Summary
Background
Epidemiologic data from studies of airway diseases, such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis indicate a gender disparity where women have worse outcomes. The explanation for this is largely unknown. We hypothesize that female sex hormones play a role in this gender disparity, predisposing women to more exacerbations and decreased lung function post-puberty.
Objective
In Cystic Fibrosis, to determine if puberty marks a point of increasing exacerbations and decreasing lung function in women relative to men.
Methods
Using the United States Cystic Fibrosis Foundation Patient Registry, we used linear regression to compare lung function and rate of pulmonary exacerbations in men versus women before and after puberty.
Results
Of 5,137 subjects who met inclusion criteria, 2,689 were male and 2,448 were female. Average age of puberty was found to be 13.2 ± 2.2 years in men and 11.2 ± 2.0 years of age in women. Percent predicted FEV1 pre-and post-puberty were no different between males versus females (P = 0.44 pre-puberty and P = 0.16 post-puberty). In contrast, women had a significantly higher rate of pulmonary exacerbations post-puberty than men (1.17 ± 1.35 exacerbations per year in women versus 0.95 ± 1.27 in men; P < 0.001) despite controlling for morphometrics, co-morbidities, and microbiologic variables.
Conclusion
After puberty, the rate of pulmonary exacerbations increased in adolescent women relative to men with cystic fibrosis, supporting a role for sex hormones in the disease process. Further understanding of the mechanisms that modulate sex hormone receptors in airway disease may serve as future targets for therapy.
Keywords: cystic fibrosis, gender disparity, puberty, exacerbation
INTRODUCTION
Gender differences in airway diseases such as asthma, chronic obstructive pulmonary disease (COPD), and bronchiectasis have been well described. In asthma, males are disproportionately affected during childhood. This difference disappears around adolescence and females have a much higher incidence and prevalence after the onset of puberty.1–7 In the United States, more than 60% of adult asthmatics are women. Women with asthma are hospitalized more frequently and have longer hospitalizations than men and are 40% more likely to die from asthma than men.7,8 In COPD, women develop evidence of COPD after smoking fewer numbers of cigarettes per lifetime and women with severe COPD have a 50% increased risk of death compared to men.9,10 In 2000, the number of women dying from COPD surpassed the number in men.11 In non-Cystic Fibrosis related bronchiectasis, women have been shown to have a higher prevalence of the disease, particularly in conjunction with non-tuberculous mycobacterial infections, despite no known difference in immunologic susceptibility.12
In Cystic Fibrosis (CF), men and women have an equal prevalence given its autosomal recessive mode of inheritance. However, a gender disparity in CF patients has been reported where women were found to have a median life expectancy that was nearly 3 years shorter than men with a 60% increased risk of death in certain age groups.13 Women with CF have also been shown to have earlier acquisition of Pseudomonas aeruginosa, one of the most common and virulent pathogens in CF, which is associated with an accelerated rate of decline once acquired.14 The etiology of this gender gap remains elusive and several factors such as nutritional status, CF related diabetes (CFRD), pulmonary function, airway microbiology, and sex hormones have been implicated in various studies.13–19
Given that puberty represents the defining point of sex hormone production in young men and women, we set out to determine if this marks a point of change in clinical outcomes between men and women with CF. The high rate of pulmonary exacerbations in adolescents with CF, and the pre-existing registry of outcomes in patients with CF allowed for a unique opportunity to evaluate lung function and exacerbation rate in men and women pre- and post-puberty. We performed a cohort study using the United States Cystic Fibrosis Foundation Patient Registry (CFFPR) to determine if the decline in lung function or rate of pulmonary exacerbations changes differentially in men versus women with CF after puberty versus pre-puberty while adjusting for important variables known to impact outcomes. Our hypothesis was that lung function would decline and rate of pulmonary exacerbations would increase in CF women relative to men after puberty.
MATERIALS AND METHODS
Study Population
We studied individuals between the ages of 9 and 19 registered in the CFFPR over a 30-year time period, between January 1, 1978 through December 31, 2007. The US CFFPR contains demographic and clinical data on patients receiving care at CFF-accredited centers in the United States. A description of the database has been previously published. Briefly, with patient consent, CFF-accredited care centers complete standardized data at every CF patient visit and annually. The CFFPR captures over 90% of CF patients in the United States.20
Assessment of Age of Puberty
Given the lack of data on age of menarche or tanner stages in the data base, peak height velocity (PHV) was used to determine age of puberty as has been described previously.21,22 The height velocity in centimeters per year was calculated for each subject using all available data between the ages of 9 through 19.
