Abstract
Patients with Type 2 diabetes mellitus (T2DM) are at high risk of developing cardiovascular disease (CVD). Treatment of diabetic dyslipidemia, comprised mainly of hypertriglyceridemia, and low HDL-C, with either statin or fibrate monotherapy, is moderately effective at reversing the abnormal lipid levels, but does not completely reverse the risk of CVD. Combination therapy with a statin and fibrate more effectively treats diabetic dyslipidemia; however, neither the impact on CVD risk nor the safety profile of statin–fibrate combined treatment had been tested in a large randomized trial. The Action to Control Cardiovascular Risk in Diabetes (ACCORD)-Lipid trial tested the hypothesis that combination therapy with a fibrate and statin would more effectively prevent major CVD events in a high-risk population of patients with T2DM compared with statin monotherapy. In ACCORD-Lipid, over 5000 patients were treated with fenofibrate plus simvastatin versus simvastatin alone. Although combination therapy did not significantly reduce CVD event rates in the ACCORD-Lipid cohort as a whole, a predefined subgroup of participants with the combination of significant hypertriglyceridemia and low HDL-C experienced a 31% lower event rate with combination therapy. Post hoc analyses conducted in similar subsets in previous fibrate monotherapy trials were concordant with these findings in ACCORD-Lipid. Combination therapy was well tolerated and safe, with no detectable increase in myopathy. The implications of the ACCORD-Lipid findings for the treatment of dyslipidemia in patients with T2DM are discussed.
Keywords: coronary heart disease, diabetes, dyslipidemia, fenofibrate, HDL-C, simvastatin, stroke, triglyceride
Rationale & design of ACCORD-Lipid
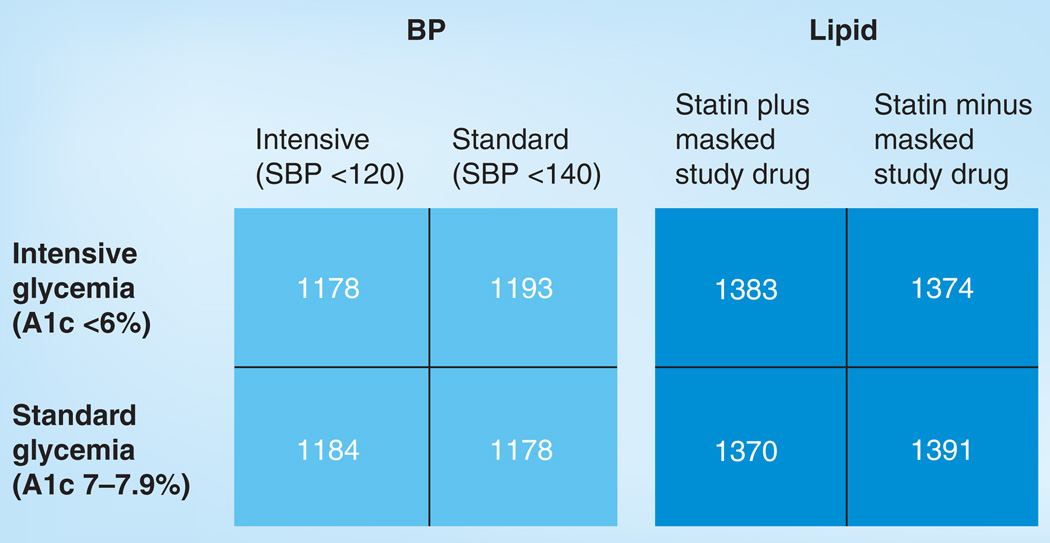
Patients with Type 2 diabetes mellitus (T2DM) are at a high risk of developing atherosclerotic cardiovascular disease (CVD), particularly older patients with T2DM and those with multiple risk factors for CVD. This latter group has a risk of CVD events that is approximately equivalent to that of nondiabetic patients with established CVD [1]. Individuals with T2DM and established CVD are at very high risk of recurrent CVD events [2]. These observations led the Adult Treatment Panel (ATP) to designate T2DM as a coronary disease equivalent in terms of treatment goals, and to recommend aggressive risk factor management in such patients [2]. The current approach to reduce CVD risk in patients with T2DM is a strategy of modifying multiple risk factors including lipids, blood pressure and blood glucose; however, the ideal target levels for each of these treatments had not been clearly defined. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, sponsored by the NIH and conducted in the USA and Canada, examined the effects of intensive modification of blood glucose, blood pressure and lipids on CVD events in a population of patients with T2DM who were at high risk of such events [3]. ACCORD-Lipid was one of three interventions in the overall ACCORD trial. Participants who qualified for the overall ACCORD glycemia trial were then assigned to participate in either the blood pressure or lipid substudy in a double 2×2 design (Figure 1). This allowed independent testing of the blood pressure and lipid hypotheses within the overall framework of the ACCORD glycemia trial. The hypothesis tested in ACCORD-Lipid was whether, in the context of good glycemic control, a strategy that used combination therapy with a fibrate and statin to increase HDL-C and decrease triglycerides, in addition to LDL-C lowering, reduced CVD risk more than a strategy that used statin monotherapy to achieve LDL-C lowering. The fibrate used in ACCORD-Lipid was fenofibrate and the statin was simvastatin.
Figure 1. ACCORD study design.
The overall ACCORD study was a double 2×2 randomized trial design. Using baseline BP or lipids, participants were randomly assigned to either intensive or standard BP or lipid treatment and then underwent a second randomization to either standard or intensive glycemic control. This figure shows the number of participants randomized to various combinations of glycemia, BP and lipid treatment. A total of 2753 participants were randomized to statin plus placebo and 2765 participants were randomized to statin plus fenofibrate in the lipid component of ACCORD.
A1c: Hemaglobin A1c; BP: Blood pressure; SBP: Systolic blood pressure.
The ACCORD trial was conducted at 77 clinical sites in the USA and Canada between the years 2001 and 2009. The ACCORD-Lipid results were published in the Spring of 2010 [4]. The results of the ACCORD glycemia and blood pressure trials were published separately [5,6]. In ACCORD-Lipid, 5518 patients with T2DM were randomly assigned to receive fenofibrate or placebo on a background of treatment with simvastatin 20 or 40 mg/day. Participants were followed for an average of 4.7 years and the effect of the study intervention on rates of occurrence of the primary CVD end point (fatal and nonfatal coronary heart disease and stroke) was assessed. The rationale for the selection of combination therapy as the intensive lipid intervention for ACCORD was based on the observation that the absolute rates of CVD events are high in patients with T2DM [1,7,8], and remain so even after significant reductions are achieved by treatment with either statin or fibrate monotherapy [9–12]. Together with the high prevalence of hypertriglyceridemia and low HDL-C among patients with T2DM [13,14] and the ability of combination therapy with a fibrate and statin to correct these lipid abnormalities [15], this made combination therapy an attractive intervention for testing in ACCORD-Lipid.
