Abstract
Choline carboxylates, ChCm, with m = 2–10 and choline oleate are known as biocompatible substances, yet their influence on biological membranes is not well-known, and the effect on human skin has not previously been investigated. The short chain choline carboxylates ChCm with m = 2, 4, 6 act as hydrotropes, solubilizing hydrophobic compounds in aqueous solution, while the longer chain choline carboxylates ChCm with m = 8,10 and oleate are able to form micelles.
In the present study, the cytotoxicity of choline carboxylates was tested using HeLa and SK-MEL-28 cells. The influence of these substances on liposomes prepared from dipalmitoylphosphatidylcholine (DPPC) was also evaluated to provide insights on membrane interactions. It was observed that the choline carboxylates with a chain length of m > 8 distinctly influence the bilayer, while the shorter ones had minimal interaction with the liposomes.
Keywords: Choline ionic liquids, Biological membrane, Cytotoxicity
1. Introduction
The investigation of the toxicity of ionic liquids and surfactants is a very important field, since it was found that commonly used cations in ionic liquids like imidazolium or pyridinium are toxic in nature [1]. Further, choline compounds are forbidden in cosmetical products according to the European Cosmetic Directive 76/768/EEC, because they are assigned as quaternary ammonium compounds, which are known as phase transfer catalysts with intrinsic irritation potential [2]. In order to boost the utilization of choline compounds in future applications, cytotoxicity tests were performed to evaluate the actual skin irritation potential. Previous toxicity experiments considered the charge, the number of ethoxy groups [3] and the hydrophilicity, but in the case of surfactants it is also very important to consider the critical micelle concentration (cmc) [1,3–5]. The cytotoxicity of ionic surfactants is mainly caused by the monomers [3,6] and consequently, the IC50 values – concentrations required to cause death in 50% of the cell population – are usually found to be below the cmc. To check this for our systems, we also measured the cmc. In a previous study Petkovic et al. [7] investigated the toxicity of choline carboxylates ChCm with m = 2–10 by carrying out tests with filamentous fungi as a model for eukaryotic organisms. Choline carboxylates were found to exert only minor toxic activity on these organisms. Also Muhammad et al. found just slight cytotoxic effects on the human breast cancer cell line MCF-7 for choline carboxylates ChCm with m = 2,3,4, 6 [8].
The present study is focused on the influence of choline carboxylates on biological cell membranes. The aim of the paper is to determine if there is a correlation between IC50 values and the substrate concentration at which an interaction of the substance with the liposomes is found. Although the culture and buffer media are different, the primary interaction of interest is membrane disruption caused by choline carboxylate penetration, which should be possible to discern in both environments.
The IC50 values were determined for two human cell lines, namely cervix carcinoma cells (HeLa) and keratinocytes (SK-MEL-28). [9–13]. Cytotoxicity was analysed for short chain choline carboxylates ChCm with a chain length of m = 2–10 and their respective sodium equivalents. To further investigate the influence of the double bond in an alkyl chain on the cytotoxicity, IC50 values of choline oleate and choline octadecanoate were determined. Further, the impact of an additional carboxylate group was examined by analyzing the cytotoxicity of choline bicarboxylates, like choline succinate (ChbiC4), choline glutarate (ChbiC5) and choline adipate (ChbiC6) and their sodium equivalents (Fig. 1).
Fig. 1.
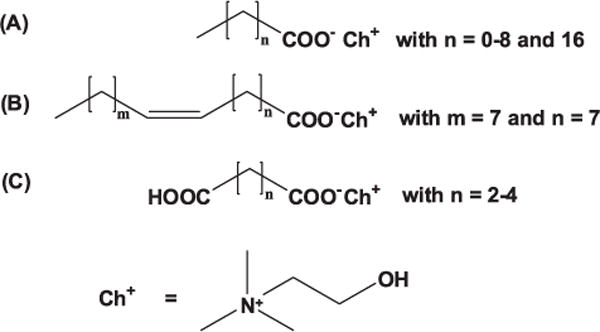
Cytotoxicity was measured of choline carboxylates ChCm with m = 2–10 and 18 (A), of choline oleate (B) and of choline bicarboxylate ChbiCm with m = 4–6 (C) and in addition of their respective sodium equivalents.
To better understand interfacial interactions with membranes, the influence of the choline carboxylates ChCm with m = 2–10 and choline oleate on the gel to liquid crystalline transition of cooperativity behavior of DPPC liposomes, a basic biological membrane analog, was investigated at the biological pH of 7.4.
