Abstract
The objective of the study was to determine if there are sex-based differences in the prevalence and clinical outcomes of subclinical peripheral artery disease (PAD). We evaluated the sex-specific associations of ankle–brachial index (ABI) with clinical cardiovascular disease outcomes in 2797 participants without prevalent clinical PAD and with a baseline ABI measurement in the Health, Aging, and Body Composition study. The mean age was 74 years, 40% were black, and 52% were women. Median follow-up was 9.37 years. Women had a similar prevalence of ABI < 0.9 (12% women versus 11% men; P=0.44), but a higher prevalence of ABI 0.9–1.0 (15% versus 10%, respectively; P < 0.001). In a fully adjusted model, ABI < 0,9 was significantly associated with higher coronary heart disease (CHD) mortality, incident clinical PAD and incident myocardial infarction in both women and men. ABI < 0.9 was significantly associated with incident stroke only in women. ABI 0.9–1.0 was significantly associated with CHD death in both women (hazard ratio 4.84, 1.53–15.31) and men (3.49, 1.39–8.721. However, ABI 0.9–1.0 was significantly associated with incident clinical PAD (3.33, 1.44–7.70) and incident stroke (2.45, 1.38–4.35) only in women. Subclinical PAD was strongly associated with adverse CV events in both women and men, but women had a higher prevalence of subclinical PAD.
Keywords: women, sex-specific, peripheral artery disease, epidemiology
Introduction
Peripheral artery disease (PAD) is a major public health problem in the USA, and is conservatively estimated to be present in 8 million Americans.1–4 As the population ages, PAD prevalence will continue to rise. PAD is a significant cause of lower extremity functional impairment and can lead to ulceration, gangrene and limb loss. Asymptomatic (subclinical) PAD is also clinically important. Subclinical PAD – defined as an abnormal ankle–brachial index (ABI) in the absence of clinical symptoms – is an independent predictor of increased cardiovascular (CV) and all-cause mortality.5–18 A meta-analysis demonstrated that inclusion of ABI into cardiovascular disease (CVD) risk stratification using the Framingham risk score would reclassify 19% of men and 36% of women into a different risk category and modify treatment recommendations.19
Although it is generally accepted that women have a lower rate of CVD compared with men until the seventh decade of life,20 several community-based studies have demonstrated a higher prevalence of PAD in women compared with men.1,2,21–25 Investigators from both the Cardiovascular Health Study (CHS) and the Multi-Ethnic Study of Atherosclerosis (MESA) found subclinical PAD to be more prevalent in women compared with men, with women being over-represented in the 0.9–1.0 ABI category.11,26 Although most studies have focused on a traditional cut-point of ABI < 0.9 to define PAD, substantial literature demonstrates elevated CV risks with ABI ≤ 1.0.11,12,18,19 As a result, the American College of Cardiology Foundation/American Heart Association Task Force updated the guidelines for management of patients with PAD in 2011, and now considers ABI values of 0.9–1.0 to be abnormal.27
Subclinical PAD may be unique among CVD risk factors in having a higher prevalence in women compared with men. However, it is not clear whether women with subclinical PAD have the same, increased or decreased risk of adverse CV events compared with men. If the CV risk associated with subclinical PAD is the same in women and men, but the prevalence is higher in women, subclinical PAD would account for a greater attributable risk for CVD among women compared with men. To address this gap in knowledge, our current study investigated the association between ABI and adverse CV outcomes in women and men in the Health, Aging, and Body Composition (Health ABC) Study. We hypothesized that women would have a higher prevalence of subclinical PAD (ABI ≤ 1.0) compared with men, but that subclinical PAD would have similar associations with adverse CV events in both sexes.
Methods
Study population
The Health, Aging, and Body Composition (Health ABC) study was designed to evaluate the causes of functional decline among well-functioning older adults. Between March 1997 and April 1998, 3075 men and women aged 70–79 years were recruited at the University of Pittsburgh, Pittsburgh, PA and the University of Tennessee, Memphis, TN; 1584 participants (52%) were women and 1281 (42%) were black. Details of the Health ABC study design and recruitment procedures have been previously described.28 At baseline, eligible participants were free of disability in performing mobility-related activities of daily living and free of functional limitation (defined as any difficulty walking a quarter of a mile or climbing up 10 stairs without resting). We also excluded any participant with a self-reported history of claudication or a history of lower extremity bypass surgery or angioplasty. This analysis included all persons without prevalent PAD and with a baseline ABI measurement (n = 2797).