Data Collection
Several demographic and clinical characteristics were examined. Chi-squared or Fisher’s exact test was used for categorical variables and independent groups t-test for continuous variables. Mean age of CF diagnosis, race (Caucasian, other), Cystic Fibrosis Transmembrane conductance Regulator (CFTR) genotype (homozygous for F508delta, heterozygous for F508delta, no F508delta), and mean sweat chloride test value were evaluated. To assess whether advances in CF management impacted post-pubertal decline in lung function, the study population was subdivided into birth cohorts 1978–1987 and 1988–1997. We also looked at possible influences of CFRD, pancreatic insufficiency, and P. aeruginosa infection on outcomes given that these variables are known to impact outcomes in CF. CFRD, pancreatic insufficiency and P. aeruginosa acquisition after the age of 19 was considered negative at puberty. A subject was considered to have CFRD if found to have a positive response for diabetes or CFRD in annual data set prior to age 19. P. aeruginosa was defined as positive if subject ever had two or more positive results entered in the registry.
Analysis
The primary outcomes of interest were average percent predicted forced expiratory volume in 1 second (FEV1%). and number of pulmonary exacerbations per year pre- and post-puberty. FEV1 was analyzed as percent predicted. A pulmonary exacerbation was defined as a change in respiratory status that required treatment with intravenous antibiotics and/or was called a “pulmonary exacerbation” on the CFFPR encounter form. Lung function 10 years pre- and post-peak height velocity was examined and FEV1% and rate of exacerbations before and after puberty were compared between males and females.
Average lung function and number of exacerbations per year before and after puberty were calculated for each subject, respectively. The differences in outcomes between males and females were determined using linear regression with the primary outcomes adjusted for covariates including race, age of puberty, CFRD, pancreatic insufficiency, mean sweat chloride test, genotype, birth cohort, P. aeruginosa acquisition, and BMI Z-score, which was based on National Center for Health Statistics reference curves and computed using the anthropometric statistical package of the Center for Disease Control.23
Univariate analysis of each variable for pulmonary exacerbation was done first. Covariates with P < 0.05 in the univariate analysis were included in the multivariate model. The rate of pulmonary exacerbations was log transformed due to the non-normal distribution. Thus, the exponentiated regression coefficients were the estimates of rate ratios. The analysis for the rate of pulmonary exacerbations was reported as rate ratios with a 95% confidence interval (95% CI). Rate ratios of greater than one indicate an increase in rate of exacerbations.
A P-value < 0.05 was considered statistically significant for all analyses. Analysis was performed using SAS 9.1 (SAS Institute Inc., Cary, NC). The Institutional Review Board at Washington University and subsequently the University of Texas Southwestern Medical Center approved the study.
RESULTS
Cohort Characteristics
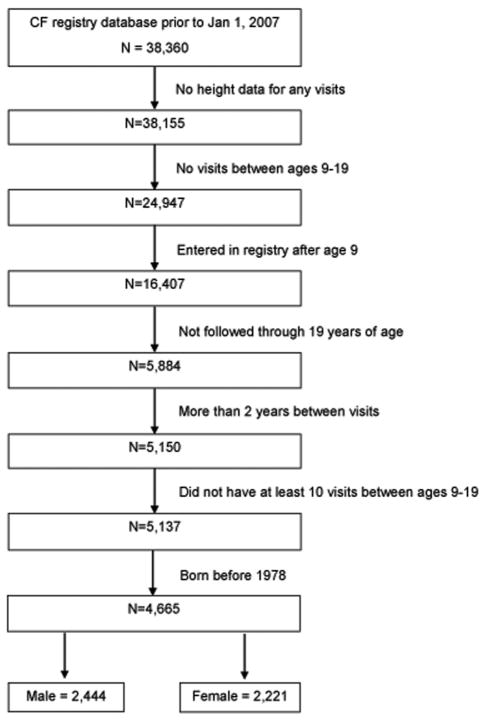
This study was done to assess whether puberty impacts lung function decline in CF patients and to compare rates of exacerbation pre- versus post-puberty. The cohort contained 38,360 patients registered in the CF database between January 1, 1978 through December 31, 2007 (Fig. 1). Since peak height velocity was the primary marker for determining age of puberty, we excluded patients with no height data for any visits (205) and those with no visits during the ages of 9–19 (13,208). Of the remaining 24,947 patients, we then excluded those who entered the registry after age 9 (8,540) or those who were not followed through 19 years of age (10,523) in order to capture those with early or late onset puberty. We also excluded patients with more than 2 years between visits (734) and those that did not have at least 10 visits between ages 9–19 (13) in order to ensure sufficient height information between the ages of 9 and 19 to accurately assess age of puberty. Lastly, because we excluded subjects without data between the ages of 9–19, severely ill patients who died in the teenage years may have been excluded. Given that the median survival in CF in 1966 was age 10 and in 1972 was 17, we excluded the earliest cohort in the registry, those entered prior to January 1, 1978.24 The final study group consisted of 4,665 patients, with 2,444 males and 2,221 females.
Fig. 1.
Flow chart of male and female cohorts from the Cystic Fibrosis Foundation Patient Registry.