To ensure that the toxicity results only from the surfactant monomer, and is not influenced by the formation of micelles, the critical micelle concentration of choline octanoate, choline decanoate and choline oleate was determined by conductivity and surface tension measurements. The hydrotrope behavior of the short chain choline carboxylates like choline acetate, choline butanoate and choline hexanoate was investigated. Sodium alkyl-benzene sulfonates and sodium butyl monoglycolsulfate (NaBMGS) are used to extract non-polar, water insoluble phyto-constituents selectively due to permeabilization of the cell. Also choline hexanoate is able to dissolve suberin out of cork by cleavage of the ester bonds in suberin [14].
2. Materials and methods
2.1. Synthesis
The synthesis of choline acetate was done by neutralization of acetic acid (Merck, purity 100%) with 45% methanolic choline hydroxide solution (Sigma–Aldrich). The obtained salt was recrystallized twice in diethylether. A white salt was obtained after lyophilization and drying under vacuum atmosphere.
Choline carboxylates (ChCm) with m = 4, 5, 6, 7, 8, 9,10, choline oleate and choline bicarboxylates (ChbiCm) with m = 4, 5, 6 were synthesized according to the synthesis route of Petkovic et al. but with minor modifications [7]. In contrast to the synthesis of Petkovic et al. [7] the choline carboxylate ionic liquid was lyophilized and then dried for more than two weeks on a high vacuum pump. Heating during this procedure was skipped to avoid decomposition of the choline cation.
The following chemicals were used: Oleic acid (Sigma–Aldrich, purity ≥ 99%), propionic acid (Sigma–Aldrich, purity ≥ 99.5%), butyric acid (Sigma–Aldrich, purity ≥ 99%), valeric acid (Sigma–Aldrich, purity ≥ 99%), hexanoic acid (Sigma–Aldrich, purity ≥ 99%), heptanoic acid (Merck, purity ≥ 99%), octanoic acid (Sigma–Aldrich, purity ≥ 99%), nonanoic acid (Sigma–Aldrich, purity ≥ 97%), decanoic acid (Alfa Aesar, purity = 99%), adipic acid (Alfa Aesar, purity ≥ 99%), glutaric acid (Alfa Aesar, purity ≥99%), succinic acid (Alfa Aesar, purity ≥ 99%) and 80 wt% aqueous choline bicarbonate solution (Sigma–Aldrich, stored at 2°C to avoid decomposition and without stabilizer).
The purity of the choline carboxylates was evaluated with electro-spray mass spectroscopy (ThermoQuest Finnigan TSQ 7000 instrument) (ES-MS) and 1H and 13C NMR measurements (Bruker Avance 300 spectrometer at 300 MHz using tetramethylsilane (TMS) as internal standard) were performed. Coulometric Karl-Fischer titration was performed on an Abimed MCI analyzer (Model CA-02) to determine the water content.
The sodium salts were prepared by adding an equimolar amount of 1 M sodium hydroxide solution (Merck) to the corresponding carboxylic acid. The sodium salts were lyophilized (>24h) and dried under vacuum atmosphere. For the synthesis the above mentioned carboxylic acids were used. Sodium octanoate (Sigma–Aldrich, purity ≥ 99%), sodium decanoate (Sigma–Aldrich, purity ≥ 98%) and sodium oleate (Sigma–Aldrich, purity = 99%) were bought. The melting temperatures of the choline based ionic liquids are given in the SI.
2.2. UV–Vis measurements
To determine the concentration of hydrotrope in water at which the solubilization of hydrophobic dye in the aqueous hydrotrope solution of choline acetate, choline butanoate or choline hexanaote increase, Disperse Red 13 was solubilized as hydrophobic dye. All the solubilization experiments and the UV–Vis measurements were performed in a thermostated room at 25 ± 0.2 °C. Different concentrated aqueous hydrotrope solutions were prepared and saturated with Disperse Red 13. After stirring for 24 h the excess of dye was removed. The amount of the dissolved Disperse Red 13 was determined with UV–Vis measurements on a Varian Cary 3E spectrophotometer. The absorption was measured at the wavelength of 503 nm [15]. The calibration curve with defined amounts of Disperse Red 13 was prepared by dissolving Disperse Red 13 in absolute ethanol (Baker, purity ≥ 99.9%).
2.3. Conductivity
A valuable method to determine the critical micelle concentration cmc and also to determine the micelle ionization degree α at the cmc is to measure the concentration dependent specific conductivity κ of an aqueous choline carboxylate solution ChCm with m = 8, 10 and oleate. An autobalance conductivity bridge (Konduktometer 702, Knick), arranged with a Consort SK41 T electrode cell was used to measure the specific conductivity. For the calibration 0.01 M, 0.1 M and 1 M potassium chloride solutions were needed to determine the cell constant at 25 °C and 40 °C [16]. The concentration dependent conductivity was evaluated at 25 °C and 40 °C. A break in the slope of the plot of concentration versus the specific conductivity κ marks the cmc of the respective choline carboxylates (see Supplementary Information (SI)). The accuracy of the cmc values is ±4%. We are aware that the culture medium would cause a minor decrease in the cmc because of the high ionic strength. However, this slight shift would not change our qualitative conclusions.
