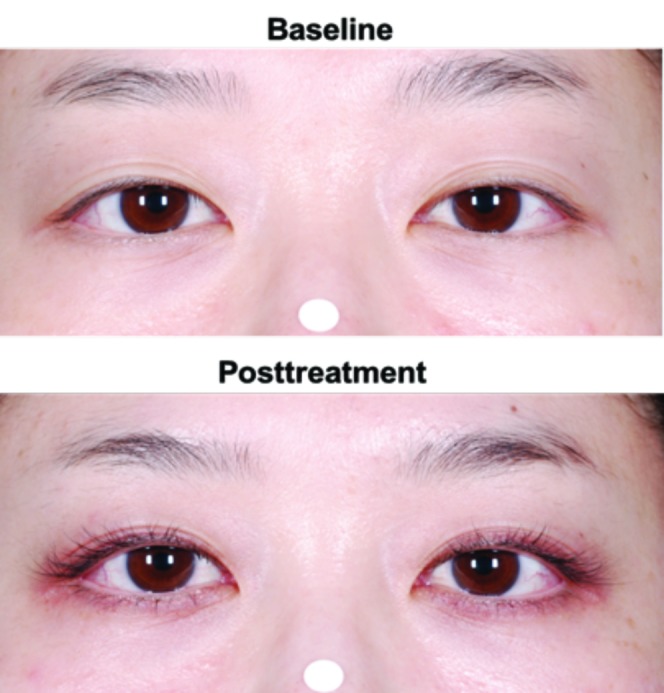
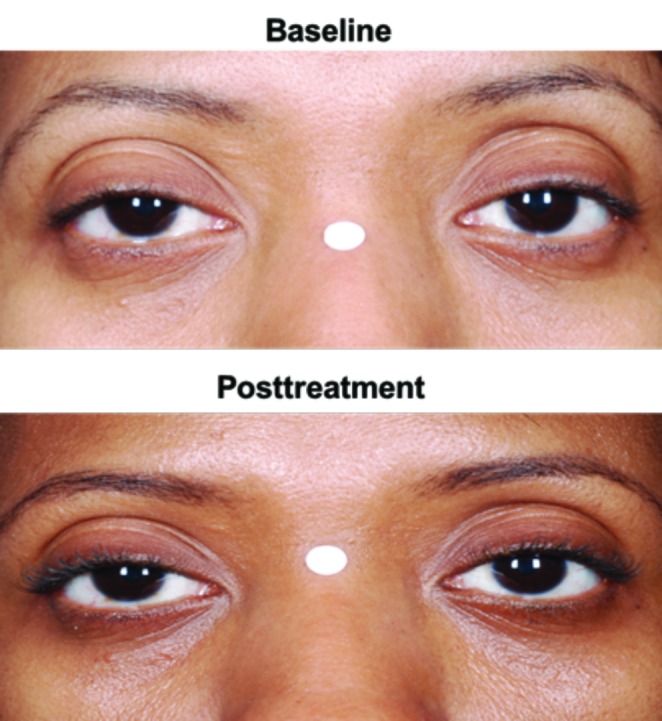
Examples of skin hyperpigmentation in the periorbital area (A) in an Asian subject and (B) in an African American subject who received bimatoprost 0.03%
Figure 5.
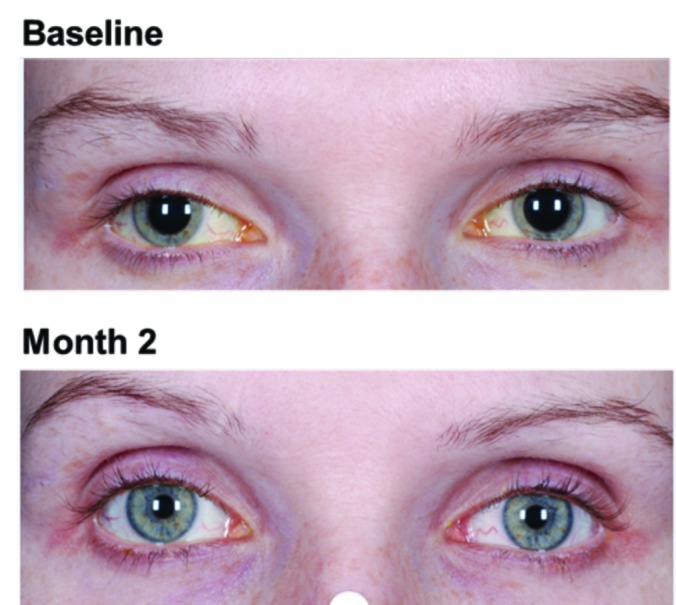
Example of enophthalmos reported for one subject who received bimatoprost 0.03%
Figure 6.