The demographic and clinical characteristics of the study population are shown (Table 1). In comparing men versus women, there was no significant difference in age of CF diagnosis, race, mean sweat test value, genotype, prevalence of pancreatic insufficiency, or birth cohort distribution. However, rate of CFRD was significantly higher in females affecting 13.9% while only 8.4% of the males were diabetic. Interestingly, the prevalence of P. aeruginosa was higher in women and men. Finally, the Body Mass Index (BMI) Z-score post-puberty was found to be significantly lower in the women relative to the men.
TABLE 1.
Baseline Characteristics of Male and Female Cohorts
| Males (n = 2,444) | Females (n = 2,221) | P-value | |
|---|---|---|---|
| Caucasian, n (%) | 2,340 (95.7) | 2,125 (95.7) | 0.91 |
| Sweat test, mean ± SD | 103 ± 17 | 103 ± 18 | 0.32 |
| Age of diagnosis, mean ± SD1 | 18.0 ± 32.2 | 18.5 ± 32.9 | 0.56 |
| Genotype, n (%) | 0.44 | ||
| No F508delta | 169 (8.3) | 165 (8.7) | |
| Heterozygous for F508delta | 741 (36.3) | 648 (34.3) | |
| Homozygous for F508delta | 1,134 (55.4) | 1,075 (56.9) | |
| Date of birth, n (%) | 0.78 | ||
| 1988–1997 | 237 (9.7) | 210 (9.5) | |
| 1978–1987 | 2,207 (90.3) | 2,011 (90.5) | |
| Pancreatic insufficient, n (%)2 | 2,186 (89.4) | 1,985 (89.4) | 0.94 |
| P. aeruginosa, n (%)2 | 2,287 (93.6) | 2,108 (94.9) | 0.05 |
| Body mass index (Z-score), mean ± SD3 | |||
| Pre-puberty | −0.33 ± 0.87 | −0.36 ± 0.85 | 0.33 |
| Post-puberty4 | −0.78 ± 1.35 | −0.42 ± 0.91 | <0.001 |
| CF-related diabetes, n (%)2 | 204 (8.4) | 308 (13.9) | <0.001 |
| FEV1%, n (%)4 | 0.55 | ||
| ≥90% | 1,136 (47.2) | 1,021 (47.0) | |
| 70–89% | 868 (36.1) | 764 (35.1) | |
| 40–69% | 376 (15.6) | 357 (16.4) | |
| <40% | 26 (1.1) | 32 (1.5) | |
Age of diagnosis in months.
Pancreatic insufficient, P. aeruginosa, CF-related diabetes diagnosed after age 19 were considered negative at puberty.
Body mass index Z-scores calculated using the anthropometric statistical package of the Centers for Disease Control pre- and post-puberty.
FEV1%, percent predicted forced expiratory volume in the first second pre-puberty.
Distribution of Peak Height Velocity
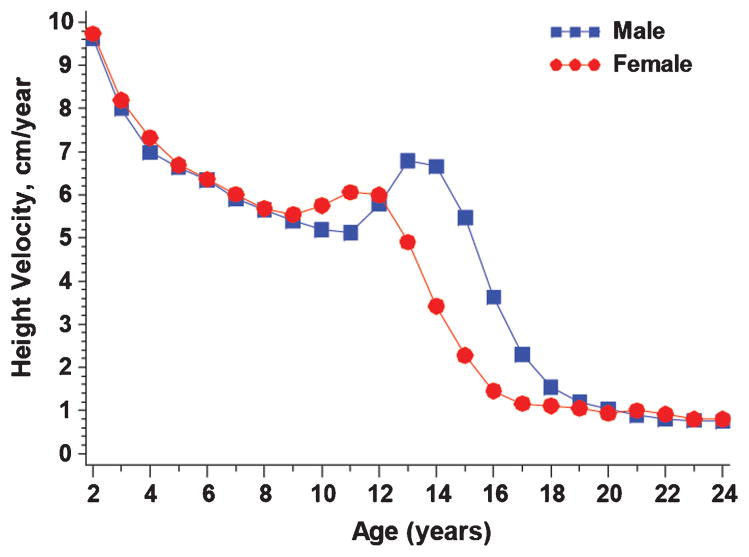
Using peak height velocity as the marker of puberty, average age of puberty in males with cystic fibrosis was 13.1 ± 2.1 and that in females was 11.1 ± 1.9 (Fig. 2). There was a significant difference in the average ages of puberty between the two genders, however this is similar to the ages of puberty for males and females in the general population found in other reports to be 13.4 in boys and 11.8 in girls using peak height velocity.25,26
Fig. 2.
Distribution of peak height velocity. Shown is height velocity (cm/year) in males versus females from age 2 through 24 years of age.