For the calculation of the micelle ionization degree α at the cmc Eq. (8) presented by Evans [17] was used:
| (8) |
λCh+ is the counterion conductivity given by Fleming as 42.0 S cm2 [18]. N represents the amount of surfactant molecules in the micelle and S1 the slope before the cmc and S2 the slope above the cmc. The micelle ionization degree α could only be calculated for choline oleate. Choline octanoate and choline decanoate are at the limits of Eq. (8) and present too small slopes.
2.4. Surface tension
To determine the critical micelle concentration of choline octanoate, choline decanoate and choline oleate at 25 °C and 40 °C the concentration dependent surface tension σ was measured. The temperature accuracy was 25 °C± 0.1 °C and 40°C±0.1 °C. The measurements were performed with a Krüss tensiometer (model K100 MK2) using a platinum-iridium ring. The data acquisition works automatically and records the surface tension as a function of the concentration. The surface tension was measured during dilution of the soap solution with degassed, bidistilled water. The data correction was done according to the procedure performed by Harkins and Jordan [19].
2.5. Cytotoxicity on HeLa and SK-MEL-28 cells
Cytotoxicity of the compounds was determined on SK-MEL-28 (keratinocytes, CLS 300337) and HeLa (cervix carcinoma, ATCC CCL17) cells using MTT assay introduced by Mosmann et al. [19] and modified by Vlachy et al. [21] with the only exception that in this study culture media did not contain Amphotericin B and Penicillin G/Streptomycinsulfate [20]. For the stock solutions, the substances were directly dissolved in the corresponding culture media. The cells were exposed for 68 h to the test compounds followed by incubation with MTT for four hours. For each ionic liquid in solution the IC50 value (in molL−1) was determined. This value represents the concentration of tested substance that lowers MTT reduction by 50% relative to the untreated control. The IC50 value was calculated for each substance from a concentration-response curve, which was based on eight different concentrations. The assay was performed in triplicate and repeated independently three to five times. The results are expressed as the averaged IC50 values ±standard deviation (SD) (see Fig. 4 and Table 3). The dose-response curves of the substances are shown in SI. We are aware that IC50 values are inhibitory concentrations and therefore their interpretation as a measure of cytotoxicity must be taken with caution. However, IC50 values of HeLa and SK-Mel Cells are frequently used to estimate skin irritation power and cytotoxicity, in the manner that we have used here.
Fig. 4.
IC50 values of choline (Δ) and sodium (▲) carboxylates and choline (O) and sodium (•) bicarboxylates obtained on (A) SK-MEL-28 and (B) HeLa cells as a function of alkyl chain length m.
Table 3.
IC50 values of choline oleate, sodium oleate and choline octadecanoate (ChC18) determined for HeLa and SK-MEL-28 cells.
| Ionic liquid | HeLa IC50 [mmol L−1 ] | SK-MEL-28 IC50 [mmolL−1] |
|---|---|---|
| Choline oleate | 0.467 ± 0.034 | 0.521 ±0.051 |
| Sodium oleate | 0.263 ± 0.027 | 0.813 ± 0.198 |
| ChC18 | 0.832 ± 0.143 | 0.116 ± 0.015 |
2.6. Calorimetry of model membranes
Large, unilamellar vesicles of DPPC were prepared by extrusion as described by Weaver et al. [22], using membranes with a pore size of ≤0.2 μm. Briefly, lyophilized DPPC powder was hydrated with 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer at pH 7.40, and 70°C, then incubated for 30 min. Solutions were extruded through 100nm Nucleopore® track-etched polycarbonate filters (Whatman, Pleasanton, CA) that had been mounted in a mini-extruder (Avanti Polar Lipids, Alabaster, AL). Liposomes were then refrigerated at 4°C for a minimum of 16 h before use.
A MicroCal VP-DSC microcalorimeter (MicroCal, Northhampton, MA) was used for thermal analysis. An aliquot of the stock lipid vesicle solution was combined with experimental compounds in 20 mM HEPES buffer at pH 7.40 in a 1:1 ratio by volume. A control (lipid only) thermal scan was acquired for each tested compound. After a 15 min equilibration period at 10°C, samples were scanned from 20°C to 50°C at 60°C/hour. Reversibility was determined by rescanning after cooling to the initial temperature. Tests were prepared with choline carboxylates ChCm with chain length m = 2, 3, 4, 5, 6, 7, 8, 9, 10 and choline oleate.