An additional goal of ACCORD-Lipid was to determine the safety of combined fenofibrate–simvastatin therapy. With current treatment guidelines emphasizing LDL-C as the primary treatment target in patients at risk of CVD, statin treatment has become highly prevalent [2,16,17]. With wider use of statins, fibrate therapy is increasingly being considered within the context of statin therapy. Reports of myositis and rhabdomyolysis in patients treated with combined statin–fibrate therapy led to concerns over the safety of this combination [18,19]. Although myopathy was primarily observed with a combination of statin and gemfibrozil, it was generally assumed that concomitant fenofibrate treatment would also increase the risk of myopathy. The actual extent of the risk of myopathy with combination therapy has been extremely difficult to determine due to limited use in the setting of controlled clinical trials. With over 5000 patients participating, half of whom were assigned to combination treatment with simvastatin and fenofibrate, ACCORD-Lipid was the largest such cohort ever assembled, representing over 25,000 patient years of exposure. Thus, ACCORD-Lipid provides a crucial assessment of the safety of statin–fibrate combination therapy.
Effect of fenofibrate on plasma lipoproteins in ACCORD-Lipid
The addition of fenofibrate to statin therapy resulted in a modest but significant increase in HDL-C and a substantial reduction in plasma triglycerides. At the 1-year visit, mean HDL-C increased from 0.98 to 1.05 mmol/l (38.0–40.4 mg/dl), an increase of 6.3% in the fenofibrate treatment group [4]. This effect of fenofibrate was maintained throughout the follow-up period. HDL-C levels in the placebo (simvastatin only) group also increased from 0.99 to 1.02 mmol/l (38.2–39.4 mg/dl) at the first annual visit, a 3.1% increase. However, HDL-C continued to increase in the placebo group during the follow-up period, such that by the end of the study the relative difference in HDL-C between the two groups had narrowed to 1.7% (0.02 mmol/l or 0.7 mg/dl absolute difference) [4]. Insofar as statins have some effect on increasing HDL-C, the improvement in the placebo group may reflect the use of simvastatin in increasing doses as the trial progressed [20]. Alternatively, intensified glycemic therapy in the glycemia portion of ACCORD resulted in sustained improvements in HbAlc levels that could have affected HDL-C levels favorably in the placebo group. In either case, this would indicate an absence of these effects in the fenofibrate group. Plasma levels of LDL-C were 10.8% lower in both groups at the first annual visit and progressively decreased as statin therapy was intensified over the remainder of the trial, reaching levels between 2.0 and 2.1 mmol/l (77.7–83.0 mg/dl) by the third to seventh annual visits [4]. There was no difference in LDL-C concentrations between the fenofibrate and placebo groups. Median triglyceride levels decreased from 1.85 to 1.37 mmol/l (164.0–121.0 mg/dl) in the fenofibrate group between baseline and the first annual visit, a 21.2% decrease [4]. Triglyceride levels remained in this range throughout the follow-up period in the fenofibrate group. At the first annual visit, triglyceride levels were only 1% lower in the placebo group but continued to fall over the remainder of the trial, such that by the final visit triglyceride levels in the placebo group were 8.7% lower than baseline (13.5% relative difference between placebo and fenofibrate groups). As was the case with HDL-C, the basis for the continued fall in triglyceride levels in the placebo group is unclear, but could have resulted from a ‘unique’ effect of simvastatin and/or improved glycemic control of plasma triglyceride concentrations in the placebo group. Thus, in ACCORD-Lipid, fenofibrate effectively increased HDL-C and lowered triglycerides. However, owing to continued improvements in the placebo group over the course of the study, the difference between the two groups narrowed as the trial progressed. In both groups, LDL-C was effectively and equally reduced to levels consistent with current treatment guidelines [2,20,21].
Safety & tolerability of combination fenofibrate-simvastatin therapy in ACCORD-Lipid
By the end of the ACCORD follow-up period, adherence to the study intervention remained high, with 77.3 and 81.3% of ACCORD-Lipid participants remaining on blinded fenofibrate or placebo, respectively [4]. Participants were asked at each visit to report any out of the ordinary muscle aches or pains. Slightly more than 40% of participants reported muscle symptoms during the trial; however, this did not differ between the fenofibrate and placebo groups (40.1 vs 40.5%, respectively) (Table 1). Reports of muscle pain were rarely associated with elevation in creatine phosphokinase in either the fenofibrate or placebo groups (0.3% in both groups). Similarly, investigatonal report of myositis or rhabdo-myolysis was also infrequent and equal in both groups (0.1%) (Table 1). Thus, combination therapy with fenofibrate and simvastatin was not associated with increased risk of myopathy.
Table 1.
Safety profile of combination simvastatin-fenofibrate therapy in the ACCORD-Lipid trial
| Adverse event | Fenofibrate (n = 2765) |
Placebo (n = 2753) |
p-value |
|---|---|---|---|
| Unusually severe muscle aches/pain | 1110(40.1%) | 1115(40.5%) | 0.79 |
| Unusually severe muscle aches/pain with CPK >5 × ULN | 7 (0.3%) | 8 (0.3%) | 0.79 |
| Investigator reported myositis/rhabdomyolysis | 4(0.1%) | 3(0.1%) | 1.00 |
| ALT>5×ULN | 16(0.6%) | 6 (0.2%) | 0.03 |
| Investigator reported hepatitis | 3(0.1%) | 0 (0.0%) | 0.25 |
| Serum creatinine >115 µmol/l (>1.3 mg/dl) (women) | 235 (27.9%) | 157(18.7%) | <0.001 |
| Serum creatinine >133 µmol/l (>1.5 mg/dl) (men) | 698 (36.7%) | 350(18.5%) | <0.001 |
| Any gallbladder-related event | 7 (0.3%) | 5 (0.2%) | 0.57 |
All patients were treated with 20–40 mg simvastatin. Results are self-reports of unusual muscle pain or weakness, major adverse events and results of routine safety laboratory tests.
ALT: Alanine aminotransferase; CPK: Creatine phosphokinase; ULN: Upper limits of central laboratory normal range. Data taken from [4].
In this regard, it is important to note that prior studies indicating increased risk of myopathy with combined fibrate–statin therapy were largely conducted prior to the approval of fenofibrate for use in the USA in 1998 and primarily reflect the use of gemfibrozil in combination with a statin [22,23]. This is an important distinction insofar as gemfibrozil has a significant pharmacokinetic interaction with most statins, inhibiting their metabolism via glucuronidation and oxidation of statin hydroxyacids [24,25]. Fenofibrate does not share this effect and does not significantly impact plasma levels of simvastatin or other statins [24,26]. Thus, as suggested by more recent reports [27], ACCORD-Lipid indicates that combination therapy with fenofibrate and simvastatin has an excellent safety profile. An additional advantage of fenofibrate in patients with T2DM is that gemfibrozil, unlike fenofibrate, is a potent inhibitor of CYP2C8, the enzyme responsible for the metabolism of many oral hypoglycemic agents including rosiglitazone, pioglitazone, repaglanide and glyburide [25].