Lung Function and Pulmonary Exacerbation Rate Before and After Puberty
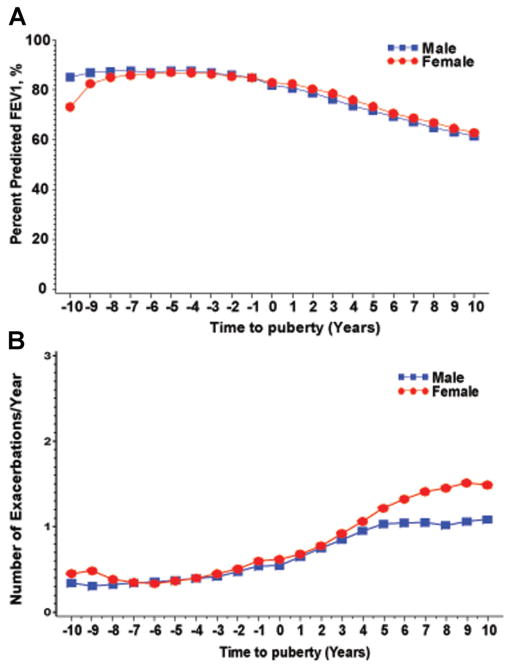
FEV1% and average number of pulmonary exacerbations in men versus women were examined before and after the onset of puberty (Fig. 3). Time 0 marks the age of puberty. FEV1% was similar in both genders pre-puberty and did not change significantly after puberty (87.0 in males and 86.6 in females pre-puberty, P = 0.42; 72.5 in males and 72.5 in females post-puberty, P = 0.16; Table 2). The rate of FEV1% decline did become more steep in the women post-puberty than pre-puberty relative to then men, changing from −0.62 to −2.27 in males to −0.27 to −2.37 in females, but was not statistically different. In addition, females demonstrated a significantly higher number of exacerbations after puberty with a mean of 1.23 ± 1.46 exacerbations per year, as opposed to 0.99 ± 1.38 exacerbations per year in males.
Fig. 3.
Lung function and pulmonary exacerbation rate before and after puberty. (A) Average percent predicted FEV1 in males and females 10 years before puberty and 10 years after puberty. Time 0 marks age of puberty per subject per cohort. (B) Mean number of pulmonary exacerbations per year in males and females 10 years before puberty and 10 years after puberty. Time 0 marks age of puberty per subject per cohort.
TABLE 2.
Lung Function and Pulmonary Exacerbation Rate Pre- and Post-Puberty
| Males (n = 2,444) | Females (n = 2,221) | P-value | |
|---|---|---|---|
| FEV1%, n | |||
| Pre-puberty | 87.0 ± 17.6 | 86.6 ± 18.7 | 0.42 |
| Post-puberty | 72.5 ± 21.4 | 72.5 ± 20.5 | 0.16 |
| Change in FEV% per year | |||
| Pre-puberty | −0.62 ± 2.96 | −0.27 ± 3.90 | 0.002 |
| Post-puberty | −2.27 ± 4.40 | −2.37 ± 5.00 | 0.49 |
| Pulmonary exacerbations, n (%) | |||
| Pre-puberty | 0.41 ± 0.73 | 0.45 ± 0.76 | 0.10 |
| Post-puberty | 0.99 ± 1.38 | 1.23 ± 1.46 | < 0.001 |
Univariate Analysis of Pulmonary Exacerbations
Annual number of pulmonary exacerbations before and after puberty was calculated for each subject based on the individual’s age of puberty. The difference in outcomes between males and females was determined using linear regression. In males, the number of exacerbations per year post puberty was 0.24 lower than that seen in females (Table 2). There was no association between annual number of exacerbations and race, pancreatic insufficiency or genotype (Table 3). The rate of exacerbations for cystic fibrosis patients with diabetes was higher annually compared to those without diabetes (2.32, 95% CI: 1.94–2.77, P < 0.001). A significantly higher rate of exacerbations was also noted in patients infected with P. aeruginosa (7.1, 95% CI: 5.61–8.99). The study population was divided into two birth cohorts at 10-year intervals; 1978–1987 and 1988–1997. Using the 1968–1977 cohort as the reference group, it was noted that the annual rate of exacerbation was lower in the later birth cohort. This could represent the advances in management of cystic fibrosis over time. A higher BMI was associated with fewer numbers of exacerbations per year. We also found that a higher number of exacerbations pre-puberty was a potential predictor of higher rate of exacerbations in the post-pubertal period.
TABLE 3.