3. Results and discussion
3.1. Hydrotrope behavior
There are two opposing forces, which are the decisive factors in determining whether a substance belongs to the group of hydrotropes, which show little self-aggregation, or to surfactants, which aggregate at the critical micelle concentration (cmc) to micelles [23]. This is on the one hand the energy which is needed to transfer the hydrophobic tails of the amphiphil into the interior of the micelle and on the other hand the repulsion force of the head-groups [24]. In this study, the hydrophobicity varies with the chain length. It was found that choline carboxylates with chain length of m ≥ 8 behave as surfactants, whereas with m < 8 they behave like hydrotropes [25].
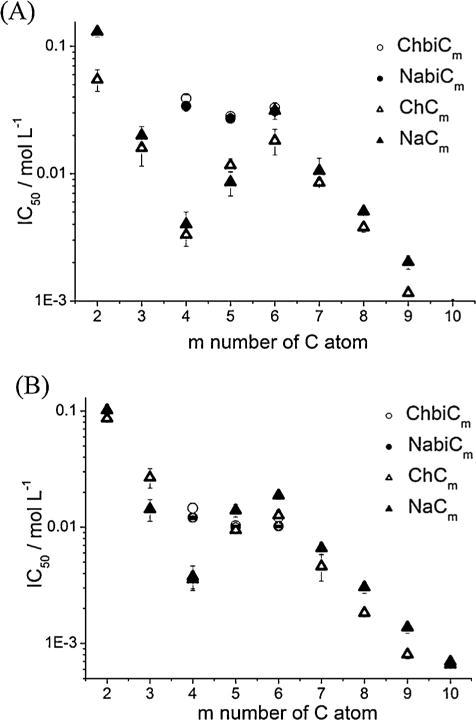
Above a certain threshold concentration of hydrotrope in water, often called minimal hydrotrope concentration MHC (≈1 M)[26], solubilization of a hydrophobic, hardly water soluble compound in the aqueous hydrotrope solution increases exponentially. To evaluate this MHC, the departure from linearity of the solubilization curve has to be determined [15]. For many amphiphilic molecules this concentration is hard to determine because the increase of solubilized hydrophobic molecules, here hydrophobic dye Disperse Red 13, occurs exponentially even at low concentration. Thus, the term minimal hydrotrope concentration is controversial.[26] The MHC of sodium xylenesulfonate (SXS), sodium p-toluenesulfonate and sodium butylmonoglycolsulfate (NaBMGS) is 0.4 M, 0.35 M, and 0.8 M, respectively. To compare the solubilizing value of choline carboxylates ChCm with m = 2,4,6 with commonly used hydrotropes, the concentration dependent optical densities obtained from UV-Vis measurements, resulting from the dissolved amount of Disperse Red 13 in the aqueous hydrotrope solutions, were determined. The optical density increases exponentially above a certain threshold concentration (Fig. 2(A)), namely 1.75 M for choline hexanoate, 3.5 M for choline butanoate and 5.4 M for choline acetate. These values are much higher than the MHCs found for the common hydrotropes. Also comparing the optical densities of Disperse Red 13 solutions at the aqueous hydrotrope concentration of 2.248 M, it is observed that Disperse Red 13 is sparingly soluble in the choline carboxylates ChCm with m = 2, 4, 6 compared to the very efficient hydrotrope SXS (Table 1) [26].
Fig. 2.
Aqueous hydrotrope concentration of ChCm with m = 2, 4, 6 versus the optical density (O. D.503nm) value of the dissolved amount of Disperse Red 13 in the solution taken at a wavelength of 503 nm.
Table 1.
Optical density O. D. values of Disperse Red 13 saturated aqueous hydrotrope solutions at 25 °C and a hydrotrope concentration of 2.248 mol/L. The values were measured at 503 nm.
| Hydrotrope | Optical Density O.D.503nm |
|---|---|
| CHC6 | 54.0 |
| ChC4 | 0.2 |
| ChC2 | ≤ 0.2 |
| SXS | 347.0 (Bauduin et al., 2005) |
Compared to choline hexanoate and choline butanoate, sodium hexanoate and sodium butanoate are able to self-aggregate in aqueous solution and show a cmc of 1.57 M (20°C) and 3.5 M (25°C), respectively [27,28]. These values are in the same concentration range as found for the MHCs of the choline ones.
