Introduction
KEY TEACHING POINTS
|
Long QT syndrome (LQTS) is a disorder of cardiac repolarization characterized by a prolonged QT interval on electrocardiogram (ECG). LQTS usually is referred to as a monogenic disorder.1 QT interval in the population is variable,2 which leads to many “borderline” cases and makes definitive diagnosis difficult.1 The utility of clinical genetic screening in congenital LQTS has been demonstrated,3 although issues remain with variants of unknown significance (VUS), false positives, and false negatives.4 The study and diagnosis of LQTS are additionally complicated by a high degree of variable penetrance, making genotyping a suboptimal predictor of clinical outcome and complicating risk stratification.5 Genetic testing companies provide some guidance on the relevance of the mutations identified. In the genetic screening performed in this study through FAMILION, mutations are classified as class I mutations, including deleterious and probable deleterious mutants. Class II are possible deleterious mutations or mutations of unknown significance (VUS), and class III are polymorphisms.
Most reported cases of congenital LQTS have a single affected allele.3 Here we describe a family in which only the proband presented with LQTS clinically, but genetic testing revealed that all 5 family members studied carried at least 1 of 3 potential LQT mutations: 2 LQT2 mutations in the potassium channel hERG (class I mutation: S654G; class II mutation: A913V) and 1 LQT3 mutation in the cardiac sodium channel SCN5A (class I mutation: F1596I). Our objective was to use functional characterization and computational modeling to determine the contribution to the LQT phenotype of 3 potential LQT mutations identified in a single lineage. We hypothesized this family presents a polygenic case of LQTS in which an additive effect of the mutations leads to the LQT phenotype.
Case report
Subjects completed a questionnaire that included general health information, history of syncope, and medications. Resting 12-lead ECGs were obtained. Symptom-limited exercise tests were performed by the proband and his 2 sisters. QT interval was measured in multiple leads and corrected for heart rate using the Bazett formula.
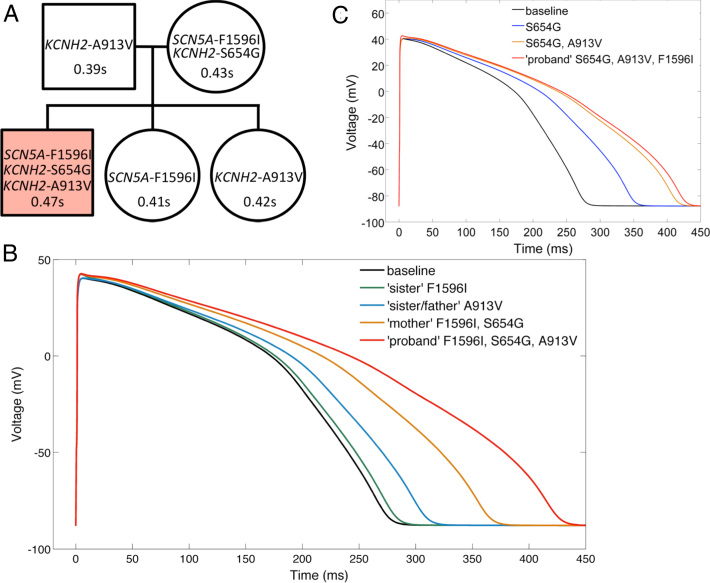
The proband in this study is a healthy 15-year-old boy with no history of syncope diagnosed with LQTS after presport ECG screening. He currently is symptom-free on propranolol. Neither the proband nor any of his family members had any history of syncope, disease, or chronic medication use. Baseline resting ECG parameters and genetic mutations are listed in Table 1 and shown in Figure 3A. Multiple resting ECGs of the proband recorded before his referral to this center showed QTc as high as 0.48 second. On presentation, QTc was 0.47 second. On exercise test, QTc was 0.45 second lying and 0.46 second standing. QTc shortened during exercise and gradually prolonged back to 0.47 second at the end of recovery. T waves showed slight notching.
Table 1.