With regard to other potential adverse effects associated with fibrate therapy noted in previous studies, reports of gallbladder-related events, pancreatitis or hepatitis did not differ between the groups. By contrast to the experience in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, neither pulmonary embolism nor deep vein thrombosis was observed in ACCORD-Lipid [4,28]. Elevations of serum liver enzymes (more than five-times the upper limits of normal) did occur with higher frequency among fenofibrate-treated patients, but rates were very low (0.6 vs 0.2%) (Table 1) [4].
Mild-to-moderate increases in serum creatinine occurred more frequently in the fenofibrate treatment group (Table 1). That fenofibrate treatment can result in moderate, reversible increases in serum creatinine, particularly in patients with moderate renal insufficiency, has been known for some time [29]. The exact mechanism of the increase in plasma creatinine following fenofibrate therapy is unknown. The rise in serum levels has been attributed to increased creatinine production rather than reduced renal function [30], although other studies have demonstrated reduced creatinine clearance and glomerular filtration rates following fenofibrate therapy [31]. In the FIELD study, a subset of participants were re-examined at 8 weeks following discontinuation of fenofibrate and reversal of the creatinine elevation was found [28]; similar analyses are being conducted in ACCORD-Lipid. The available evidence suggests that despite increased creatinine, the overall effect of fenofibrate therapy on renal function may, in fact, be protective, at least as evidenced by reduced albuminuria [4,28,32]. Similarly, fenofibrate has been shown to increase plasma levels of homocysteine, an amino acid whose levels have been linked by epidemiologic studies to increased risk of atherosclerosis [33]. Although baseline homocysteine levels predicted the risk of CVD events in the Diabetes Atherosclerosis Intervention Study (DAIS), the fenofibrate-mediated increase in homocysteine levels did not attenuate the effects of the drug on angiographic progression of coronary atherosclerosis or clinical outcomes [34]. However, in the Finnish substudy of FIELD, increases in homocysteine were associated with reduced effects of fenofibrate on HDL-C and apoA-I [35].
Effect of combination therapy on cardiovascular outcomes in ACCORD-Lipid
The primary outcome measure in ACCORD was the first occurrence of major fatal and nonfatal CVD events (coronary heart disease and stroke). Over an average of 4.7 years of treatment, the rate of CVD events was 2.24% per year in the fenofibrate group versus 2.41% per year in the placebo group (hazard ratio: 0.92; 95% CI: 0.79–1.08; p = 0.32) [4]. Thus, in the entire study cohort, addition of fenofibrate to simvastatin treatment provided no significant additional benefit over that of simvastatin alone. When a study such as ACCORD-Lipid fails to confirm its primary hypothesis it is important to carefully examine the results in order to determine why. ACCORD included significant numbers of women (30.7%) and minorities (31.6%), as well as participants with a wide range of lipid values. Reduced responses to fenofibrate in terms of CVD events among one or more of these groups might have diluted the overall effect in the entire cohort. In addition, the response may have been affected by other factors including age, prior CVD or assignment to intensive versus standard therapy in the glycemia arm. To detect any heterogeneity in response to fenofibrate, the ACCORD-Lipid investigators examined the occurrence of the primary CVD outcomes by treatment group in ten prespecified subgroups based on these baseline characteristics [4]. There was no evidence of heterogeneity of response to fenofibrate therapy in terms of the primary end point in subgroups defined by age, prior CVD, baseline HbAlc or glycemia-arm assignment. By contrast, there was strong evidence of heterogeneity of response by sex, with men appearing more likely to benefit compared with women (interaction p-value = 0.011) [4]. Indeed, there was a suggestion that fenofibrate might have been detrimental to women overall. The ACCORD investigators were surprised by this finding; it was not observed in the FIELD study [28]. Of potential importance, the primary outcome rate of 6.6% over 4.7 years of follow-up for women in ACCORD-Lipid on placebo was much lower than expected and only 50% of the rate in men on placebo. Further analyses will be required in order to gain greater insight into this finding.
There were also trends for heterogeneity by race and baseline lipid concentrations. White subjects seemed to have a better outcome with fenofibrate than non-white subjects (interaction p-value = 0.09). The basis for this is also under investigation.
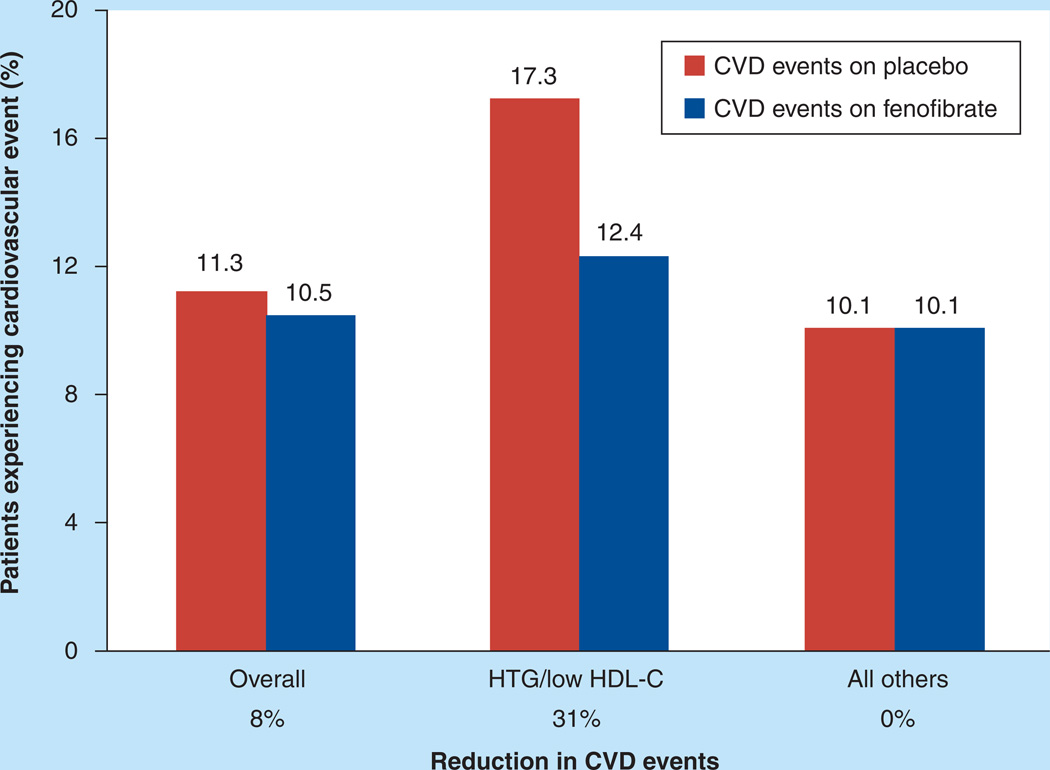
In addition, when the primary outcome was examined by tertiles of baseline lipids, there was evidence of heterogeneity in responses when study participants who entered the study with a plasma triglyceride level in the top third (≥2.30 mmol/l or ≥204 mg/dl) and a HDL-C level in the bottom third (≤0.88 mmol/l or ≤34 mg/dl) were compared with all other participants; here, there was a strong trend suggestive of a better response in the group with significant dyslipidemia (interaction p-value = 0.057). Two points of note: first, the cardiovascular event rate was much higher in the placebo group with significant dyslipidemia than in the placebo group without dyslipidemia (17.3 vs 10.1%); and second, fenofibrate treatment was associated with a 31% lower event rate in the group with significant dyslipidemia compared with placebo (Figure 2) [4]. Thus, in this dyslipidemic subgroup, combination therapy with fenofibrate and simvastatin appeared to reduce CVD events. This subgroup represented 17% of the overall ACCORD-Lipid study cohort. Importantly, the heterogeneity by gender observed in the overall ACCORD-Lipid cohort was not apparent within this subgroup [4].