3.2. Critical micelle concentration (cmc)
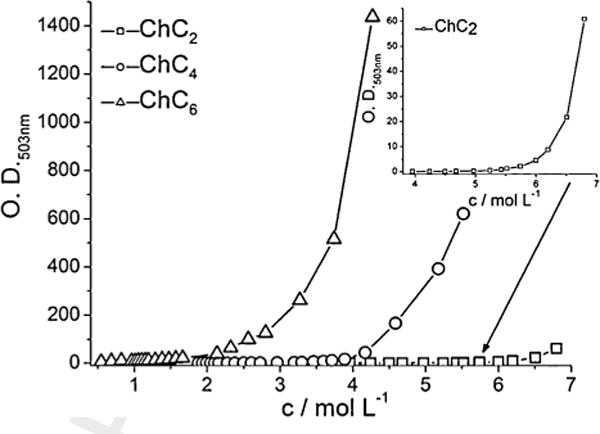
The cmc is further influenced by several factors e.g. by the presence of salts or organic compounds in the solution, the structure of the surfactant, the existence of a second liquid phase, the chain length and temperature. In this study, the influence of the temperature (25°C and 40°C), structure (choline stearate vs. choline oleate) and chain length (m = 8, 10) of choline carboxylates on the cmc was investigated. It is known that the free energy of micellization, which reflects the chain length of a surfactant, is proportional to the logarithm of the activities in dilute solutions. In dilute solutions the activity can be approximated by the concentration [23,29]. Therefore the logarithm of the cmc shows a linear relation to the surfactant chain length m (Eq. (1)).
| (1) |
A is a characteristic value at a distinct temperature for the head-group. B is a constant at the respective temperature. [23,29] Klein et al. [25] found for long chain choline carboxylates ChCm with m = 12, 14, 16 at 25°C a value of 1.9 for A and 0.29 for B as it is typical for monoionic headgroups. This equation fits also very well with the cmcs found for choline octanoate and choline decanoate at 25°C (Fig. 3). Potassium carboxylates possess the same values for A and B at 25°C [29].
Fig. 3.
Linear dependence of the logarithm of the cmc of potassium, sodium and choline carboxylates of the alkyl chain length m. (25°C: KC8 [31], KC10 [31], NaC8 [31], NaC10 [31], ChC12 [24], ChC14 [24], ChC16 [24]; 40°C: KC8 [31], NaC10 [31]).
As shown in Fig. 3, the cmc of choline carboxylate surfactants is not significantly affected by exchanging the choline cation for sodium or potassium nor by changing the temperature. Normally, the cmc of alkali carboxylates decreases with increasing size of the alkali counterion [29] and also with decreasing counterion-headgroup binding. Taking into account Collinsćoncept [30,31] the counterion-headgroup interaction should increase in the order Ch+ < K+ < Na+, and the cmc should also increase. As seen in Fig. 3, the influence of the counterion is negligible in dilute solutions [29].
It is known from the literature that the cmc should increase with a double bond in the alkyl chain [29]. This could be also confirmed in the case of choline oleate. The cmc of choline octadecanoate could not be measured at 25°C due to the high Krafft temperature of 40°C [25]. The above stated Eq. (1) predicts a value of 0.45 mM for the cmc of choline octadecanoate at 25°C. The cmc increases for choline oleate (Table 2), which contains a double bond in the alkyl chain compared to choline octadecanoate. Comparing the cmc of sodium oleate (cmc = 2.64 mM [32]) and potassium oleate (cmc = 0.80 mM [32]) at 25°C with the one of choline oleate no systematic influence of the counterion could be found. For choline oleate, the micelle ionization degree α at the cmc could be calculated (see Table 2). The value is in the same range as found for the long chain choline carboxylates [25].
Table 2.
Critical micelle concentration cmc of choline octanoate, choline decanoate and choline oleate (Choleate) obtained from conductivity and surface tension measurements at 25°C and 40°C. The micelle ionization degree α at the cmc was calculated from the conductivity measurements.
| cmca/mM | cmcb/mM | α | |
|---|---|---|---|
| ChC8 25°C | 383.0 | 324.9 | – |
| ChC8 40°C | 393.8 | 360.0 | – |
| ChC10 25°C | 103.3 | 75.0 | – |
| ChC10 40°C | 106.8 | 87.2 | – |
| Choleate 25°C | 1.6 | 1.3 | 0.30 |
| Choleate 40°C | 1.7 | 1.6 | 0.24 |
Critical micelle concentration cmc of choline octanoate, choline decanoate and choline oleate (Choleate) obtained from conductivity measurements.
Critical micelle concentration cmc of choline octanoate, choline decanoate and choline oleate (Choleate) obtained from surface tension measurements.
3.3. Cytotoxicity
The application of choline carboxylates in cosmetical formulation is currently forbidden due to the European Cosmetic Directive 76/768/EEC (Annex II/168) [33].
In order to check the usability of the choline carboxylates and choline bicarboxylates in pharmaceutical formulations or other applications and to obtain the IC50 value of the different choline carboxylates and choline bicarboxylates, the substances were tested for their cytotoxic effects. Cytotoxicity was determined performing MTT assays on HeLa and SK-MEL-28 keratinocyte cells according to the procedure described by Mosmann et al. [20] and modified by Vlachy et al. [21].