Clinical features of the family studied
| Age (years) | Sex | Mutations | Heart rate (bpm) | QT (seconds) | QTc (seconds) | |
|---|---|---|---|---|---|---|
| Proband | 15 | M | SCN5A-F1596I | 93 | 0.38 | 0.47 |
| hERG-S654G | ||||||
| hERG-A913V | ||||||
| Mother | 39 | F | SCN5A-F1596I | 71 | 0.39 | 0.43 |
| hERG-S654G | ||||||
| Father | 40 | M | hERG-A913V | 61 | 0.29 | 0.39 |
| Sister 1 | 13 | F | SCN5A-F1596I | 83 | 0.35 | 0.41 |
| Sister 2 | 7 | F | hERG-A913V | 83 | 0.36 | 0.42 |
Figure 3.
In silico effect of mutations on action potential duration (APD). A: Family tree of affected long QT (LQT) lineage. QTc at screening is indicated in seconds. Shading denotes the long QT syndrome (LQTS)–diagnosed proband carrying all 3 mutations. B: Predicted effect of family mutation status on APD. C: Isolating the effect of hERG-S654G and both hERG mutations from the proband’s simulated action potential shows all but approximately 15 ms of the prolongation is attributable to hERG-S654G and hERG-A913V together.
Sister 1 (SCN5A-F1596I) had normal QTc at baseline (0.41 second) and with both lying (0.40 second) and standing (0.44 second). There was no significant prolongation of QTc during exercise or recovery. T-wave morphology was normal. Sister 2 (KCNH2-A013V) had normal QTc at baseline (0.42 second). With lying and standing, QTc increased from 0.42 to 0.46 second. QTc increased to 0.48 second during early exercise and reached 0.47 second during recovery. T waves showed notching. The T waves of the mother and father showed normal morphology.
Genetic testing was performed using the FAMILION test (Transgenomic Inc, New Haven, CT). The proband was tested for mutations in the genes implicated in LQT1-13. Family members were tested for the known class I and II mutations reported in the proband. Genetic testing revealed that every member carries at least 1 class I or class II mutation, although only the proband displays a prolonged QTc (Figure 3A). The KCNH2/hERG-S654G mutation is classified by the FAMILION genetic testing as class I. hERG-A913V is scored as class II by FAMILION. Both mutations have been potentially linked to arrhythmia, although they have not been functionally characterized.6, 7 SCN5A-F1596I was scored as class I by FAMILION. It has been potentially linked with arrhythmia but not shown to be pathogenic.8 In order to assess which mutation(s) is at the source of the LQT phenotype and identify which family members may be at risk, functional characterization of the mutations was performed (see Online Supplementary Material for detailed methods of the functional characterization and in silico modeling).
Functional analysis of SCN5A-F1596I
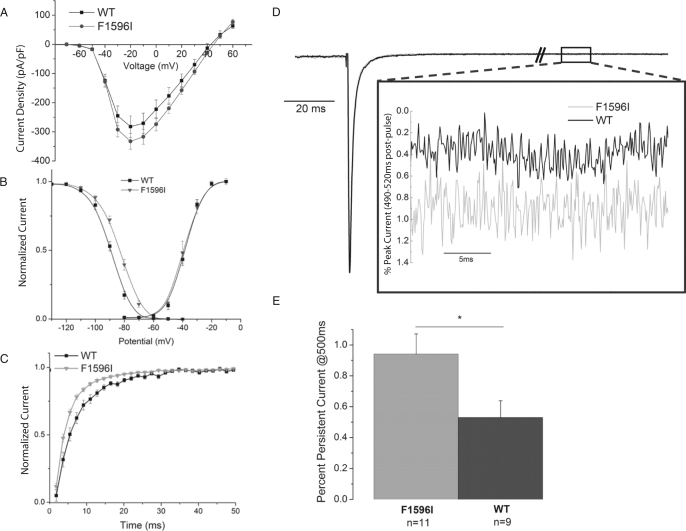
The SCN5A-F1596I mutation was expressed and characterized in HEK293 cells (Table 2). Peak current density was equal to wild type (WT; Figure 1A). Steady-state inactivation shifted slightly +4.9 mV (Figure 1B). Activation V0.5 and k were unchanged (Figure 1B). The time constant of recovery from inactivation was faster than WT (Figure 1C). Persistent current of SCN5A-F1596I relative to peak current (0.9%) was increased compared to WT (0.5%; Figures 1D and 1E). These changes, although modest, are the signature of an LQT3 mutation.