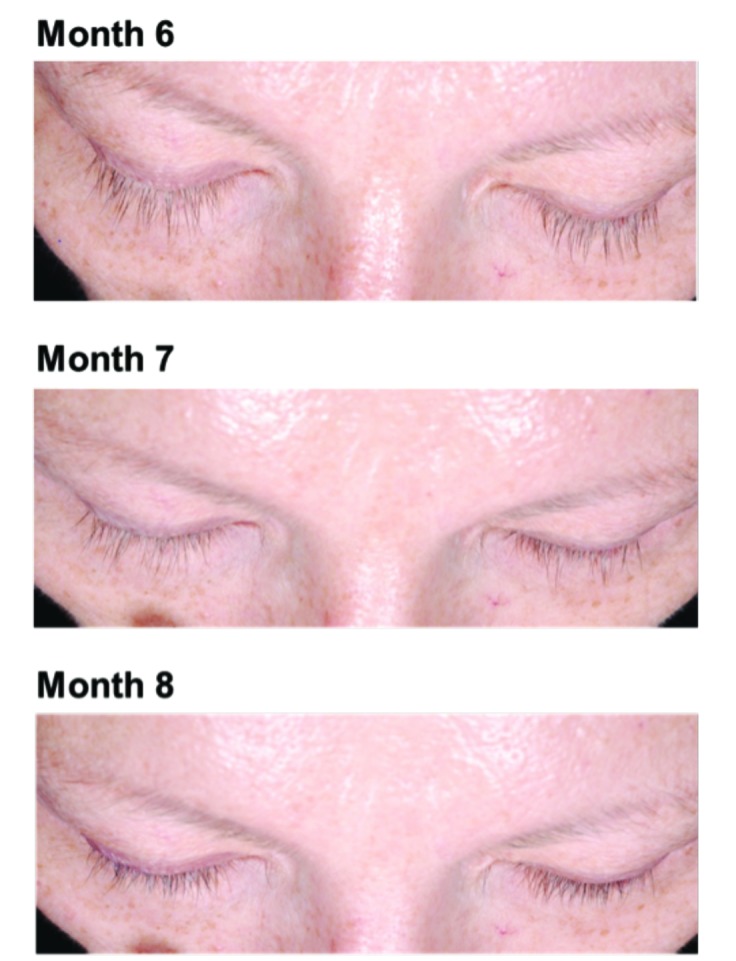
Example of madarosis reported for one subject who received bimatoprost 0.03%.
Serious AEs. Serious AEs occurred in 4.4 percent and 2.4 percent of bimatoprost-treated and vehicle-treated subjects in the overall population, respectively; however, none were eye disorders and none were considered by investigators to be treatment-related. One death that was deemed to be probably due to pulmonary embolism and cardiac arrest occurred in a postchemotherapy subject in the bimatoprost 0.03% group in Study 6. The investigator considered the death to be unrelated to treatment.
Other safety assessments. No clinically meaningful changes were reported in best-corrected visual acuity, vital signs, or physical examinations. Three pregnancies in bimatoprost-treated subjects were reported (one each from Studies 1, 4, and 6), all of which culminated in healthy infants with no delivery complications.
Safety analysis by demographics. Through four months, differences in the incidence of AEs by age, sex, and race were evaluated between subjects treated with bimatoprost 0.03% and vehicle-treated subjects in the idiopathic hypotrichosis and in the postchemotherapy subpopulations. The incidence appeared to be similar to that of the overall population, although the number of subjects in each subgroup of the postchemotherapy population was often too small to make definitive comparisons. For the subgroups with a large enough population to draw reliable conclusions, safety analysis through the end of treatment generally showed that the overall incidence of AEs reported in the subgroups was similar to that of the overall population.