Univariate Analysis of Pulmonary Exacerbations Per Year After Puberty
| Rate ratio (95% CI) | P-value | |
|---|---|---|
| Gender: female | 1.40 (1.25–1.57) | <0.001 |
| Caucasian race | 1.31 (0.99–1.73) | 0.06 |
| Sweat test | 1.006 (1.003–1.010) | <0.001 |
| Age of CF diagnosis | 0.996 (0.994–0.998) | <0.001 |
| Genotype | ||
| No F508delta | Ref. | |
| Heterozygous F508delta | 1.21 (0.95–1.53) | 0.12 |
| Homozygous F508delta | 1.25 (1.00–1.57) | 0.05 |
| Date of birth cohort (1988–1997 vs. 1978–1987) | 0.74 (0.61–0.89) | 0.002 |
| Pancreatic insufficiency | 1.17 (0.98–1.41) | 0.09 |
| P. aeruginosa infection | 7.1 (5.61–8.99) | <0.001 |
| Pre-puberty BMI Z-score | 0.68 (0.63–0.72) | <0.001 |
| CFRD | 2.32 (1.94–2.77) | <0.001 |
| Age of puberty | 1.04 (1.01–1.06) | 0.006 |
| Pre-puberty pulmonary exacerbations per year | 1.59 (1.56–1.64) | <0.001 |
Multivariate Analysis of Pulmonary Exacerbations
All potential risk factors that were found to be significant were then analyzed using a multivariate model. Adjustment for all of the other risk factors, alone or in combination, did not reduce the gender-related relative risk for pulmonary exacerbations from its unadjusted value. In this fully adjusted model, age of puberty, CFRD, P. aeruginosa infection, average BMI pre-puberty and average number of exacerbations pre-puberty were all significantly associated with an increase in the number of exacerbations per year (Table 4).
TABLE 4.
Multivariate Analysis of Pulmonary Exacerbations Per Year After Puberty
| Rate ratio (95% CI) | P-value | |
|---|---|---|
| Gender: female | 1.48 (1.32–1.66) | <0.001 |
| Sweat test | 1.003 (1.000–1.006) | 0.039 |
| Age of CF diagnosis, month | 0.997 (0.995–0.999) | 0.002 |
| Age of puberty, years | 1.04 (1.01–1.07) | 0.004 |
| CF-related diabetes | 1.72 (1.46–2.02) | <0.001 |
| P. aeruginosa | 4.20 (3.38–5.23) | <0.001 |
| BMI Z-score, pre-puberty | 0.80 (0.75–0.85) | <0.001 |
| Pre-puberty pulmonary exacerbations per year | 1.53 (1.49–1.57) | <0.001 |
DISCUSSION
Our data show a significant increase in rate of pulmonary exacerbations in CF women post-puberty relative to men. The separation appears to begin approximately 6 years after the onset of puberty. We did not find a change in FEV1% in women post-puberty, which may be due to the fact that the difference in rate of decline in FEV1% over time is not sufficient to detect a difference in FEV1% over a 10-year observation period. We did, however, note a trend toward a steeper decline in FEV1% over time post-puberty in women relative to men. We also showed that the prevalence of P. aeruginosa, a major contributor to morbidity and mortality in CF, was higher in women relative to men, as has been suggested previously.14 Finally, we show that the age of puberty in CF patients is not notably different than that of the general population when peak height velocity is used as the marker of puberty.26
We acknowledge that there may be several explanations for our findings to consider. First, women may seek care more commonly than men resulting in an increased rate of diagnosis of exacerbations, and not a true difference in disease. However, we did assess number of visits in men versus women and found both groups to have a median number of 10 visits in the 10 years post-puberty. Second morphometric features including body habitus and airway size may lead to differences in outcome, however, we have controlled for BMI using Z-scores and still found a predisposition toward exacerbations in women. Third, differences in adherence to medical therapy that occurs particularly during late adolescence and early adulthood may result in the increased rate of pulmonary exacerbations in women. CF studies have not thoroughly addressed gender based differences in adherence, however, a previous study did show that gender was not a significant factor for treatment adherence in CF.27 Finally, though we cannot infer a causal relationship from our study, there remains the possibility that sex hormones modulate components of the mucociliary apparatus and immune function, contributing to a gender disparity in rate of pulmonary exacerbations remains.
Though the mechanism by which women with CF have increased pulmonary exacerbations and worse outcomes than men is unknown, the role of sex hormones including estrogen and progesterone remains a viable explanation. There is a host of in vitro data and some in vivo data to suggesting that estrogen and progesterone modulate factors involved in airway clearance and host defense. Estrogen and progesterone receptors are known to be present in the lungs. Estrogen has been shown to decrease airway surface liquid in airway epithelium via modulation of CFTR, non CFTR chloride channels, ENaC (Epithelial sodium channel) and other sodium channels on airway epithelial cells.28–31 Estrogen has also been shown to increase mucin expression the airways, particulary Muc 5B.32 Recent work also showed that estrogen promotes conversion of P. aeruginosa from a non-mucoid to mucoid phenotype and that CF exacerbations in women peak at point of high estrogen in the ovulatory cycle.19 In addition, progesterone has been shown to decrease cilia beat frequency in human airway epithelial cells by approximately 40%, all of which may contribute to impaired mucociliary clearance.33 While estrogen may act as a proinflammatory agent by increasing IL-17 and neutrophil counts in the lungs of mice infected with P. aeruginosa, conflicting data has shown a decrease in IL-8 production from airway epithelium in the presence of estrogen.34 Recent human studies have also shown incongruent outcome data on use of oral contraceptive pills in women with CF.19,35,36 Therefore, the direct relationship between sex hormones such as estrogen and progesterone on respiratory indices remains unknown without a prospective study evaluating this in detail.