Ankle–brachial index measurement
The participant was recumbent or semirecumbent for at least five minutes before measuring the blood pressure. Blood pressures were measured using standard blood pressure cuffs, a conventional mercury sphygmomanometer, and an 8-MHz Doppler ultrasound probe. Pressures were taken in the right arm (brachial artery) and both ankles (posterior tibial artery). The ABI was calculated by dividing the systolic blood pressure (SBP) at the ankle by the SBP of the arm. Measures were performed twice, and the results were averaged. The lower value between the two legs was used in the analysis.29 Participants who could not have the lower extremity arteries occluded before 300 mmHg were recorded as ‘unable to reach occlusion blood pressure.’ This consisted of 68 participants (41 men and 27 women) and these participants were included in the ABI ≥ 1.3 group. Participants with open wounds, ulcers or rashes were excluded from ABI measurement.
CVD outcomes
All Health ABC participants were contacted by telephone every six months and asked directed questions to elicit information about hospitalizations, angioplasty or surgery. They were also assessed at annual clinic visits. In addition, participants were asked to report any hospitalizations or CV events as they occurred. Coronary heart disease (CHD) death was defined as death due to myocardial infarction (MI), coronary insufficiency or ischemic heart disease. Incident clinical PAD was defined as (a) the development of a blockage or ulcerated plaque on ultrasound/angiogram, (b) Loss of lower extremity pulse on Doppler exam, (c) positive exercise test for lower extremity claudication, (d) Surgery, angioplasty or thrombolysis for PAD, or (e) amputation ≥1 toes or part of the lower extremity due to ischemia or gangrene. Incident stroke was defined as a nonfatal cerebrovascular accident or cerebrovascular disease as an underlying cause of death. Incident MI included a nonfatal MI or fatal MI as the immediate cause of death.
Covariates
Covariates included sociodemographic variables (age, race, study site and education), co-morbidities (diabetes mellitus [DM], hypertension [HTN] and chronic obstructive pulmonary disease) and physical and biological parameters, including smoking status, body mass index (BMI), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides and cystatin C. Diabetes was defined by a self-reported diagnosis, a fasting glucose ≥ 126 mg/dL or by the use of insulin or oral hypoglycemic medications. Hypertension was defined as SBP > 140 mmHg, diastolic blood pressure of >90 mmHg or by the use of any antihypertensive medications. We estimated the glomerular filtration rate with the cystatin C CKD-EPI equation without demographic coefficients (eGFRcys).30
Statistical analysis
We compared baseline characteristics of male and female participants across different categories of ABI (<0.9, 0.9–1.0, 1.01–1.29 and ≥1.3). The referent category included ABI values between 1.01 and 1.29. Baseline characteristics were evaluated for statistical significance using a t-test or chi-squared test where appropriate. Participants with prevalent disease were excluded from the analysis of each specific outcome. For each ABI category, rates of CHD death, clinical PAD, stroke and MI were calculated for men and women. Associations of ABI categories with CV events were evaluated separately for men and women in demographic adjusted (age, race, site and education) and fully adjusted (demographic adjusted as well as smoking, DM, HTN, eGFRcys, LDL and HDL) Cox proportional hazards models. The proportional hazards assumption was tested using standard residual-based techniques. All analyses were performed using S-Phis (release 8.0, Insightful Inc, Seattle, WA, USA) and SPSS statistical software (release 16.0.1, SPSS Inc, Chicago, IL, USA).