The incidence of commonly reported eye- and skin-related AEs (≥2%) was generally comparable between Caucasian and non-Caucasian subjects and between the non-Caucasian subgroups of black and Asian subjects (Table 7). However, for bimatoprost 0.03%-treated subjects, the incidence of blepharal pigmentation (8.1% vs. 0.9%) and eyelid pruritus (5.1% vs. 2.5%) was higher in non-Caucasian versus Caucasian subjects, respectively. The 19 non-Caucasian subjects reporting blepharal hyperpigmentation included nine African-American and nine Asian subjects receiving bimatoprost 0.03%. Conversely, among those receiving bimatoprost 0.03%, most of the non-Caucasian subjects reporting eyelid pruritus (9 of 12) were African American. Four cases of skin hyperpigmentation were reported in non-Caucasian subjects treated with bimatoprost 0.03%; all four subjects were self-identified as African American and all but one case occurred within the first four months of treatment. No incident of skin hyperpigmentation was reported in Caucasian subjects, although there was one report of skin discoloration. The incidence of upper respiratory infection appeared higher in Caucasian versus non-Caucasian subjects (2.9% vs. 1.3%) and the incidence of nasopharyngitis was higher in non-Caucasian versus Caucasian subjects (6.4% vs. 1.8%).
TABLE 7.
Most common eye- and skin-related adverse events (≥2% in either treatment group) reported by race through the end of treatment in the six pooled studies
| ADVERSE EVENT, N (%) | OVERALL | CAUCASIAN | NON-CAUCASIAN | AFRICAN AMERICAN | ASIAN | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BIM 0.03% (n=680) | VEH (n=379) | BIM 0.03% (n=444) | VEH (n=190) | BIM 0.03% (n=236) | VEH (n=189) | BIM 0.03% (n=75) | VEH (n=53) | BIM 0.03% (n=135) | VEH (n=123) | |
| Overall (all categories) | 322 (47.4) | 130 (34.3) | 208 (46.8) | 59 (31.1) | 114 (48.3) | 71 (37.6) | 39 (52.0) | 16 (30.2) | 62 (45.9) | 49 (39.8) |
| Conjunctival hyperemia | 43 (6.3) | 5 (1.3) | 29 (6.5) | 1 (0.5) | 14 (5.9) | 4 (2.1) | 7 (9.3) | 2 (3.8) | 6 (4.4) | 1 (0.8) |
| Eyelids pruritus | 23 (3.4) | 8 (2.1) | 11 (2.5) | 4 (2.1) | 12 (5.1) | 4 (2.1) | 9 (12.0) | 4 (7.5) | 2 (1.5) | 0 |
| Erythema of eyelid | 22 (3.2) | 3 (0.8) | 16 (3.6) | 2 (1.1) | 6 (2.5) | 1 (0.5) | 2 (2.7) | 0 | 1 (0.7) | 0 |
| Blepharal pigmentation | 23 (3.4) | 2 (0.5) | 4 (0.9) | 0 (0.0) | 19 (8.1) | 2 (1.1) | 9 (12.0) | 0 | 9 (6.7) | 2 (1.6) |
| Punctate keratitis | 22 (3.2) | 6 (1.6) | 17 (3.8) | 4 (2.1) | 5 (2.1) | 2 (1.1) | 2 (2.7) | 1 (1.9) | 3 (2.2) | 1 (0.8) |
| Eye pruritus | 15 (2.2) | 3 (0.8) | 10 (2.3) | 2 (1.1) | 5 (2.1) | 1 (0.5) | 3 (4.0) | 0 | 2 (1.5) | 1 (0.8) |
| Dry eye | 14 (2.1) | 3 (0.8) | 8 (1.8) | 2 (1.1) | 6 (2.5) | 1 (0.5) | 3 (4.0) | 0 | 3 (2.2) | 1 (0.8) |
| Eye discharge | 4 (0.6) | 2 (0.5) | 0 | 0 | 3 (1.3) | 0 | 0 | 0 | 3 (2.2) | 0 |
| Skin hyperpigmentation | 4 (0.6) | 0 | 0 | 0 | 4 (1.7) | 0 | 4 (5.3) | 0 | 0 | 0 |
| Radiation skin injury | 8 (1.2) | 2 (0.5) | 2 (0.5) | 0 | 6 (2.5) | 2 (1.1) | 0 | 0 | 4 (3.0) | 2 (1.6) |
BIM=bimatoprost; URT=upper respiratory tract; VEH=vehicle
When subjects were grouped according to age, those aged 45 to 65 years who were treated with bimatoprost (n=401) experienced a higher incidence of overall AEs than did subjects younger than 45 years (n=241) (50.1% vs. 42.6%, respectively), whereas, when receiving vehicle, the overall incidence of AEs was lower in subjects aged 45 to 65 years versus those younger than 45 years (33.2% vs. 36.2%, respectively). The overall incidence of eye disorder AEs among subjects treated with bimatoprost was similar in these age groups (25.7% of subjects aged <45 years and 27.4% of subjects aged 45-65 years). Subjects aged 45 to 65 years experienced a higher incidence versus younger subjects of conjunctival hyperemia (8.0% vs. 4.6%), eyelid pruritis (4.0% vs. 2.9%), erythema of the eyelid (3.7% vs. 1.2%), and punctate keratitis (3.5% vs. 2.9%) following treatment with bimatoprost, respectively. Subjects in the bimatoprost group aged 45 to 65 years experienced a lower incidence versus younger subjects of blepharal pigmentation (2.2% vs. 5.8%) and dry eye (1.0% vs. 3.7%), respectively. The number of subjects older than 65 years was insufficient to draw meaningful comparisons (bimatoprost group, n=38; vehicle group, n=24). Similarly, only 16 male subjects were included in each treatment group in the overall population; thus, sample sizes were too small to provide any clinically meaningful comparisons.