In general, cell tests with keratinocytes are a good in vitro method to obtain a reliable forecast of skin irritation potential [34,35]. To get reliable cytotoxicity results with in vitro tests, it is necessary that the substance is completely soluble in the culture media [3]. The choline carboxylates ChCm with m = 8, 10 and oleate show Krafft temperatures lower than 0°C [36]. The Krafft temperature of choline nonanoate and the shorter chain carboxylates should be in the same range, because the Krafft temperature decreases with decreasing chain length [23]. The sodium salts also show Krafft temperatures below room temperature [37–39]. Choline octadecanoate possesses a Krafft temperature of TKrafft ≈40°C [25]. However, it could be dissolved directly in the culture media. In contrast, the corresponding sodium salt was not soluble in the media and could not be tested [3].
The determined IC50 values of the choline carboxylates and choline bicarboxylates, obtained from SK-MEL-28 and HeLa cells, show a cytotoxicity comparable to that one of the sodium analogs. Accordingly, the impact of the cation on the cytotoxicity is very low (Fig. 4). No correlation was found for cytotoxicity and counterion. Petkovic et al. [7] and Klein et al. [2] obtained comparable cytotoxicity data for the choline carboxylates and their respective sodium soaps. On the contrary, they got a slightly higher cytotoxicity for the sodium carboxylates [2,7]. This shows that cytotoxicity is mediated to a greater extend by the anion than by choline. It has also been reported that in tests with alkali salts the alkali cation shows no intrinsic toxicity [40]. In contrast to prior studies, the toxicity of carboxylates increases with decreasing size of the cation. This was found for alkali cations as well as for quaternary ammonium ions [35,41]. The toxicity was explained by the stronger binding of a small cation on the carboxylate headgroup compared to a larger and higher charge diffused cation, like a quaternary ammonium ion. This stronger binding resulted in ion pairs [40,42]. However, as already seen for the cmc, the difference in counterion binding is negligible in dilute solutions and so the toxicity of these carboxylate soaps is not cation specific. The overall cytotoxicity of these substances is very low. According to the T-SAR (Thinking in Structure-sActivity Relationship) concept [40, 43, 44] the cytotoxicity should increase with increasing hydrophobicity and thus with increasing chain length [42, 45, 46].
To verify this, the cytotoxicity of the even and also of the odd chain length carboxylates, which are not naturally occuring, was analysed. This should reveal if the toxicity increases monotonously with increasing chain length or if a non-monotonous behavior exists, as for physico-chemical properties (e.g. Krafft point [47]).
In both cell lines, cytotoxicity increased with increasing alkyl chain length, but not in a linear way. An exception was found for the choline carboxylates ChCm and for the sodium carboxylates NaCm with m = 4–6. In these cases, the cytotoxicity decreased with increasing chain length. This is in contrast to the results of Petkovic et al. [7] (where cytotoxic effects follow as be explained according to the stated in T-SAR concept [40,42]. Also the results are not in line with those of Muhammad et al., who reported that cytotoxicity of ChCm with m = 2, 3, 4, 6 on the human breast cancer cell line MCF-7 decreases with increasing chain length [8]. This study agrees partly with the results found by Petkovic et al. [7] where the cytotoxicity increases with increasing chain length (see m = 2–4 and 6–10) and partly with the one of Muhammad et al. [8], where the cytotoxicity decreases with increasing chain length (see m = 4–6). However, cytotoxicity often depends on the considered system and on the cell line that is used for toxicity analysis [1].
In literature, such breaks in linearity of cytotoxicity are often found for chain length m = 10–14 [2, 6, 48]. To the best of our knowledge it was not yet reported for chain lengths of m = 4–6. To check if this discontinuity in cytotoxicity in the line of choline butanoate, choline pentanoate and choline hexanoate could result from the amount of — CH2— or —CH3 groups in the alkyl chain, the cytotoxicity of choline succinate, choline glutarate and choline adipate on HeLa and SK-MEL-28 cells was investigated. Again, a break in the linear course of cytotoxicity was found for choline glutarate. This suggests a correlation to the amount of available — CH2— groups in the alkyl chain.
Furthermore, the cytotoxicity of choline oleate, sodium oleate and choline octadecanoate was analysed to investigate the influence of the double bond in an alkyl chain. Compared to the long chain choline carboxylates (ChCm with m = 11–16) the IC50 value of choline octadecanoate is in the same range for the HeLa and the SK-MEL-28 cells [49]. Also, the toxicity of choline oleate is in the range of choline octadecanoate. A specific influence of the double bond could not be found. The counterion shows also no impact on the cytotoxicity of oleate soaps, as already seen for the other carboxylates (Tables 3 and 4).
Table 4.