Table 2.
SCN5A biophysical properties
| n | Steady-state inactivation V0.5 (mV) | Steady-state inactivation slope factor k | Activation V0.5 (mV) | Activation slope factor k | Recovery from inactivation τ (ms) | |
|---|---|---|---|---|---|---|
| WT | 7 | –81.6 ± 0.9 | 5.9 ± 0.1 | –37.9 ± 3.1 | 5.1 ± 0.5 | 5.1 ± 0.2 |
| F1596I | 11 | –86.5 ± 1.1* | 6.2 ± 0.2 | –37.8 ± 6.4 | 4.2 ± 0.5 | 3.7 ± 0.4* |
P <.05 compared to wild type (WT).
Figure 1.
Biophysical properties of SCN5A-F1596I. A: Current–voltage relationship of wild type (WT; n = 7) and F1596I (n = 11). B: Steady-state activation and inactivation C: Recovery from inactivation for F1596I. D: Representative overlaid traces of a persistent current protocol for WT and SCN5A-F1596I channels. The persistent current at 500 ms is shown in the inset.E: Summary data of persistent current experiments.
Functional analysis of hERG mutations
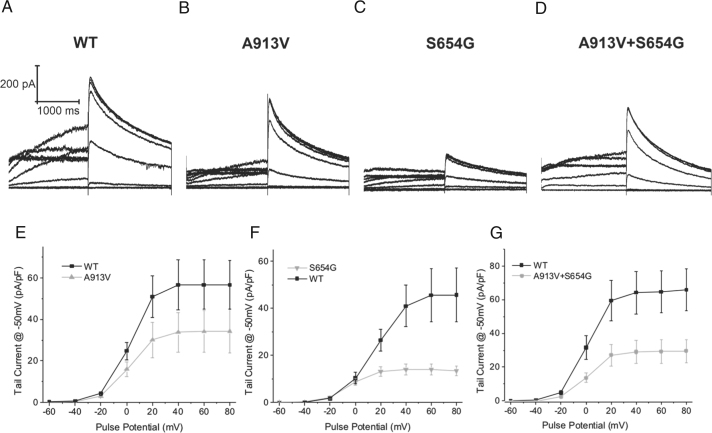
hERG constructs were characterized in HEK293 cells (Figure 2). The hERG-A913V mutation resulted in a 37% decrease in tail current (Figures 2B and 2E). hERG-S654G showed a distinct loss of function, with tail current density decreased approximately 70% (Figures 2C and 2F). Other biophysical properties were not modified for either mutant. Coexpression of both hERG-S654G and hERG-A913V resulted in a decrease in currents consistent with an average effect of both mutations (Figures 2D and 2G).
Figure 2.
Characterization of hERG-A913V and hERG-S654G. A–D: Representative current traces E: Plot of tail current from A913V (n = 12) vs wild type (WT; n = 12). P <.05 for voltages >20 mV. F: Plot of tail current from S654G (n = 9) vs WT (n = 11). P <.05 for voltages >20 mV. G: Plot of tail current from hERG-S654G+hERG-A913V (n = 11) vs WT (n = 12). P <.05 for voltages >0 mV.
Simulated effect of mutations on action potential duration
To further explore which mutation(s) was needed to explain the presence of LQT in the proband, we simulated the effects of the mutations on the human ventricular action potential in silico.9 SCN5A-F1596I was defined as an 80% increase in late current, a +5-mV shift in steady-state inactivation, and a 40% change in the time constant of recovery of inactivation. KCNH2-A913V was defined as a 37% decrease in conductance for IKr. KCNH2-S654G was defined as a 73% decrease of conductance in IKr. All changes were adjusted to account for heterozygosity. At baseline, the model gives an action potential duration at 90% repolarization (APD90) of approximately 265 ms. The sister’s SCN5A-F1596I genotype gives a slight prolongation of APD90 by <10 ms. The father/sister hERG-A913V gives an approximately 30-ms prolongation of APD90. The mother’s genotype, both SCN5A-F1596I and KCNH2-S654G, increased APD90 by approximately 75 ms. Finally, simulating the proband gave the longest APD90 at approximately 420 ms, increased by approximately 160 ms (Figure 3B).