Results
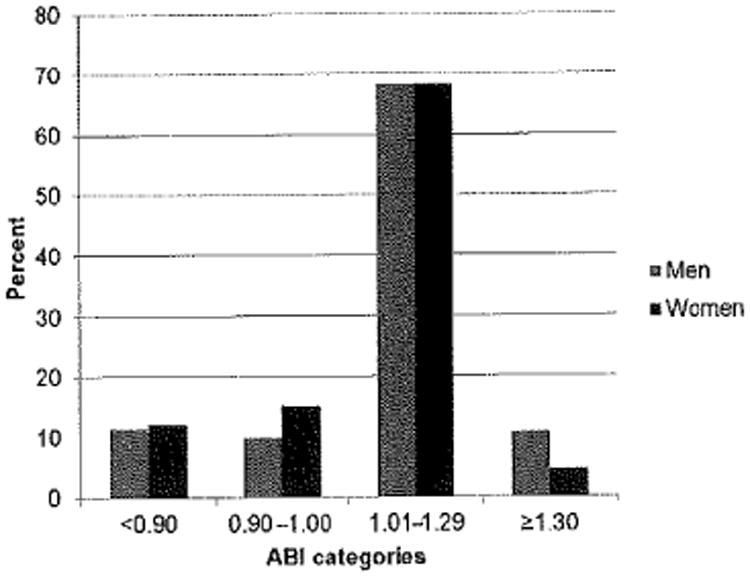
Among the 2797 participants included in this analysis, the mean age was 74 years, 52% were women and 40% were black. The characteristics most strongly associated with lower ABI categories were black race, elevated SBP, prevalent CVD and DM (Table 1). However, in women, the association with DM was only present in the ABI < 0.9 category. The mean ABI was significantly higher in men (1.10 ±0.18) compared with women (1.05 ± 0.17, P < 0.001). Men and women had a similar prevalence of ABI<0.9 (men 11%, women 12%) and ABI 1.01–1.29 (both men and women, 68%; Figure 1). However, women had a higher prevalence of ABI 0.9–1.0 compared with men (15% versus 10%, respectively, P < 0.001), and men had higher prevalence of ABI ≥ 1.3 compared with women (11% versus 5%, respectively, P < 0.001). The mean brachial pressures were significantly different among men (149 ± 20) and women (155 ± 26) with ABI <0.9 (P = 0.01); however, the mean brachial pressures did not differ in men and women in the other categories of ABI.
Table 1. Comparison of male and female participant characteristics by categories of ankle–brachial index.
| Men (N=1336) | Women (N = 1461) | |||||||
|---|---|---|---|---|---|---|---|---|
| <0.9 | 0.9–1.0 | 1.01–1.29 | ≥1.3 | <0.9 | 0.9–1.0 | 1.01–1.29 | ≥1.3 | |
| N | 151 | 129 | 912 | 144 | 177 | 220 | 998 | 66 |
| Age | 75 (3) | 74 (3) | 74 (3) | 74 (3) | 74 (3) | 73 (3) | 73 (3) | 74 (3) |
| Blacks | 84 (56) | 64 (50) | 293 (32) | 38 (26) | 114 (64) | 113 (51) | 391 (39) | 31 (47) |
| BMI | 26.8 (4.2) | 26.7 (3.9) | 27.2 (3.9) | 27.3 (4.0) | 27.6 (5.8) | 28.2 (6.1) | 27.4 (5.2) | 29.0 (6.2) |
| DM | 43 (29) | 31 (24) | 120 (13) | 21 (15) | 46 (26) | 27 (12) | 106 (11) | 11 (17) |
| SBP | 139 (20) | 140 (24) | 134 (20) | 132 (21) | 146 (26) | 140 (22) | 133 (19) | 135 (19) |
| DBF | 72 (12) | 74 (12) | 73 (11) | 71 (11) | 71 (12) | 71 (13) | 70 (12) | 69 (12) |
| LDL | 121 (32) | 123 (31) | 118 (33) | 113 (30) | 130 (35) | 127 (36) | 124 (37) | 118 (37) |
| HDL | 48 (13) | 50 (16) | 48 (14) | 47 (14) | 58 (16) | 63 (19) | 60 (17) | 60 (20) |
| Triglycerides | 117 [90,159] | 113 [78,144] | 117 [86,163] | 113 [85,169] | 116 [92,168] | 117 [91,151] | 121 [90,169] | 120 [91,169] |
| Prevalent CVD | 57 (38) | 38 (30) | 223 (25) | 28 (20) | 50 (29) | 46 (21) | 153 (16) | 11 (17) |
| Prevalent CHF | 6 (4) | 2 (2) | 14 (2) | 0 (0) | 2 (2) | 1 (1) | 6 (1) | 1 (2) |
Age = mean years (±SD); Blacks = number (%); BMI, body mass index = mean kg/m2 (±SD); DM, diabetes mellitus = number (%); SBP, systolic blood pressure = mean mmHg (±SD); DBP, diastolic blood pressure = mean mmHg (±SD); LDL, low-density lipoprotein cholesterol = mean mg/dL (±SD); HDL, high-density lipoprotein cholesterol = mean mg/dL (±SD); triglycerides = median mg/dL [IQR]; CVD, cardiovascular disease and includes history of slroke, transienl ischemic attack, or coronary heart disease; number (%); CHF, congestive heart failure; number (%)
Figure 1. Distribution of ankle–brachial index (ABI), stratified by sex.