Slope S1 and S2 obtained from the plot of the concentration versus the specific conductivity. The accuracy of the slope is 5%. The one of the calculated ionization degree α is 7%.
| 1000S1 [Scm2 mol−1] | 1000S2 [Scm2 mol−1] | A | |
|---|---|---|---|
| Choline oleate 25°C | 56.6 | 37.3 | 0.30 |
| Choline oleate 40°C | 77.1 | 44.8 | 0.24 |
3.4. Lipid bilayer interactions
DPPC membranes were exposed to choline carboxylate compounds with m = 2–10 and oleate in order to identify trends in lipid bilayer interactions [22]. As shown in Fig. 5, the temperature (Tm) and enthalpy of the melting transition (ΔH), as well as the cooperativity, were not significantly different from control up to an alkyl chain length of m = 8. Choline carboxylates with m = 9 and greater displayed significant interactions with the lipid bilayer. Choline nonanoate (m = 9) and choline decanoate (m = 10) both significantly decreased the Tm and the cooperativity of the transition, as measured by the width of the peak at the midpoint of the transition. The ΔH was not significantly altered. In the case of choline nonanoate, significant shouldering was also observed (Fig. 6). This is typically observed when the inner and outer headgroups are hydrated differentially. For choline decanoate and choline nonanoate at a concentration of 10 mM, there is significant interaction of the ionic liquid and the lipid bilayer, consistent with penetration of the compounds into the acyl chain region. At a concentration of 10 mM choline oleate significantly disrupts the liposome, evidenced by loss of the DPPC peak. The determined cmc (1.6 mM) is less than the test concentration of 10 mM, suggesting that micelles may have formed under these processing conditions. In the case of choline nonanoate the concentration dependence of the membrane interactions was also investigated. At the IC50 concentration there is only a modest depression in the Tm, but as the concentration increased, the Tm and cooperativity decreased considerably, consistent with growing membrane disruption. This would indicate a likely accumulation of choline nonanoate in the lipid bilayer.
Fig. 5.
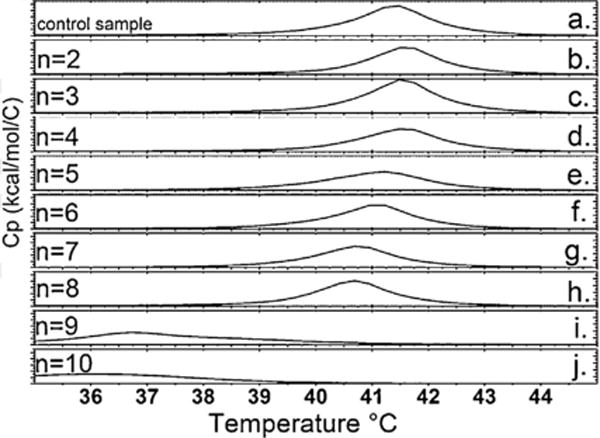
DSC thermal traces illustrating the melting point of DPPC liposomes with and without (control) a 10 mM choline carboxylate solution ChCn with n = 2–10 at pH 7.40.
Fig. 6.
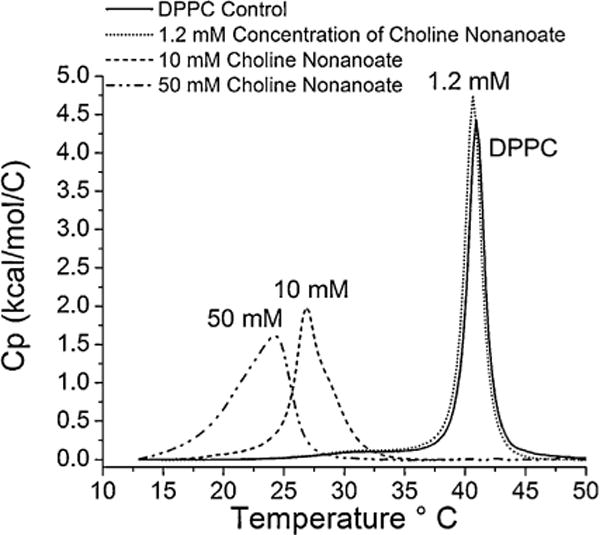
DSC thermal traces demonstrating the influence of different concentrations of choline nonanoate on the melting temperature of the DPPC liposomes from a gel phase to a liquid crystalline phase at pH 7.40: 0 mM ChC9,1.2 mM ChC9,10 mM ChC9, 50 mM ChC9.
4. Conclusion
Choline carboxylates ChCm with m = 2, 4, 6 behave like hydrotropes and dissolve Disperse Red 13 exponentially with increasing hydrotrope concentration above a certain threshold concentration. However, compared to the common hydrotropes they are not very efficient at dissolving sparingly water-soluble compounds. In contrast to the sodium analogs, choline as a counterion inhibited the self-aggregation of choline hexanoate and choline butanoate [27, 32]. Choline octanoate and choline decanoate behave as their sodium and potassium analogs, exhibiting similar cmc values. It was found that the size of the cation and the cation to carboxylate interaction in very diluted aqueous solutions show no influence on the cmc. The logarithm of the cmc decreases linearly with the chain length. With increasing temperature, the cmc values increase only slightly. Choline oleate shows an increased cmc compared to choline octadecanoate, due to its double bond. However, the cmc is not in line with the results found for alkali oleate soaps, where the cmc decreases with increasing size of the counterion [32].