Figure 2. Effect of fenofibrate on major cardiovascular events in ACCORD participants with hypertriglyceridemia and low HDL-C at baseline versus all others.
In ACCORD-Lipid there was a nonsignificant overall 8% reduction in cardiovascular events with fenofibrate therapy. In a prespecified subgroup analysis, participants with triglyceride Ivels of greater than 2.30 mmol/l (>204 mg/dl) and HDL-C levels of less than 0.88 mmol/l (<34 mg/dl; HTG/low HDL-C) experienced a 31 % reduction in events with fenofibrate therapy (nominal p = 0.032) versus no effect in the remaining participants (all others). Numbers over bars are percent of patients experiencing a primary CVD event during the ACCORD-Lipid follow-up by treatment group. The HTG/low HDL-C subgroup comprised 17% of ACCORD-Lipid participants.
CVD: Cardiovascular disease; HTG: Hypertriglyceridemia.
Adapted from [4].
Although it is biologically plausible that individuals with hypertriglyceridemia and low HDL-C might be more likely to benefit from fenofibrate therapy, as part of a panel of multiple comparisons, the results of this prespecified subset analysis should not, in and of itself, be considered definitive but rather hypothesis generating. To determine the feasibility of this hypothesis, we reviewed the results of similar subgroup analyses in several major fibrate trials conducted prior to ACCORD.
Fibrate therapy & cardiovascular outcomes: a historical perspective
Prior to the completion of ACCORD-Lipid, six major randomized clinical trials examined the impact of fibrate therapy on CVD events had been carried out (Table 2) [28,36–40]. An important distinction between these studies and ACCORD-Lipid is that fibrate alone was administered in these trials, without a concomitant statin. The outcome of these studies varied widely, with three of the studies demonstrating a significant impact on CVD events in the overall study population, whereas the remaining three, such as ACCORD-Lipid, showed no significant overall effect (Table 2). In order to fully understand the role of fibrates in the treatment of dyslipidemia, it is important to understand the reasons for these varying results. Although one might conclude from reviewing the outcomes of these trials that CVD efficacy with fibrates is agent specific, insofar as two of the three trials showing a positive effect of fibrate therapy used gemfibrozil, there are several reasons to believe that this is not the case. First, the fibrates are chemically related and share a common mechanism of action as activators of the PPAR-α nuclear receptor. Second, the fibrates exhibit comparable pharmacological profiles, including similar effects on plasma lipoproteins. Although the varying outcome of these trials cannot be completely explained, it is clear that there were major differences in the populations studied in each of these trials. This heterogeneity is probably a major factor in the differing outcomes of these trials. Specifically, trials that included substantial proportions of Caucasians and males and whose inclusion criteria favored recruitment of participants with significant dyslipidemia were more likely to have an overall positive outcome [37–39]. By contrast, fibrate therapy was less likely to show an overall effect on CVD in studies that recruited significant numbers of women and minorities, and that had either broad or no lipid inclusion criteria [4,28,36,40]. A brief summary of each of these trials with a focus on analyses of lipoprotein predictors of fibrate response is presented in the following paragraphs.
Table 2.
Cardiovascular disease prevention with fibrate therapy.
| Trial | Year reported | Drug | CHD risk reduction (primary end point) |
Ref. |
|---|---|---|---|---|
| CDP | 1975 | Clofibrate | 7% (NS) | [36] |
| WHO | 1978 | Clofibrate | 20% (p< 0.05) | [37] |
| HHS | 1987 | Gemfibrozil | 34% (p < 0.02) | [38] |
| VA-HIT | 1999 | Gemfibrozil | 22% (p < 0.006) | [39] |
| BIP | 2000 | Bezafibrate | 7.3% (p = 0.26) | [40] |
| FIELD | 2005 | Fenofibrate | 11% (p = 0.16) | [28] |
| ACCORD-Lipid | 2010 | Fenofibrate | 8%(p = 0.32) | [4] |
The impact of fibrate monotherapy on CHD risk reduction in the major randomized clinical trials completed prior to ACCORD-Lipid was variable, with some trials reporting a significant reduction and others no significant effect overall. CHD: Coronary heart disease; NS: Nonsignificant
Although the Coronary Drug Project (CDP) is perhaps best known for its niacin treatment arm, the study tested several different interventions, including dextrothyroxine, estrogen and clofibrate [36]. Both the estrogen and dextrothyroxine arms were terminated early due to excess numbers of cardiovascular or thromboembolic events. Findings of both the niacin and clofibrate arms were reported simultaneously in 1975 [36]. Neither group (niacin or clofibrate) experienced a significant reduction in total mortality, the primary study end point. Although there was a significant 15% reduction in nonfatal myocardial infarction and coronary death in the niacin group, the 7% reduction observed with clofibrate treatment was not significant [36]. The CDP exclusively recruited middle-aged men with coronary heart disease. A total of 1103 men were randomized to clofibrate treatment and 2789 to placebo. Although there were no lipid entry criteria in CDP-Clofibrate, the mean baseline cholesterol was very high (6.48 mmol/l or 250 mg/dl). Although the number of patients studied was relatively small, the number of cardiovascular end points appeared to be sufficient to provide adequate power to detect an effect of the study intervention. HDL-C was not measured; thus, no subset analyses are available. It is not entirely clear why the overall outcome was negative in this trial.