Men had higher rates of incident clinical PAD compared with women across all categories of ABI (Figure 2a). Men had higher rates of CHD death and incident MI compared with women across all categories of ABI, except in the ≥1.3 category, where the rates were higher for women (Figures 2b and c). Women had higher rates of incident stroke compared with men in the low (ABI < 0.9) and high (ABI ≥ 1.3) categories of ABI (Figure 2d). For both men and women, the ABI < 0.9 and ABI 0.9–1.0 categories had higher rates of CHD death, incident clinical PAD, MI and stroke compared with the referent group. ABI categories had a U-shaped association with CHD death, incident PAD, MI and stroke among women (Figures 2a–d).
Figure 2.
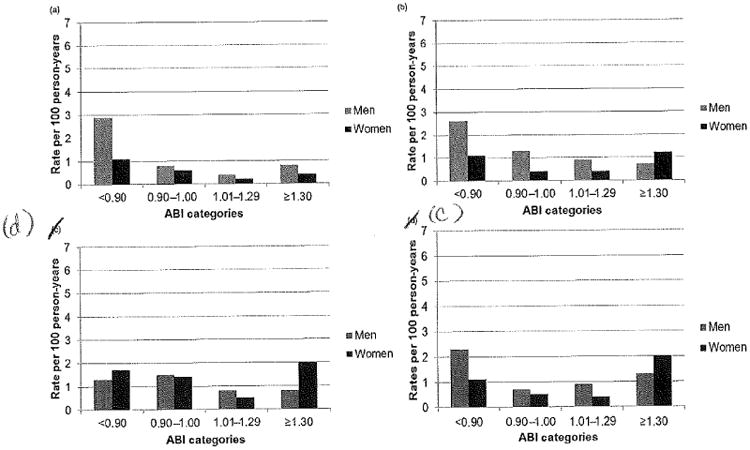
(a) Rates of incident clinical peripheral artery disease by category of ankle–brachial index, stratified by sex, (b) rates of coronary heart disease death by category of ankle–brachial index, stratified by sex, (c) rates of incident MI by category of ankle–brachial index, stratified by sex, (d) rates of incident stroke by category of ankle–brachial index, stratified by sex. ABI, ankle–brachial index; MI, myocardial infarction
In the fully adjusted Cox proportional hazards model, there were significant interactions observed between sex and ABI for the outcomes of incident MI (P = 0.03) and incident stroke (P = 0.007). Both men and women with ABI < 0.9 were at significantly higher risk for CHD death, clinical PAD and MI compared with the referent group; however, only women with ABI < 0.9 were at significantly higher risk for incident stroke (hazard ratio [HR] 2.58, 95% CI: 1.35–4.92 in women versus 1.17, 0.56–2.47 in men; Table 2). Both men (HR 3.49, 1.39–8.72) and women (HR 4.84, 1.53–15.31) with ABI 0.9–1.0 were at significantly higher risk for CHD death compared with the referent group. However, ABI 0.9–1.0 was significantly associated with incident clinical PAD (HR 3.33, 1.44–7.70) and incident stroke (HR 2.45, 1.38–4.35) only in women. Women with ABI ≥ 1.3 (n = 66) were also at significantly higher risk for incident stroke and MI, but this association was not observed in men (n = 144; Table 2). There were no significant changes to the hazard ratios when BMI and statin use were added to the fully adjusted Cox proportional hazards model.
Table 2. Association of ABI categories with cardiovascular events, stratified by sex.