DISCUSSION
In this pooled analysis of safety findings from six randomized, parallel-group clinical trials conducted in adult subjects receiving bimatoprost 0.03% for the treatment of eyelash hypotrichosis for up to 12 months, most AEs were mild in severity, were primarily ocular in nature, and occurred early in treatment. A slightly higher overall incidence of AEs in subjects treated for 12 months with bimatoprost 0.03% is consistent with the longer duration of treatment exposure compared with placebo treatment for six months. The somewhat higher incidence of AEs in both bimatoprost and vehicle-treated postchemotherapy subjects may be related to the enduring effects of chemotherapy.15,16 The most common AEs, with an incidence of at least two percent in the overall population, included conjunctival hyperemia, eyelid pruritus, blepharal pigmentation, nasopharyngitis, erythema of the eyelid, and punctate keratitis. The vast majority of these eye-related AEs occurred early in treatment and were mild in severity. Overall, ocular and other AEs rarely led to study discontinuation, with 3.2 percent of bimatoprost-treated and 2.4 percent of vehicle-treated subjects stopping therapy because of AEs.
The known pharmacologic mechanisms of bimatoprost provide a rationale for the occurrence of the most commonly reported AEs of the eyes and skin. Adverse events of particular interest with ophthalmic application of prostamide F2α analogs, such as bimatoprost, include IOP reduction, iris and skin hyperpigmentation, and enophthalmos.17,18 In patients with glaucoma, topical prostaglandins act to lower IOP by enhancing aqueous humor outflow.19 Although the same 0.03% bimatoprost concentration is used for the treatment of eyelash hypotrichosis as is used for glaucoma, the total dose applied to the eyelid margins for the treatment of hypotrichosis is only approximately five percent of the dose compared with that administered by eyedropper for the treatment of glaucoma.6 Across the pooled studies, 975 eyelash hypotrichosis subjects had routine IOP examinations and, among these subjects, fewer than two percent experienced decreased IOP through the end of treatment. The magnitude of the decrease in IOP associated with bimatoprost 0.03% was not clinically meaningful and was not associated with changes in visual acuity or other biomicroscopy or ophthalmoscopy findings. The postbaseline range from minimum to maximum IOP measurements and the range of minimum and maximum mean change from baseline in IOP across the studies were similar between the bimatoprost 0.03% and vehicle groups. The IOP measurements from these pooled data showed variability, with a range from 3 to 5mmHg, which is similar to measurements observed in two previous studies conducted in untreated, normotensive eyes.20,21 One study found that the mean IOP diurnal variation in a group of 220 normotensive individuals was 3.7mmHg and, in 84 percent of these individuals, IOP fluctuation was less than 5.0mmHg.20 A later study in 1,178 subjects found that the mean IOP fluctuation in normotensive eyes was 5.0mmHg.21 The variations in IOP associated with bimatoprost 0.03% and vehicle in this study were, thus, consistent with normal diurnal variation.