The findings of the WHO Clofibrate trial were reported in 1978 [37]. This primary prevention trial was conducted exclusively in middle-aged men recruited from three European centers (Edinburgh, Budapest and Prague). A total of 10,000 men whose cholesterol was in the upper third of the 30,000 screened individuals were randomized to either clofibrate or placebo. The mean serum cholesterol was 6.42 mmol/l (248 mg/dl) on average at study entry and was reduced by 9 % in the clofibrate treatment group (Table 2). The incidence of ischemic heart disease events was reduced by 20% in the clofibrate treatment group, primarily due to a 25% decrease in nonfatal myocardial infarction [37]. The incidence of fatal myocardial infarction was similar in both clofibrate and placebo groups.
The Helsinki Heart Study (HHS) was also a primary prevention trial in which 4081 asymptomatic Finnish male postal and railway workers were randomly assigned to treatment with either gemfibrozil or placebo [38]. The primary lipid inclusion criterion for HHS was a non-HDL-C level of greater than 5.2 mmol/l (200 mg/dl). This proved fortuitous insofar as it allowed inclusion of a significant percentage of individuals with hypertriglyceridemia. In HHS, gemfibrozil treatment was accompanied by an 11% increase in HDL-C, an 11% decrease in LDL-C and a 35% decrease in triglycerides [38]. In HHS, treatment with gemfibrozil resulted in a significant 34% reduction in the occurrence of the primary end point of myocardial infarction and coronary heart disease death (Table 2) [38]. Initial analyses indicated that the benefit of gemfibrozil treatment was greatest among those with hypertriglyceridemia [38,41], and subsequent analyses identified a group of participants with hypertriglyceridemia, low HDL-C and BMI greater than 26 as experiencing the greatest reduction in CVD risk with gemfibrozil treatment (Table 3) [42]. Very few HHS participants (135) had T2DM at entry into the study [43]. Myocardial infarction and cardiac death occurred with greater frequency among HHS participants with T2DM and although CVD event rates were lower with gemfibrozil treatment, the number of subjects with T2DM was too small to allow for valid statistical analyses to be performed [43].
Table 3.
Comparison of lipid subgroup analyses in fibrate trials.
| Study (drug) | CVD reduction -entire cohort (p-value) |
Lipid subgroup HDL-C criteria |
Lipid subgroup triglyceride criteria |
CVD reduction – lipid subgroup (p-value) |
Ref. |
|---|---|---|---|---|---|
| HHS (gemfibrozil) | −34% (0.02) | <1.09 mmol/l (<42 mg/dl)† |
≥2.31 mmol/l (≥204 mg/dl) |
−78% (0.002) | [36,42] |
| VA-HIT (gemfibrozil) |
−22% (0.006) | - | ≥2.03 mmol/l (≥180 mg/dl) |
−28% (<0.05) | [39,44] |
| BIP(bezafibrate) | −7.3% (0.24) | - | ≥2.26 mmol/l (≥200 mg/dl) |
−39.5% (0.02) | [40] |
| FIELD (fenofibrate) | −11% (0.16) | Men: <1.04 mmol/l (<40 mg/dl) Women <1.30 mmol/l (<50 mg/dl) |
≥2.31 mmol/l (≥204 mg/dl) |
−27% (0.005) | [25,49] |
| ACCORD (fenofibrate) |
−8% (0.26) | ≤0.88 mmol/l (≤34 mg/dl) |
≥2.31 mmol/l (≥204 mg/dl) |
−31% (0.032) | [4,54] |
Percentage reductions in cardiovascular event rates within fibrate trials are presented for both the entire study cohort and subgroups defined by hypertriglyceridemia and/or low HDL-C There is a consistent finding of reduced cardiovascular outcomes in these trials in subgroups defined by the presence of hypertriglyceridemia and low HDL-C regardless of whether the overall outcome was positive or negative.
BMI >26 kg/m2.
CVD: Cardiovascular disease.
The Veteran’s Affairs HDL Intervention Trial (VA-HIT) was a placebo-controlled secondary prevention trial of gemfibrozil treatment conducted in 2531 men with low HDL-C as their primary lipid abnormality [39]. The primary lipid entry criterion for VA-HIT was an HDL-C level of 1.04 mmol/l (40 mg/dl) or less and an LDL-C level of 3.63 mmol/l (140 mg/dl) or less; there was no entry criterion for triglyceride concentration [39]. In VA-HIT, gemfibrozil treatment resulted in a relatively modest (6%) increase in HDL-C and a 31% decrease in triglyceride; LDL-C was unaltered [39]. The occurrence of the combined primary end point of myocardial infarction, coronary heart disease death and stroke was reduced by 22% (p < 0.006) following 5 years of gemfibrozil treatment (Table 2). Although HDL-C was low in the entire VA-HIT cohort, there was evidence of both increased risk and greater response to gemfibrozil among those with higher triglyceride levels (>2.03 mmol/l or >180 mg/dl) (Table 3) [44]. A total of 24.5% of VA-HIT participants (627 subjects) had T2DM. The diabetes subgroup in VA-HIT experienced an approximately twofold higher occurrence of CVD events. Gemfibrozil treatment in those with T2DM resulted in a 24% reduction in CVD that was comparable with that among nondiabetics [39]. In nondiabetic VA-HIT participants, the greatest reduction in CVD events occurred among those within the highest quartile of fasting plasma insulin [12,45]. Thus, in VA-HIT, even in the presence of uniformly low HDL-C, the presence of insulin resistance and hypertriglyceridemia identified a subgroup with higher risk and who experienced greater event reduction with fibrate therapy [12,44,45]. Interestingly, despite greater CVD end point reduction, the lipid response to gemfibrozil was actually less pronounced in those with T2DM; HDL-C levels increased by 0.04 mmol/l (1.55 mg/dl) in that subgroup [12,45]. Furthermore, in the overall VA-HIT cohort, only a small fraction (23%) of CVD event reduction could be attributed to the lipid changes observed with gemfibrozil treatment [44].
The Bezafibrate Infarction Prevention (BIP) trial was a secondary prevention trial conducted in 3090 Israelis, predominantly men (90%), whose HDL-C level was less than 1.17 mmol/l (45 mg/dl) and triglyceride level was less than 3.39 mmol/l (300 mg/dl) [40]. Bezafibrate therapy resulted in a robust 18% increase in HDL-C, as well as 21 and 5% reductions in triglyceride and LDL-C levels, respectively [4o]. After an average follow-up of 6.2 years, bezafibrate treatment resulted in a nonsignificant 7.3% reduction in nonfatal and fatal myocardial infarction and sudden cardiac death (Table 2); however, those entering the trial with triglyceride levels of greater than 2.26 mmol/l (>200 mg/dl) experienced a significant 39.5% reduction in the primary study end point (Table 3) [4o]. Patients with a clinical diagnosis of T2DM were specifically excluded from participation in BIP; however, almost half of the BIP participants met diagnostic criteria for metabolic syndrome [46]. Among BIP participants, those meeting at least three criteria for the presence of metabolic syndrome (including BMI >28 kg/m2) exhibited a 25% lower rate of occurrence of the primary CVD end point with bezafibrate treatment [46,47]. Thus, in BIP, the presence of hypertriglyceridemia and other components of the metabolic syndrome predicted a positive response to bezafibrate therapy. It is interesting to note that in the St Mary’s, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention Trial (SENDCAP), a relatively small carotid ultrasound study in patients with T2DM, hypertriglyceridemia and low HDL-C, bezafibrate treatment resulted in reduced coronary heart disease events [48].