| ABI<0.9 | Men (N = 1336) | Women (N = 1461) Fully adjusted† N = 177 | ||
|---|---|---|---|---|
|
| ||||
| Demo adjusted* | Fully adjusted† N = 151 | Demo adjusted* | ||
| CHD death | 3.78 (1.63, 8.77) | 4.38 (1.81, 10.62) | 5.35 (1.73, 16.48) | 4.96 (1.53, 16.01) |
| Incident PAD | 7.85 (4.44, 13.90) | 5.77 (3.21, 10.37) | 5.99 (2.70, 13.28) | 5.56 (2.44, 12.67) |
| Incident stroke | 1.32 (0.64, 2.75) | 1.17 (0.56, 2.47) | 2.84 (1.52, 5.31) | 2.58 (1.35,4.92) |
| Incident MI | 3.21 (1.72, 5.99) | 2.26 (1.19, 4.30) | 3.57 (1.67, 7.60) | 2.55 (1.13, 5.72) |
| ABI 0.9–1.0 | N = 129 | N = 220 | ||
| CHD death | 3.07 (1.25, 7.55) | 3.49 (1.39, 8.72) | 3.36 (1.11, 10.18) | 4.84 (1.53, 15.31) |
| Incident PAD | 2.22 (0.95, 5.16) | 1.70 (0.72, 4.03) | 3.53 (1.54, 8.12) | 3.33 (1.44, 7.70) |
| Incident stroke | 1.70 (0.88, 3.31) | 1.53 (0.77, 3,03) | 2.51 (1.42, 4.45) | 2.45 (1.38, 4.35) |
| Incident MI | 1.01 (0.40, 2.57) | 0.89 (0.35, 2.30) | 1.51 (0.65, 3.53) | 1.48 (0.62, 3.49) |
| ABI 1.01–1.29 | N = 912 | N = 998 | ||
| 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |
| ABI ≥ 1.3 | N = 144 | N = 66 | ||
| CHD death | 1.24 (0.36, 4.29) | 1.13 (0.33, 3.90) | – | – |
| Incident PAD | 1.36 (0.52, 3.59) | 1.60 (0.60, 4.24) | 1.99 (0.26, 15.48) | 2.48 (0.31, 19.78) |
| Incident stroke | 0.63 (0.25, 1.60) | 0.65 (0.26, 1.63) | 4.91 (2.34, 10.31) | 4.81 (2,27, 10.20) |
| Incident MI | 1.65 (0.84, 3.25) | 1.89 (0.95, 3.76) | 8,46 (3.71, 19.30) | 9.31 (4.01, 21.63) |
ABI, ankle–brachial index; CHD, coronary heart disease; PAD, peripheral artery disease; MI, myocardial infarction
Adjusted for age, race, site, education and income
Furlher adjusted for smoking, diabetes mellitus, hypertension, cystatin C-based glomerular filtration rate, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol
Discussion
Although women have lower overall rates of CVD compared with men, subclinical PAD (ABI≤1.0 in absence of lower extremity symptoms) appears to disproportionately affect women relative to men. Early epidemiological studies used ABI < 0.9 as a traditional cut-point for elevated CV risk; however, this threshold fails to identify a large number of at-risk individuals who have elevated risk of adverse CV events with ABI ≤; 1.0.8,10–12 We found an equal prevalence of ABI < 0.9 in men and women, but a higher prevalence of ABI 0.9–1.0 in women compared with men. In our study, ABI ≤ 1.0 was associated with significantly elevated risks of CHD death, clinical PAD and stroke, and these associations were at least as strong in women compared with men. Given the higher prevalence of ABI ≤ 1.0 in women, subclinical PAD appears to be a uniquely strong risk factor in women. In addition, we found ABI ≥ 1.3 to distinguish women with extremely high risk for CV events, but the overall prevalence was low.
Our analysis confirms findings from many previous studies that subclinical PAD is a strong and independent predictor of adverse CV events. However, the results from our study build on these data by demonstrating potential sex-specific risks for individual adverse CV outcomes. There is a debate over whether ‘normal’ ABI levels are different in men compared with women, and across ethnic groups.31–33 In a fully adjusted model of 1775 healthy participants in MESA, women had approximately 0.02 lower ABI values than men, and blacks had about 0.02 lower ABI levels than white.33 Even if women do have slightly lower ‘normal’ ABI values compared with men, we have shown that the sex-independent cut-point of ABI ≤ 1.0 denotes a significantly elevated risk for adverse CV events in both sexes. The finding that ABI ≤ 1.0 was strongly associated with risk for CVD events in women argues against the idea that the higher prevalence of ABI ≤ 1.0 simply reflects a lower ‘normal’ ABI value in women.
In our study, subclinical PAD was a consistent risk factor for adverse events in both a local vascular bed (lower extremities) as well as remote vascular territories (coronary and cerebral circulations). Although ABI < 0.9 was associated with an increased risk for clinical PAD events in both men and women, ABI 0.9–1.0 was associated with increased incident clinical PAD risk only in women (compared with the referent group). This is an important observation since several studies have demonstrated that women have worse outcomes after treatment for PAD compared with men, with lower rates of bypass graft patency and increased rates of wound complications.34–37 As such, women with subclinical PAD should be considered a high-risk group that warrants appropriate preventive strategies and risk factor modification before the clinical manifestations of lower extremity claudication, rest pain or ulceration become apparent.