Conjunctival hyperemia is a common side effect associated with the topical administration of prostaglandins due to their effect on ocular vasodilation. These effects are generally mild and transient and rarely result in treatment discontinuation, even at the higher dosage of intraocular administration of bimatoprost used for glaucoma treatment.13,19 Conjunctival hyperemia was experienced by 6.3 percent of the overall bimatoprost-treated eyelash hypotrichosis population. The severity of this AE ranged from mild to severe; three subjects with idiopathic hypotrichosis discontinued because of this AE. Of these three subjects, all from Study 6, the maximum severity was mild in two subjects and severe in one subject.
Iris hyperpigmentation is believed to be an irreversible side effect caused by topical prostaglandin analog stimulation of melanocytes in the iris.17 In this pooled safety analysis, two cases of iris hyperpigmentation were reported. However, both cases were mild in severity, difficult to discern in photographs (Figure 3), and may have been affected by variability by having more than one assessor across visits. In one subject, the iris pigmentation was reported to have resolved by the end of the study. Skin pigmentation is a reversible class effect of topical prostaglandin analogs. The exact mechanism has not been elucidated, although increased melanogenesis has been suggested.17,18,22 In the overall pooled population, blepharal skin hyperpigmentation was reported in 3.4 percent of subjects treated with bimatoprost 0.03%, and the frequency was higher in non-Caucasian subjects compared with Caucasian subjects (8.1% vs. 0.9%, respectively).
Enophthalmos is a rare side effect of bimatoprost, and it has been postulated that topical prostaglandin analogs may initiate orbital fat atrophy.17,23 In glaucoma patients treated with topical prostaglandins, enophthalmos was partially or completely reversible following discontinuation of treatment.23 Of the 680 subjects who received bimatoprost for the treatment of eyelash hypotrichosis, one subject experienced enophthalmos in both eyes. In this subject, enophthalmos occurred after 57 days of study treatment, and the AE was ongoing five months after treatment discontinuation.
The incidence of madarosis was low (0.9%) in the overall bimatoprost-treated population. Prostaglandins likely interact with hair follicle prostanoid receptors to stimulate resting follicles (telogen) to become growing follicles (anagen). It is hypothesized that, in some subjects, this stimulation may result in a greater proportion of eyelashes undergoing simultaneous detachment prior to the emergence of the tips of the newly growing eyelashes, an occurrence that is temporary and reversible.
CONCLUSION
Results of this pooled analysis of six studies involving bimatoprost 0.03% for the treatment of idiopathic or chemotherapy-induced eyelash hypotrichosis for up to 12 months identified no new or unexpected safety signals. The incidence of the most common AEs (reported in ≥2% of subjects) was consistent with that previously documented in the individual clinical studies of bimatoprost 0.03% used for the treatment of eyelash hypotrichosis. Most AEs were mild in severity, were localized to the site of treatment, and were reversible with treatment cessation. Neither the serious AEs nor the death of a postchemotherapy subject were considered related to treatment. The safety profile across races was generally similar and was similar to that in the overall study population. In addition, this pooled analysis shows that longer-term treatment with bimatoprost 0.03% is not associated with an increased incidence of AEs. Taken together, these data demonstrate that daily use of bimatoprost 0.03% has a favorable safety and tolerability profile when applied topically to the upper eyelid margin.
ACKNOWLEDGMENT
Writing and editorial assistance was provided to the authors by Michelle McDermott, PharmD, and Kristin E. Larsen, PhD, of Peloton Advantage, Parsippany, New Jersey, and was funded by Allergan Inc., Irvine, California.
Footnotes
DISCLOSURE:Dr. Wirta serves as a consultant for and has received research grants from Allergan, Inc. Dr. Pariser serves as a remunerated consultant and investigator and has received research grants and honoraria from Allergan, Inc. Dr. Yoelin is a consultant and investigator for Allergan, Inc. Dr. Arase serves as an investigator for Allergan, Inc. Dr. McMichael serves as a consultant and investigator for Allergan, Inc. Dr. Weng, Ms. Mao, and Dr. VanDenburgh are employees of Allergan, Inc., and may own stock or options in that company. Dr. Demos was an employee of Allergan, Inc., at the time of the initial drafting of this manuscript. This study was sponsored by Allergan, Inc., Irvine, California. Writing and editorial assistance was provided by Michelle McDermott, PharmD, and Kristin E. Larsen, PhD, of Peloton Advantage, Parsippany, New Jersey, and was funded by Allergan, Inc. Neither honoraria nor other forms of payment were made for authorship.