Given the retrospective nature of this study, it does have inherent limitations. There was not information available regarding age of menarche, tanner stages, or hormone levels in females or males to confirm age of puberty. We defined puberty as the point of peak height velocity (PHV) between the ages of 9 through 19, based on previous studies.37,38 Timing of puberty has a 4- to 5-year variation in the general population and the marker used to define this has been varied.39,40 In girls, recording breast development can be imprecise as breast development may be stimulated independent of pituitary-gonadal activation.40 Age of menarche has been shown to only moderately correlate with breast and pubic hair development and is therefore also imprecise.41 In boys, enlargement of the testicular volume to greater than 4 ml is traditionally the first marker of puberty, however this measurement has inherent inter-observer variability. Self estimation questionnaires have also been relatively inaccurate.42 PHV is an objective biological maturity indicator and reflects the maximum velocity in stature during adolescence. CF patients have historically been reported to have a delay in average age of puberty relative to other adolescents. This pubertal delay is most marked in patients homozygous for F508delta and those with abnormal oral glucose tolerance tests.43 This maturational lag has also been correlated with a delay in attaining pubertal levels of insulin-like growth factor, luteinizing hormone, follicle-stimulating hormone, poor nutritional status, and sex steroid hormones.44 We found the average age of puberty in this CF cohort to be very similar to the general population.21 Because we aimed to focus this study on the impact of puberty on CF exacerbations and lung function, we excluded patients not followed through ages 9–19 in the registry. This may have created a bias in the data by excluding evaluation of very sick patients who died before the age of 19 and should be noted in the interpretation of these data.
Despite the limitations, our data shows a higher rate of pulmonary exacerbations in CF women relative to men that begins after puberty as well as a higher prevalence of P. aeruginosa, which has been shown to contribute to worse pulmonary outcomes.45 In fact, patients with frequent exacerbations (>1 per year) have an increased 3-year risk of death or lung transplant.46 In addition, adolescence and early adulthood is known to be a time of decline in health in CF.47 Therefore, as patients are approaching this very dynamic and difficult period, it is important for CF providers to keep this health disparity in mind. Understanding the mechanisms behind this gender disparity and whether or not sex hormones directly alter host defense is an important question remaining to be answered that may affect use of oral contraceptive in CF women and serve as a target for developing new therapies.
Acknowledgments
Cystic Fibrosis Foundation for providing the data sets. American Thoracic Career Development Award (R.J.); Washington University CTSA KL2 RR024994 UL1 RR024992 (R.J.) NIH K08 HL105671 (R.J.).
References
- 1.Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax. 1992;47:537–542. doi: 10.1136/thx.47.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Stewart P, Johansen H, McRae L, Taylor G. Sex difference in hospitalization due to asthma in relation to age. J Clin Epidemiol. 2003;56:180–187. doi: 10.1016/s0895-4356(02)00593-0. [DOI] [PubMed] [Google Scholar]
- 3.de Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162:68–74. doi: 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- 4.Nicolai T, Pereszlenyiova-Bliznakova L, Illi S, Reinhardt D, von Mutius E. Longitudinal follow-up of the changing gender ratio in asthma from childhood to adulthood: role of delayed manifestation in girls. Pediatr Allergy Immunol. 2003;14:280–283. doi: 10.1034/j.1399-3038.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 5.Venn A, Lewis S, Cooper M, Hill J, Britton J. Questionnaire study of effect of sex and age on the prevalence of wheeze and asthma in adolescence. BMJ. 1998;316:1945–1946. doi: 10.1136/bmj.316.7149.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992;268:3437–3440. [PubMed] [Google Scholar]