Elevated ABI (ABI > 1.3) has also been shown to be a risk factor for adverse CV outcomes, as the vessels in the legs become more difficult to compress with the accumulation of arterial wall calcification. Several cohort studies have demonstrated that the association of ABI with risk of CV events and death has a reverse J-shaped distribution, with increased risk at both the low and high ends of the ABI spectrum.8,11,19 Our study demonstrated high ABI to be significantly associated with increased risk for incident stroke and MI, but only in women. In this study, men with high ABI had low rates of incident CV events and similar risk as the referent group, although the confidence intervals were wide. This association between high ABI with CV risk has not been entirely consistent in the literature. The Atherosclerosis Risk in Communities (ARIC) cohort did not demonstrate any increase in CHD risk in men or women with ABI > 1.3, despite a high prevalence (10.3%) of participants with AB I> 1.3; however, this is the only study that used an automated blood pressure cuff to determine ankle pressures.6 On the other hand, the Strong Heart Study found ABI > 1.4 to predict mortality with similar strength as ABI < O.9.8 Findings from the CHS cohort demonstrated ABI > 1.4 to be associated with higher risks of all-cause mortality, but not significantly associated with fatal and non-fatal CV events.11 The sex differences for high ABI observed in our study need to be evaluated in future studies, particularly given the small number of participants with high ABI.
Our study has several limitations. The Health ABC participants are an elderly cohort free of functional limitation at baseline; hence these results may not apply to a younger cohort or to older diabetic adults with more physical impairments or co-morbidities. There is also the possibility that we may not have excluded all participants with prevalent PAD. However, all participants at study entry were able to walk at least a quarter of a mile or climb up 10 stairs without resting, had no history of lower extremity claudication, and had no history of previous lower extremity angioplasty or arterial surgery. There is also the possibility of residual confounding. Finally, we had a small number of women in the ABI ≥ 1.3 category; although this appears to be a very high-risk category, these findings should be confirmed in a cohort with a larger sample of female participants.
Conclusions
Subclinical PAD is more common in women than in men, and women with subclinical PAD represent a high-risk group for future adverse CV events, Additional studies are needed to evaluate potential prevention and treatment interventions to mitigate the high observed risks for women with subclinical PAD.
Acknowledgments
This publication was supported by NIH/NCRR/OD UCSF-CTSI grant Number KL2 RR024130 (Dr Hiramoto), NIH R01DK087961-01A1, R01AG027002-05A1 and R01AG034853 (Dr Shiipak). Its contents are the responsibility of the authors and do not necessarily represent the official views of the NIH, This research was also supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050 and NINR grant R01-NR012459, and in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Conflicts of interest: None.
References
- 1.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the united states. Am J Prev Med. 2007;32:328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Selvin R, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 4.Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Sac. 2007;55:583–9. doi: 10.1111/j.1532-5415.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) collaborative research group. Circulation. 1993;88:837–45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 6.Weatherley BD, Nelson JJ, Heiss G, et al. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study, 1987–2001. BMC Cardiovasc Disord. 2007;7:3. doi: 10.1186/1471-2261-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman AE, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Healdi Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vase Biol. 1999;19:538–45. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 8.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 9.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ. 1996;313:1440–4. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooi JD, Kester AD, Staffers HE, Rinkens PE, Knottnerus JA, van Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7-year follow-up study. J Clin Epidemiol. 2004;57:294–300. doi: 10.1016/j.jclinepi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the cardiovascular health study. Circulation. 2006;113:388–93. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 12.Ono K, Tsuchida A, Kawai H, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol. 2003;14:1591–8. doi: 10.1097/01.asn.0000065547.98258.3d. [DOI] [PubMed] [Google Scholar]