REFERENCES
- 1.Woodward DF, Phelps RL, Krauss AH, et al. Bimatoprost: a novel antiglaucoma agent. Cardiovasc Drug Rev. 2004;22:103–120. doi: 10.1111/j.1527-3466.2004.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 2.Higginbotham EJ, Schuman JS, Goldberg I, et al. One-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286–1293. doi: 10.1001/archopht.120.10.1286. [DOI] [PubMed] [Google Scholar]
- 3.Whitcup SM, Cantor LB, VanDenburgh AM, Chen K. A randomised, double masked, multicentre clinical trial comparing bimatoprost and timolol for the treatment of glaucoma and ocular hypertension. Br J Ophthalmol. 2003;87:57–62. doi: 10.1136/bjo.87.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams RD, Cohen JS, Gross RL, et al. Long-term efficacy and safety of bimatoprost for intraocular pressure lowering in glaucoma and ocular hypertension: year 4. Br J Ophthalmol. 2008;92:1387–1392. doi: 10.1136/bjo.2007.128454. [DOI] [PubMed] [Google Scholar]
- 5.Law SK. Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthalmol. 2010;4:349–358. doi: 10.2147/opth.s6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;3:3948. doi: 10.2147/ccid.s5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones D. Enhanced eyelashes: prescription and over-the-counter options. Aesthetic Blast Surg. 2011;35:116–121. doi: 10.1007/s00266-010-9561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris CL, Stinnett S, Woodward J. The role of bimatoprost eyelash gel in chemotherapy-induced madarosis: an analysis of efficacy and safety. Int J Trichology. 2011;3:84–91. doi: 10.4103/0974-7753.90809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Bsychooncology. 2008;17:317–328. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 10.Hesketh PJ, Batchelor D, Golant M, et al. Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support Care Cancer. 2004;12:543–549. doi: 10.1007/s00520-003-0562-5. [DOI] [PubMed] [Google Scholar]
- 11.Smith S, Fagien S, Whitcup SM, et al. Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J Am Acad Dermatol. 2012;66:801–806. doi: 10.1016/j.jaad.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Harii K, Arase S, Tsuboi R, et al. Bimatoprost for eyelash growth in Japanese subjects: two multicenter controlled studies. Aesthetic Blast Surg. 2014;38:451460. doi: 10.1007/s00266-014-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirta D, VanDenburgh AM, Weng E, et al. Long-term safety evaluation of bimatoprost ophthalmic solution 0.03%: a pooled analysis of six double-masked, randomized, active-controlled clinical trials. Clin Ophthalmol. 2011;5:759–765. doi: 10.2147/OPTH.S17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaser DA, Hossain P, Perkins W, et al. Long-term safety and efficacy of bimatoprost solution 0.03% application to the eyelid margin for the treatment of idiopathic and chemotherapy-induced eyelash hypotrichosis: a randomised controlled trial. Br J Dermatol. doi: 10.1111/bjd.13443. 2014 Oct 9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imperia PS, Lazarus HM, Lass JH. Ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 1989;34:209–230. doi: 10.1016/0039-6257(89)90105-7. [DOI] [PubMed] [Google Scholar]
- 16.Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51:19–40. doi: 10.1016/j.survophthal.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Hollo G. The side effects of the prostaglandin analogues. Expert OpinDrugSaf. 2007;6:45–52. doi: 10.1517/14740338.6.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Aim A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008;53(suppl 1):S93–105. doi: 10.1016/j.survophthal.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Cracknell KP, Grierson I. Prostaglandin analogues in the anterior eye: their pressure lowering action and side effects. Exp Eye Res. 2009;88:786–791. doi: 10.1016/j.exer.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Drance SM. The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol. 1960;64:494–501. doi: 10.1001/archopht.1960.01840010496004. [DOI] [PubMed] [Google Scholar]
- 21.David R, Zangwill L, Briscoe D, et al. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol. 1992;76:280–283. doi: 10.1136/bjo.76.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapur R, Osmanovic S, Toyran S, Edward DP. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541–1546. doi: 10.1001/archopht.123.11.1541. [DOI] [PubMed] [Google Scholar]
- 23.Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol. 2011;55:22–27. doi: 10.1007/s10384-010-0904-z. [DOI] [PubMed] [Google Scholar]