The FIELD trial was a placebo-controlled mixed primary and secondary prevention trial using fenofibrate in 9795 patients with T2DM that was conducted in Australia, Finland and New Zealand [28]. FIELD recruited patients with T2DM who had a total cholesterol of between 3 and 5 mmol/l (116–193 mg/dl) and a total cholesterol:HDL-C ratio of 4.0 or greater [28]. Triglyceride levels were between 1 and 5 mmol/l (88–442 mg/dl). Among previous fibrate trials, FIELD is the most similar to ACCORD-Lipid; it was conducted exclusively among patients with T2DM and included significant numbers of women (37%). On the other hand, by contrast to ACCORD-Lipid, where 31.6% of participants were non-white, relatively few FIELD participants (7%) were non-white. In addition, FIELD recruited a lower percentage of participants with previous CVD (22 vs 37% in ACCORD-Lipid). In FIELD, fenofibrate therapy resulted in changes in HDL-C and triglyceride levels (5% increase and 29% decrease, respectively) comparable with those observed in ACCORD-Lipid [28]. The fenofibrate treatment group also experienced a 12% decrease in LDL-C. Similar to ACCORD-Lipid, fenofibrate treatment was associated with a nonsignificant 11% overall reduction in the primary outcome of first myocardial infarction or coronary heart disease death (Table 2) [28]. In subsequent post hoc analyses of the influence of various components of the metabolic syndrome on CHD outcomes in FIELD, a subset (21%) of participants with the baseline characteristic of combined low HDL-C levels of less than 1.04 mmol/l in men and less than 1.30 mmol/l in women (<40 and <50 mg/dl, respectively) and hypertriglyceridemia (≥2.3 mmol/l or ≥204 mg/dl) experienced 27% fewer cardiovascular events with fenofibrate treatment (Table 3) [49].
In summary, although the overall impact of fibrate therapy on CVD in large clinical trials has varied, most likely owing to differences in the populations studied, within each of these trials there is a clear and consistent finding of CVD risk reduction with fibrate therapy in a subgroup of participants characterized by the presence of significant hypertriglyceridemia and/or low HDL-C (summarized in Table 3). Thus, in contrast to statins, which effectively reduce CVD risk across a wide range of LDL values, the cardiovascular benefit of fibrates appears to be concentrated in a specific population of patients (i.e., those with significant hypertriglyceridemia and low HDL-C).
Effect of fenofibrate therapy on microvascular disease in FIELD &ACCORD-Lipid
Although the ability of any therapy to reduce CVD events is doubtlessly important, in the case of T2DM, the ability to impact microvascular disease (e.g., nephropathy, retinopathy, lower extremity ischemia and amputation) is also quite important, insofar as these disorders are significant contributors to morbidity and mortality. There is accumulating evidence that fibrates positively impact microvascular disease in patients with T2DM. In both FIELD and ACCORD-Lipid, fenofibrate reduced the development and progression of albuminuria, indicating a positive impact on the progression of diabetic renal disease (Table 4) [4,28]. In addition, in both studies, fenofibrate treatment reduced the progression of diabetic retinopathy as reflected by progression of retinopathy on fundiscopic examination and need for laser photocoagulation (Table 4) [50,51]. In a particularly intriguing post hoc analysis, the FIELD investigators observed that fenofibrate use resulted in a significant reduction in the risk of lower extremity amputation (hazard ratio: 0.64; 95% CI: 0.44–0.94), primarily minor amputation in participants without known large-vessel disease [52]. Thus, independent of considerations regarding CVD prevention, the presence of diabetic nephropathy, retinopathy and/or disordered lower extremity microcirculation may warrant consideration of fibrate therapy in patients with T2DM.
Table 4.
Effect of fenofibrate treatment on progression of diabetic microvascular disease in FIELD and ACCORD-Lipid.
| Study | Microvascular outcomes |
Placebo group, n (%) |
Fenofibrate group, n (%) |
Percentage change (p-value) |
Ref. |
|---|---|---|---|---|---|
| FIELD | Albuminuria progression | 539(11) | 466(9.5) | −13.6 | [28] |
| FIELD | Albuminuria regression | 400(8.2) | 462 (9.4) | +14.6(0.0022)† | [28] |
| FIELD | Retinopathy (laser treatment) |
238 (4.9) | 164(3.4) | −31.1 (0.0002) | [50] |
| ACCORD | Microalbuminuria (post-randomization) |
1137(41.6) | 1050(38.2) | −8.1 (0.01) | [4] |
| ACCORD | Macroalbuminuria (post-randomization) |
337(12.3) | 289(10.5) | −14.6(0.03) | [4] |
| ACCORD | Diabetic retinopathy progression‡ |
80(10.2) | 52(6.5) | −36.3(0.006) | [51] |
In both FIELD and ACCORD-Lipid, fenofibrate reduced progression of diabetic retinopathy and nephropathy.
Combined end point of participants regressing or not progressing with fenofibrate therapy
Combined end point of progression of retinopathy on funduscopic examination and laser photocoagulation.
What is the role of fibrate therapy in the treatment of diabetic dyslipidemia?
With the completion of the FIELD and ACCORD-Lipid trials, we now have a large body of evidence regarding the effects of fibrates, specifically fenofibrate, on both macrovascular and microvascular disease in patients with T2DM. In addition, ACCORD-Lipid provides important information on the safety of combined statin-fibrate therapy. Clinical trials provide strong evidence of a cardiovascular benefit from statin therapy in patients with T2DM [9,11,53]. These results, together with the relatively modest overall effect of fenofibrate on CVD events in FIELD [28] or ACCORD-Lipid [4], serve to validate the current practice of using statin therapy as the initial approach to reducing CVD events in high-risk patients, including those with T2DM [2,16,17,21]. On the other hand, the consistent observation of significant reductions in CVD events with fibrate therapy in patient subgroups characterized by significant hypertriglyceridemia and low HDL-C in ACCORD-Lipid and other trials suggests that the addition of fibrate therapy may further reduce CVD risk in patients with T2DM in whom hypertriglyceridemia and low HDL-C persist despite effective LDL treatment with statins. Specifically, patients in whom triglyceride levels remain greater than 2.26 mmol/l (>200 mg/dl) and HDL-C levels of less than 1.04 mmol/l (< 40 mg/dl) following statin therapy may benefit from the addition of a fibrate. ACCORD-Lipid demonstrated that this approach is safe for the combination of fenofibrate and up to 40 mg/day of simvastatin. By contrast, the potential for gemfibrozil to interact with statins raises serious safety concerns if combined with any statin at greater than minimal doses. Furthermore, both FIELD and ACCORD-Lipid suggest that the use of fenofibrate may provide a benefit when used to slow progression of microvascular disease, specifically albuminuria and retinopathy, in patients with T2DM.