- 13.Abbott RD, Petrovitch H, Rodriguez BL, et al. Ankle/brachial blood pressure in men >70 years of age and the risk of coronary heart disease. Am J Cardiol. 2000;86:280–4. doi: 10.1016/s0002-9149(00)00914-0. [DOI] [PubMed] [Google Scholar]
- 14.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kulp DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004;109:1089–94. doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- 15.Kornitzer M, Dramaix M, Sobolski J, Degre S, De Backer G. Ankle/arm pressure index in asymptomatic middle-aged males: an independent predictor of ten-year coronary heart disease mortality. Angiotogy. 1995;46:211–9. doi: 10.1177/000331979504600304. [DOI] [PubMed] [Google Scholar]
- 16.Vogt MT, Carney JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465–9. [PubMed] [Google Scholar]
- 17.Heald CL, Fowkes FG, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: systematic review. Atherosclerosis. 2006;189:61–9. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–12. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham risk score to predict cardiovascidar events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosca L, Manson JE, Sutherland SE, Langer RD, Manolio T, Barrett-Connor E. Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association Writing Group. Circulation. 1997;96:2468–82. doi: 10.1161/01.cir.96.7.2468. [DOI] [PubMed] [Google Scholar]
- 21.Kullo IJ, Bailey KR, Kardia SL, Mosley TH, Jr, Boerwinkle E, Turner ST. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Artenopathy (GENOA) study. Vasc Med. 2003;8:237–42. doi: 10.1191/1358863x03vm511oa. [DOI] [PubMed] [Google Scholar]
- 22.Zheng ZJ, Rosamond WD, Chambless LE, et al. Lower extremity arterial disease assessed by ankle-brachial index in a middle-aged population of African Americans and Whites: the Adierosclerosis Risk in Communities (ARIC) study. Am J Prev Med. 2005;29:42–9. doi: 10.1016/j.amepre.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Gofin R, Kark JD, Friedlander Y, et al. Peripheral vascular disease in a middle-aged population sample. The Jerusalem Lipid Research Clinic Prevalence study. Isr J Med Set. 1987;23:157–67. [PubMed] [Google Scholar]
- 24.Sigvant B, Wiberg-Hedman K, Bergqvist D, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–91. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Jiang Y, Wang J, Fan L, Li X, Hu FB. Prevalence of peripheral arterial disease and its association with smoking in a population-based study in Beijing, China. J Vasc Surg. 2006;44:333–8. doi: 10.1016/j.jvs.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 27.2011 ACCF/AHA focused update of the guideline for die management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2011;124:2020–45. doi: 10.1161/CIR.0b013e31822e80c3. [DOI] [PubMed] [Google Scholar]
- 28.See National Institute of Aging. National Institutes of Health Health ABC; 2010. http://wAVW.grc.nia.noh.gov/branches/ledb/healthabc/index.htm.
- 29.Madero M, Wassel CL, Peralta CA, et al. Cystatin c associates with arterial stiffness in older adults. J Am Soc Nephrol. 2009;20:1086–93. doi: 10.1681/ASN.2008030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin c alone and in combination with serum creatinine: a pooled analysis of 3418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Bailey K, Kullo I. Ethnic differences in ankle brachial index are present in individuals without peripheral arterial disease. Atheroscler Supp. 2009;10 doi: 10.1016/j.ijcard.2011.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.London GM, Guerin AP, Pannier B, Marchais SJ, Stimpel M. Influence of sex on arterial hemodynamics and blood-pressure – role of body height. Hypertension. 1995;26:514–9. doi: 10.1161/01.hyp.26.3.514. [DOI] [PubMed] [Google Scholar]
- 33.Aboyans V, Criqui MH, McClelland RL, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: The Multi-Edinic Study of Atherosclerosis (MESA) J Vase Surg. 2007;45:319–27. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen LL, Brahmanandam S, Bandyk DF, et al. Female gender and oral anticoagulants are associated with wound complications in lower extremity vein bypass: an analysis of 1404 operations for critical limb ischemia. J Vasc Surg. 2007;46:1191–7. doi: 10.1016/j.jvs.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen LL, Hevelone N, Rogers SO, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009;119:123–30. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AhChong AK, Chiu KM, Wong M, Yip AW. The influence of gender difference on die outcomes of infrainguinal bypass for critical limb ischaemia in Chinese patients. Eur J Vasc Endovasc Surg. 2002;23:134–9. doi: 10.1053/ejvs.2001.1564. [DOI] [PubMed] [Google Scholar]
- 37.Enzler MA, Ruoss M, Seifert B, Berger M. The influence of gender on the outcome of arterial procedures in the lower extremity. Eur J Vasc Endovasc Surg. 1996;11:446–52. doi: 10.1016/s1078-5884(96)80180-8. [DOI] [PubMed] [Google Scholar]