- 7.Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11:24. doi: 10.1186/1472-6874-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trawick DR, Holm C, Wirth J. Influence of gender on rates of hospitalization, hospital course, and hypercapnea in high-risk patients admitted for asthma: a 10-year retrospective study at Yale-New Haven Hospital. Chest. 2001;119:115–119. doi: 10.1378/chest.119.1.115. [DOI] [PubMed] [Google Scholar]
- 9.Birring SS, Brightling CE, Bradding P, Bradding P, Entwisle JJ, Vara J, Grigg AJ, Wardlaw P, Pavord ID. Clinical, radiologic, and induced sputum features of chronic obstructive pulmonary disease in nonsmokers: a descriptive study. Am J Respir Crit Care Med. 2002;166:1078–1083. doi: 10.1164/rccm.200203-245oc. [DOI] [PubMed] [Google Scholar]
- 10.Celli B, Vestbo J, Jenkins CR, Jones PW, Ferguson GT, Calverley PM, Yates JC, Anderson JA, Willits LR, Wise RA Investigators of the TORCH Study. Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease. The TORCH experience. Am J Respir Crit Care Med. 2011;183:317–322. doi: 10.1164/rccm.201004-0665OC. [DOI] [PubMed] [Google Scholar]
- 11.Han MK, Postma D, Mannino ND, Giardino S, Buist JL, Curtis DM, Martinez FJ. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176:1179–1184. doi: 10.1164/rccm.200704-553CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrissey BM, Harper RW. Bronchiectasis: sex and gender considerations. Clin Chest Med. 2004;25:361–372. doi: 10.1016/j.ccm.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol. 1997;145:794–803. doi: 10.1093/oxfordjournals.aje.a009172. [DOI] [PubMed] [Google Scholar]
- 14.Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48:1041–1049. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 15.Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care. 2005;28:2141–2144. doi: 10.2337/diacare.28.9.2141. [DOI] [PubMed] [Google Scholar]
- 16.Olesen HV, Pressler T, Hjelte L, Mared L, Lindblad A, Knudsen PK, Laerum BN, Johannesson M Scandinavian Cystic Fibrosis Study Consortium. Gender differences in the Scandinavian cystic fibrosis population. Pediatr Pulmonol. 2010;45:959–965. doi: 10.1002/ppul.21265. [DOI] [PubMed] [Google Scholar]
- 17.Nick JA, Chacon CS, Brayshaw SJ, Jones MC, Barboa CM, St Clair CG, Young RL, Nichols DP, Janssen JS, Huitt GA, et al. Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2010;182:614–626. doi: 10.1164/rccm.201001-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma N, Bush A, Buchdahl R. Is there still a gender gap in cystic fibrosis? Chest. 2005;128:2824–2834. doi: 10.1378/chest.128.4.2824. [DOI] [PubMed] [Google Scholar]
- 19.Chotirmall SH, Smith SG, Gunaratnam C, Cosgrove S, Dimitrov BD, O’Neill SJ, Harvey BJ, Greene CM, McElvaney NG. Effect of estrogen on pseudomonas mucoidy and exacerbations in cystic fibrosis. N Engl J Med. 2012;366:1978–1986. doi: 10.1056/NEJMoa1106126. [DOI] [PubMed] [Google Scholar]
- 20.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 21.Abbassi V. Growth and normal puberty. Pediatrics. 1998;102:507–511. [PubMed] [Google Scholar]
- 22.Aswani N, Taylor CJ, McGaw J, Pickering M, Rigby AS. Pubertal growth and development in cystic fibrosis: a retrospective review. Acta Paediatr. 2003;92:1029–1032. [PubMed] [Google Scholar]
- 23.Centers for Disease Control Division of Nutrition. Anthropometric statistical package, batch/entry file conversion program. Atlanta, GA: Agency for International Development Office of Nutrition; 1990. (Publication no. RSSA #BST-1064-12-HC-2174-03) [Google Scholar]
- 24.Warwick WJ, Pogue RE, Gerber HU, Nesbitt CJ. Survival patterns in cyctic fibrosis. J Chronic Dis. 1975;28:609–622. doi: 10.1016/0021-9681(75)90074-0. [DOI] [PubMed] [Google Scholar]
- 25.Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- 26.Iuliano-Burns S, Mirwald RL, Bailey DA. Timing and magnitude of peak height velocity and peak tissue velocities for early, average, and late maturing boys and girls. Am J Hum Biol. 2001;13:1–8. doi: 10.1002/1520-6300(200101/02)13:1<1::AID-AJHB1000>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Masterson TL, Wildman BG, Newberry BH, Omlor GJ. Impact of age and gender on adherence to infection control guidelines and medical regimens in cystic fibrosis. Pediatr Pulmonol. 2010;46:295–301. doi: 10.1002/ppul.21366. [DOI] [PubMed] [Google Scholar]
- 28.Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, Fricks I, Young SL, Tarran R. 17beta-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest. 2008;118:4025–4035. doi: 10.1172/JCI33893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AK, Schultz BD, Katzenellenbogen JA, Price EM, Bridges RJ, Bradbury NA. Estrogen inhibition of cystic fibrosis transmembrane conductance regulator-mediated chloride secretion. J Pharmacol Exp Ther. 2000;295:195–204. [PubMed] [Google Scholar]