Current guidelines suggest that additional therapy may be considered in high-risk patients in whom hypertriglyceridemia and low HDL-C persist despite effective reduction of LDL-C with statin therapy [2,16]. Available options include intensified lifestyle change, intensified statin therapy or addition of niacin, fish oil or fibrate. Although the ACCORD-Lipid findings support this approach, specifically the addition of fenofibrate, definitive proof of a benefit from this strategy is lacking. The cardiovascular efficacy of combined statin–niacin therapy is currently being tested in ongoing trials (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes AIM-HIGH] and Treatment of HDL to Reduce the Incidence of Vascular Events [HPS2-THRIVE]). A definitive clinical trial is needed that tests the ability of combined fibrate–statin therapy to reduce CVD events in patients with hypertriglyceridemia and low HDL-C.
Conclusion & future perspective
Taken in the context of prior fibrate trials, ACCORD-Lipid supports the hypothesis that intensive combination therapy with a statin plus a fibrate may reduce CVD risk in T2DM patients with significant dyslipidemia (both hypertriglyceridemia and low HDL-C). A randomized trial conducted in patients with these lipid abnormalities is needed in order to provide definitive proof for this hypothesis. Pending the design and completion of such a trial, practitioners should use available evidence to guide decision making regarding the use of combination lipid therapy in patients at high risk of CVD. ACCORD has also shown that, despite aggressive modification of major risk factors, including glucose, blood pressure and lipids, patients with T2DM continue to experience high rates of major CVD events. The implication of this finding is that additional pathways play a role in the pathogenesis of CVD in patients with T2DM. More effective prevention of CVD in patients with T2DM will require the identification of those pathways and development of novel interventions that directly target them.
Executive summary.
Patients with Type 2 diabetes mellitus are at a high risk of cardiovascular disease
-
▪
Diabetic dyslipidemia, primarily hypertriglyceridemia and low HDL-C, is associated with a high risk of cardiovascular disease (CVD)
-
▪
Statins effectively reduce CVD risk in patients with Type 2 diabetes mellitus (T2DM); however, significant risk of CVD remains despite statin therapy
-
▪
Combination statin–fibrate therapy offers the potential of addressing multiple lipid abnormalities comprising diabetic dyslipidemia
-
▪
The effect of this approach on CVD is unproven and there are concerns regarding the safety of combination therapy.
The ACCORD-Lipid trial tested the efficacy of combined statin-fibrate treatment on cardiovascular disease events in patients with Type 2 diabetes mellitus at a high risk of cardiovascular disease
-
▪
In the Action to Control Cardiovascular Risk in Diabetes (ACCORD)-Lipid trial, 5518 patients with T2DM were randomly assigned to receive fenofibrate or placebo on a background of statin (simvastatin) therapy
-
▪
After an average follow-up of 4.7 years, major cardiovascular events (fatal and nonfatal coronary heart disease and stroke) were 8% lower in the fenofibrate–simvastatin group versus the placebo–simvastatin group (p = 0.32).
-
▪
CVD events were reduced by 31 % with fenofibrate treatment in a prespecified subgroup of ACCORD-Lipid participants whose baseline triglyceride levels were in the upper fertile (>2.30 mmol/l or >204 mg/dl) and HDL-C levels in the lower fertile (<0.88 mmol/l or <34 mg/dl) of the study population
-
▪
This finding is consistent with similar subgroup analyses of previous fibrate monotherapy trials
-
▪
The occurrence of muscle-related adverse events in ACCORD-Lipid was low overall and did not differ between the two treatment groups
-
▪
Progression of diabetic microvascular disease (e.g., retinopathy and albuminuria) was reduced by fenofibrate therapy.
Conclusion
-
▪
Although the ACCORD-Lipid study findings do not support the wide application of combination fibrate–statin therapy in patients with T2DM, subgroup analyses from ACCORD-Lipid and previous fibrate trials suggest that the presence of hypertriglyceridemia and low HDL-C identifies a subgroup of patients in whom CVD events may be reduced with combination fibrate–statin therapy
-
▪
ACCORD-Lipid demonstrates that combination therapy with fenofibrate and simvastatin (up to 40 mg/day) is safe
-
▪
When viewed in the context of prior fibrate trials, ACCORD-Lipid provides evidence in support of the use of fenofibrate in patients with T2DM in whom significant hypertriglyceridemia and low HDL-C persist despite statin therapy
-
▪
The presence of diabetic nephropathy, retinopathy and/or disordered lower extremity microcirculation may warrant consideration of fibrate therapy in patients with T2DM.
Footnotes
Financial & competing interests disclosure
Marshall Elam is a consultant for Pfizer and Solvay and is a member of the speakers bureau of Abbott and Merck/Schering-Plough. Henry Ginsberg is on the advisory board of Merck, Merck/Schering–Plough and BMS/AstraZeneca; is a consultant for Merck, Abbott/AstraZeneca, BMS, Roche, Isis/Genzyme, GlaxoSmithKline, Novatis, Pfizer and Regeneron/SanofiAventis; has received research grants from Merck, ISI/Genzyme, Roche and AstraZeneca; and is a member of the speakers bureau of Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with Type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E.▪ Updated US Adult Treatment Panel (ATP) recommendations for hypercholesterolemia treatment.
- 3.Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am. J. Cardiol. 2007;99:211–331. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in Type 2 diabetes mellitus. N Engl. J. Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282.▪▪ Reports the primary outcome results of the ACCORD-Lipid study.
- 5.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in Type 2 diabetes mellitus. N. Engl, J. Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in Type diabetes. N. Engl. J. Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of Type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch. Intern. Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 8.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 9.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 11.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in Type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS)multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 12.Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT) Arch. Intern. Med. 2002;162:2597–2604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 13.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol. Metab. Clin. North Am. 2006;35:491–510. doi: 10.1016/j.ecl.2006.06.002. VII-VIII. [DOI] [PubMed] [Google Scholar]
- 14.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial) Am. J. Cardiol. 2005;95:462–468. doi: 10.1016/j.amjcard.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Genest J, McPherson R, +Frohlich J, et al. 2009 Canadian Cardiovascular Society/ Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Can. J. Cardiol. 2009;25:567–579. doi: 10.1016/s0828-282x(09)70715-9.▪ Contemporary Canadian hypercholesterolemia treatment guidelines.
- 17.Home P, Mant J, Diaz J, Turner C. Management of Type 2 diabetes: summary of updated NICE guidance. BMJ. 2008;336:1306–1308. doi: 10.1136/bmj.39560.442095.AD.▪ European treatment guidelines for the management of Type 2 diabetes, including treatment of dyslipidemia.