To assess what combination of mutations is the key factor in the LQT phenotype seen only in the proband, we also modeled the isolated effect of hERG-S654G and then of both hERG mutations combined (Figure 3C). This combination leads to a prolongation of 145 ms, about 15 ms short of the proband’s predicted APD, demonstrating that his LQT phenotype is due mainly to the dual hERG mutant genotype. Because no other family members presented with this unlucky combination, our results suggest this combination explains the LQTS seen in the proband, who therefore presents with a polygenic case of LQTS.
Discussion
The decrease in IKr seen with hERG-S654G is consistent with an LQT2 mutation.2, 10 It had a significant impact on simulated APD (Figure 3C). Based on these data, we concur with the classification of this mutation as a class I and believe that the presence of this mutation alone could be sufficient for LQTS. However, no family members had that genotype, and even the mother who carries this mutation along with the SCN5A mutant does not have LQT.
The hERG-A913V mutation produced minor deficits (73% that of WT at the homozygous state; Figure 2E). This mutation was classified as a VUS (class II) by FAMILION. Our biophysical characterization shows very minor changes; carrying hERG-A913V along with a WT allele would bring the net current to 85% of WT. It seems this mutation alone is insufficient to lead to an LQT phenotype, experimentally and phenotypically. However, this raises the important possibility that the presence of multiple VUS with minor biophysical defects could be additive and could combine to produce a prolongation of APD, leading to an LQT phenotype.
An important additive effect is notable upon comparison of the hERG-A913V+hERG-WT condition (father/sister) with the hERG-A913V+hERG-S654G proband. In the former, the presence of hERG-A913V contributes to an approximately 30-ms prolongation of APD. In the latter, hERG-A913V compounds an already present reduction in IKr (hERG-S654G) and prolongs APD by approximately 60 ms (Figures 3B and 3C), showing a greater than additive effect of dual mutation status on APD.
Implications for the family
Overall, the data suggest that, after the proband, the mother may be at highest risk for developing arrhythmias. There is good agreement between clinical QTc and predicted APD (Figure 3). However, a strict comparison between QTc and APD is imperfect, as highlighted by the father and a daughter, both of whom carry hERG-A913V but have differing QTc (0.39 and 0.42 second, respectively). Confounding factors include gender, variable penetrance, heart rate, and other unexplored disease-modifying genes. Indeed, although of doubtless use, in vitro, in silico, or genetic studies of LQT mutations may remain secondary in utility to the individual patient’s phenotype in risk assessment.11
It seems that in the absence of carrying all 3 mutations (as in the proband), minor deficits caused by SCN5A-F1596I and hERG-A913V in the other family members are insufficient to induce an LQT phenotype. This may be related to repolarization reserve.12 The cell maintains sufficient repolarizing capacity to overcome any induced deficits. Indeed, stepwise decreases of IKr lead to a disproportionate prolongation of APD for dual hERG mutation status (Figures 3B and 3C). Additionally, the contribution of unexplored polymorphisms carried by unaffected family members cannot be discounted. Ultimately, from our data, it seems possible that the unlucky proband’s compound heterozygosity at hERG combined with a mutation in SCN5A is sufficient to result in clinical LQTS.
Implications for the clinician
Despite the utility of clinical genetic screening in congenital LQTS,7 distinguishing benign variants from pathologic mutations is complicated by both false positives and negatives, and incomplete penetrance.4 Genotyping becomes a suboptimal predictor of clinical outcome and therefore is mainly used by clinicians to confirm the genotype of the disease in affected individuals or to rule out nonaffected nonmutation carriers from further follow-up. Issues remain for a third group: apparently nonaffected mutation carriers. This study represents an additional complexity in that the family presented with 3 separate potential LQTS mutations and only 1 affected individual. We used functional characterization and in silico modeling to explore the role of these 3 mutations and show that this is likely a case of polygenic LQTS in which the presence of a VUS with modest biophysical defects becomes important only in the presence of another disease-causing mutation.