- 30.Sweezey N, Tchepichev S, Gagnon S, Fertuck K, O’Brodovich H. Female gender hormones regulate mRNA levels and function of the rat lung epithelial Na channel. Am J Physiol. 1998;274:C379–C386. doi: 10.1152/ajpcell.1998.274.2.C379. [DOI] [PubMed] [Google Scholar]
- 31.Laube M, Kuppers E, Thome UH. Modulation of sodium transport in alveolar epithelial cells by estradiol and progesterone. Pediatr Res. 2011;69:200–205. doi: 10.1203/PDR.0b013e3182070ec8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi HJ, Chung YS, Kim HJ, Moon UY, Choi YH, Van Seuningen I, Baek SJ, Yoon HG, Yoon JH. Signal pathway of 17beta-estradiol-induced MUC5B expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2009;40:168–178. doi: 10.1165/rcmb.2007-0377OC. [DOI] [PubMed] [Google Scholar]
- 33.Jain R, Ray JM, Pan JH, Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol. 2011;46:446–453. doi: 10.1165/rcmb.2011-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chotirmall SH, Greene CM, Oglesby IK, Thomas W, O’Neill SJ, Harvey BJ, McElvaney NG. 17Beta-estradiol inhibits IL-8 in cystic fibrosis by up-regulating secretory leucoprotease inhibitor. Am J Respir Crit Care Med. 2010;182:62–72. doi: 10.1164/rccm.201001-0053OC. [DOI] [PubMed] [Google Scholar]
- 35.Fitzpatrick SB, Stokes DC, Rosenstein BJ, Terry P, Hubbard VS. Use of oral contraceptives in women with cystic fibrosis. Chest. 1984;86:863–867. doi: 10.1378/chest.86.6.863. [DOI] [PubMed] [Google Scholar]
- 36.Kernan NG, Alton EW, Cullinan P, Griesenbach U, Bilton D. Oral contraceptives do not appear to affect cystic fibrosis disease severity. Eur Respir J. 2012;41:67–73. doi: 10.1183/09031936.00018712. [DOI] [PubMed] [Google Scholar]
- 37.Delemarre-van de Waal HA, van Coeverden SC, Rotteveel J. Hormonal determinants of pubertal growth. J Pediatr Endocrinol Metab. 2001;14:1521–1526. [PubMed] [Google Scholar]
- 38.Klentrou P, Ludwa IA, Falk B. Factors associated with bone turnover and speed of sound in early and late-pubertal females. Appl Physiol Nutr Metab. 2011;36:707–714. doi: 10.1139/h11-085. [DOI] [PubMed] [Google Scholar]
- 39.Tanner JM, O’Keeffe B. Age at menarche in Nigerian school girls, with a note on their heights and weights from age 12 to 19. Hum Biol. 1962;34:187–196. [PubMed] [Google Scholar]
- 40.Wehkalampi K, Silventoinen K, Kaprio J, Dick DM, Rose RJ, Pulkkinen L, Dunkel L. Genetic and environmental influences on pubertal timing assessed by height growth. Am J Hum Biol. 2008;20:417–423. doi: 10.1002/ajhb.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biro FM, Huang B, Crawford PB, Lucky AW, Striegel-Moore R, Barton BA, Daniels S. Pubertal correlates in black and white girls. J Pediatr. 2006;148:234–240. doi: 10.1016/j.jpeds.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Hergenroeder AC, Hill RB, Wong WW, Sangi-Haghpeykar H, Taylor W. Validity of self-assessment of pubertal maturation in African American and European American adolescents. J Adolesc Health. 1999;24:201–205. doi: 10.1016/s1054-139x(98)00110-4. [DOI] [PubMed] [Google Scholar]
- 43.Johannesson M, Gottlieb C, Hjelte L. Delayed puberty in girls with cystic fibrosis despite good clinical status. Pediatrics. 1997;99:29–34. doi: 10.1542/peds.99.1.29. [DOI] [PubMed] [Google Scholar]
- 44.Arrigo T, Rulli I, Sferlazzas C, De Luca F. Pubertal development in cystic fibrosis: an overview. J Pediatr Endocrinol Metab. 2003;16:267–270. [PubMed] [Google Scholar]
- 45.Buzzetti R, Alicandro G, Minicucci L, Notarnicola S, Furnari ML, Giordano G, Lucidi V, Montemitro E, Raja V, Magazzu G, et al. Validation of a predictive survival model in Italian patients with cystic fibrosis. J Cyst Fibros. 2012;11:24–29. doi: 10.1016/j.jcf.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 46.de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, Paterson N, Jackson M, Lougheed MD, Kumar V, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66:680–685. doi: 10.1136/thx.2011.161117. [DOI] [PubMed] [Google Scholar]
- 47.Vandenbranden SL, McMullen A, Schechter MS, Pasta DJ, Michaelis RL, Konstan MW, Wagener JS, Morgan WJ, McColley SA Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Lung function decline from adolescence to young adulthood in cystic fibrosis. Pediatr Pulmonol. 2012;47:135–143. doi: 10.1002/ppul.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]