- 18.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024–1028. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 19.Ballantyne CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin therapy in high-risk patients. Arch. Intern. Med. 2003;163:553–564. doi: 10.1001/archinte.163.5.553. [DOI] [PubMed] [Google Scholar]
- 20.Ginsberg HN, Bonds DE, Lovato LC, et al. Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am. J. Cardiol. 2007;99:561–671. doi: 10.1016/j.amjcard.2007.03.024.▪ Describes the evolution of the ACCORD-Lipid treatment protocol that occurred in response to changing treatment guidelines.
- 21.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 23.Chang JT, Staffa JA, Parks M, Green L. Rhabdomyolysis with HMG-CoA reductase inhibitors and gemfibrozil combination therapy. Pharmacoepidemiol. Drug Saf. 2004;13:417–426. doi: 10.1002/pds.977. [DOI] [PubMed] [Google Scholar]
- 24.Prueksarhanont T, Tang C, Qiu Y, Mu L, Subramanian R, Lin JH. Effects of fibrates on metabolism of statins in human hepatocytes. Drug Metab. Dispos. 2002;30:1280–1287. doi: 10.1124/dmd.30.11.1280. [DOI] [PubMed] [Google Scholar]
- 25.Corsini A, Bellosta S, Davidson MH. Pharmacokinetic interactions between statins and fibrates. Am. J. Cardiol. 2005;96:44K–49K. doi: 10.1016/j.amjcard.2005.08.007. discussion 34K-35K. [DOI] [PubMed] [Google Scholar]
- 26.Bergman AJ, Murphy G, Burke J, et al. Simvastatin does not have a clinically significant pharmacokinetic interaction with fenofibrate in humans. J. Clin. Pharmacol. 2004;44:1054–1062. doi: 10.1177/0091270004268044. [DOI] [PubMed] [Google Scholar]
- 27.Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am. J. Cardiol. 2005;95:120–122. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 28.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with Type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 29.Lipscombe J, Lewis GF, Cattran D, Bargman JM. Deterioration in renal function associated with fibrate therapy. Clin. Nephrol. 2001;55:39–44. [PubMed] [Google Scholar]
- 30.Hottelart C, El Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002;92:536–541. doi: 10.1159/000064083. [DOI] [PubMed] [Google Scholar]
- 31.Forsblom C, Hiukka A, Leinonen ES, Sundvall J, Groop PH, Taskinen MR. Effects of long-term fenofibrate treatment on markers of renal function in Type 2 diabetes: the FIELD Helsinki substudy. Diabetes Care. 2010;33:215–220. doi: 10.2337/dc09-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in Type 2 diabetes: results from the Diabetes Atherosclerosis Intervention Study (DAIS) Am. J. Kidney Dis. 2005;45:485–493. doi: 10.1053/j.ajkd.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Westphal S, Dierkes J, Luley C. Effects of fenofibrate and gemfibrozil on plasma homocysteine. Lancet. 2001;358:39–40. doi: 10.1016/S0140-6736(00)05271-5. [DOI] [PubMed] [Google Scholar]
- 34.Genest J, Frohlich J, Steiner G. Effect of fenofibrate-mediated increase in plasma homocysteine on the progression of coronary artery disease in Type 2 diabetes mellitus. Am. J. Cardiol. 2004;93:848–853. doi: 10.1016/j.amjcard.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Taskinen MR, Sullivan DR, Ehnholm C, et al. Relationships of HDL cholesterol, ApoA-I, and ApoA-II with homocysteine and creatinine in patients with Type 2 diabetes treated with fenofibrate. Arterioscler. Thromb. Vase. Biol. 2009;29:950–955. doi: 10.1161/ATVBAHA.108.178228. [DOI] [PubMed] [Google Scholar]
- 36.Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- 37.A co-operative trial in the primary prevention of ischaemic heart disease using clofibrate. Report from the Committee of Principal Investigators. Br. Heart J. 1978;40:1069–1118. doi: 10.1136/hrt.40.10.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N. Engl. J. Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 39.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N. Engl. J. Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 40.Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 41.Manninen V, Elo MO, Frick MH, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. JAMA. 1988;260:641–651. [PubMed] [Google Scholar]
- 42.Tenkanen L, Manttari M, Manninen V. Some coronary risk factors related to the insulin resistance syndrome and treatment with gemfibrozil. Experience from the Helsinki Heart Study. Circulation. 1995;92:1779–1785. doi: 10.1161/01.cir.92.7.1779. [DOI] [PubMed] [Google Scholar]
- 43.Koskinen P, Manttari M, Manninen V, Huttunen JK, Heinonen OP, Frick MH. Coronary heart disease incidence in NIDDM patients in the Helsinki Heart Study. Diabetes Care. 1992;15:820–825. doi: 10.2337/diacare.15.7.820. [DOI] [PubMed] [Google Scholar]
- 44.Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 45.Robins SJ, Rubins HB, Faas FH, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT) Diabetes Care. 2003;26:1513–1517. doi: 10.2337/diacare.26.5.1513. [DOI] [PubMed] [Google Scholar]
- 46.Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch. Intern. Med. 2005;165:1154–1160. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 47.Robins SJ, Bloomfield HE. Fibric acid derivatives in cardiovascular disease prevention: results from the large clinical trials. Curr. Opin. Lipidol. 2006;17:431–439. doi: 10.1097/01.mol.0000236370.27508.9d. [DOI] [PubMed] [Google Scholar]
- 48.Elkeles RS, Diamond JR, Poulter C, et al. Cardiovascular outcomes in Type 2 diabetes. A double-blind placebo-controlled study of bezafibrate: the St. Mary’s, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) Study. Diabetes Care. 1998;21:641–648. doi: 10.2337/diacare.21.4.641. [DOI] [PubMed] [Google Scholar]
- 49.Scott R, O’Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with Type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543.▪▪ Examines the impact of various components of the metabolic syndrome on cardiovascular risk and fenofibrate response in the FIELD study.
- 50.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 51.Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in Type 2 diabetes. N. Engl. J. Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288.▪▪ Describes the effect of fenofibrate therapy on progression of diabetic retinopathy in the ACCORD trial.
- 52.Rajamani K, Colman PG, Li LP, et al. Effect of fenofibrate on amputation events in people with Type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;313:1780–1788. doi: 10.1016/S0140-6736(09)60698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sever PS, Poulter NR, Dahlof B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with Type 2 diabetes: Anglo–Scandinavian Cardiac Outcomes Trial – lipid-lowering arm (ASCOT-LLA) Diabetes Care. 2005;28:1151–1157. doi: 10.2337/diacare.28.5.1151. [DOI] [PubMed] [Google Scholar]
- 54.Elam MB, Lovato LC, Byington RP, et al. Abstract 19724: hypertriglyceridemia and low HDL-C predicts fenofibrate response in the ACCORD-Lipid trial. Circulation. 2010;122:A19724. [Google Scholar]