Crucially, our experiments with SCN5A-F1596I show it seems insufficient to cause disease.13 This, along with the unaffected status of the carrier daughter (Figure 3A), makes it likely that SCN5A-F1596I alone does not cause LQTS despite it being classified as a class I mutation. This demonstrates the importance of functional characterization and highlights that the algorithms used by genetic testing companies to classify mutations are not necessarily accurate.
Although all mutations identified in this family were classified as likely LQT mutations (1 was VUS), functional characterization and in silico modeling suggest that this family actually presents with a case of polygenic LQTS in which a VUS with minor biophysical defects combined with a more severe mutation actually produces additive effects leading to a prolonged QTc. Importantly, the functional characterization and in silico modeling support the clinical phenotype of this family in which only 1 individual is affected. This study highlights that functional characterization is extremely pertinent to correlate the role of the mutations to the clinical phenotype and should remain an important part of genetic mutation classifications. Our results also suggest that clinicians should be cautious when using solely the genetic screening results and classification for decision-making.
Footnotes
This work was supported by National Institutes of Health Grant R01 (HL094450 and HL124245) to Dr. Deschênes; American Heart Association Great Rivers Pre-Doctoral Fellowship 12PRE11940047 to M. Hoshi; and National Institutes of Health Grant T32 GM007250 to M. Hoshi.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.hrcr.2015.02.011.
Appendix
Supplementary materials
Supplementary Material
References
- 1.Bokil N.J., Baisden J.M., Radford D.J., Summers K.M. Molecular genetics of long QT syndrome. 2010;101:1–8. doi: 10.1016/j.ymgme.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz P.J., Stramba-Badiale M., Crotti L. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napolitano C., Priori S.G., Schwartz P.J., Bloise R., Ronchetti E., Nastoli J., Bottelli G., Cerrone M., Leonardi S. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. JAMA. 2005;294:2975–2980. doi: 10.1001/jama.294.23.2975. [DOI] [PubMed] [Google Scholar]
- 4.Kapa S., Tester D.J., Salisbury B.A., Harris-Kerr C., Pungliya M.S., Alders M., Wilde A.A.M., Ackerman M.J. Genetic testing for long-QT syndrome: distinguishing pathogenic mutations from benign variants. Circulation. 2009;120:1752–1760. doi: 10.1161/CIRCULATIONAHA.109.863076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giudicessi J.R., Kapplinger J.D., Tester D.J., Alders M., Salisbury B.A., Wilde A.A.M., Ackerman M.J. Phylogenetic and physicochemical analyses enhance the classification of rare nonsynonymous single nucleotide variants in type 1 and 2 Long-QT syndrome. Circ Cardiovasc Genet. 2012;5:519–528. doi: 10.1161/CIRCGENETICS.112.963785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullally J., Goldenberg I., Moss A., Lopes C., Ackerman M., Zareba W., McNitt S., Robinson J., Benhorin J., Kaufman E., Towbin J., Barsheshet A. Risk of life-threatening cardiac events among patients with long QT syndrome and multiple mutations. Heart Rhythm. 2013;10:378–382. doi: 10.1016/j.hrthm.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tester D.J., Will M.L., Haglund C.M., Ackerman M.J. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Olesen M., Yuan L., Liang B. High prevalence of long QT syndrome–associated SCN5A variants in patients with early-onset lone atrial fibrillation. Circ Cardiovasc Genet. 2012;5:450–459. doi: 10.1161/CIRCGENETICS.111.962597. [DOI] [PubMed] [Google Scholar]
- 9.O’Hara T., Virág L., Varró A., Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehnart S.E., Ackerman M.J., Benson D.W. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases Workshop Consensus Report About the Diagnosis, Phenotyping, Molecular Mechanisms, and Therapeutic Approaches for Primary Cardiomyopathies of Gene Mutations Affecting Ion Channel Function. Circulation. 2007;116:2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman E.S., McNitt S., Moss A.J. Risk of death in the long QT syndrome when a sibling has died. Heart Rhythm. 2008;5:831–836. doi: 10.1016/j.hrthm.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden D.M. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 13.Bennett P., Yazawa K., Makita N., George A. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material