Cytotoxicity on HeLa and SK-MEL-28 cells is not influenced by the cation, but is very dependent on the anion. A double bond, as seen for choline oleate, has no influence on toxicity. Cytotoxicity of the choline carboxylates ChCm and sodium carboxylates NaCm with m = 2, 3, 4, 5, 6, 7, 8, 9, 10 increased with increasing chain length, but in a non-linear way. The discontinuity in cytotoxicity could result from the amount of —CH2— groups in the alkyl chain, because it was also observed in the row of choline bicarboxylates ChbiCm with m = 4, 5, 6 for choline glutarate (ChbiC5), which possesses also three — CH2— groups like choline butanoate (two — CH2— and one —CH3—). Although the lack of a consistent trend is interesting, the most important point is that the choline carboxylates ChCm with m = 2, 3, 4, 5, 6, 7, 8 are only slightly toxic, and they do not penetrate the bilayer or only slightly interact with the bilayer of DPPC liposomes. The longer chain choline carboxylates ChCm with m = 9 and 10 and choline oleate penetrate into the liposome bilayer. At choline nonanoate concentrations above the IC50 value DSC scans suggested accumulation of nonanoate in the bilayer. However, according to the cytotoxicity tests with the HeLa and SK-MEL-28 cell line, all the choline carboxylates and bicarboxylates can be considered as harmless. Finally, the liposomes were exposed to choline carboxylate concentrations of 10 mM or higher. The discontinuity in cytotoxicity found for the cell lines was not elucidated with DPPC membrane interaction studies but subtle trends were observed that suggest that choline carboxylates with different chain lengths interact with biological membranes to different extents when used in concentrations around or higher than the IC50 value (Fig. 4(A)).
Supplementary Material
Acknowledgments
We would like to thank Prof. Jörg Heilmann (Pharmaceutical Biology, University of Regensburg) for enabling cell toxicity studies to be performed at his cell culture laboratory and Gabrielle Brunner (Pharmaceutical Biology, University of Regensburg) for technical assistance. Furthermore, we would like to thank the “Fonds der Chemischen Industrie” for supporting a scholarship to Doris Rengstl. This project was supported in part by Grant No. R01 GM101796 from the National Institutes of Health to Gloria Elliott.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.colsurfb.2014.09.057.
References
- 1.Petkovic M, Seddon KR, Rebelo LPN, Silva Pereira C. Chem Soc Rev. 2011;40:1383. doi: 10.1039/c004968a. [DOI] [PubMed] [Google Scholar]
- 2.Klein R. Ph.D. Thesis. Universität Regensburg; Regensburg: 2011. Choline Applied as Counterion-A Strategy for the Design of Biocompatible Surfactants and Green Ionic Liquid. [Google Scholar]
- 3.Scaife MC. Int J Cosmet Sci. 1982;4:179. doi: 10.1111/j.1467-2494.1982.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 4.Gloxhuber C. Arch Toxicol. 1974;32:245. doi: 10.1007/BF00330108. [DOI] [PubMed] [Google Scholar]
- 5.Holmes MC. Curr Opin Colloid Interface Sci. 1998;3:485. [Google Scholar]
- 6.Schott H. J Pharm Sci. 1973;62:341. doi: 10.1002/jps.2600620242. [DOI] [PubMed] [Google Scholar]
- 7.Petkovic M, Ferguson JL, Gunaratne HQN, Ferreira R, Leitao MC, Seddon KR, Rebelo LPN, Pereira CS. Green Chem. 2010;12:643. [Google Scholar]
- 8.Muhammad N, Hossain MI, Man Z, El-Harbawi M, Bustam MA, Noaman YA, Mohamed Alitheen NB, Ng MK, Hefter G, Yin C-Y. J Chem Eng Data. 2012;57:2191. [Google Scholar]
- 9.Chiba K, Makino I, Ohuchi J, Kasai Y, Kakishima H, Tsukumo K, Uchiyama T, Miyai E, Akiyama J, Okamoto Y, Kojima H, Okumura H, Tsurumi Y, Usami M, Katoh K, Sugiura S, Kurishita A, Sunouchi M, Miyajima A, Hayashi M, Ohno Y. Toxicol in Vitro. 1999;13:189. doi: 10.1016/s0887-2333(98)00072-1. [DOI] [PubMed] [Google Scholar]
- 10.Eagle H. J Exp Med. 1955;102:595. doi: 10.1084/jem.102.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant RL, Yao C, Gabaldon D, Acosta D. Toxicology. 1992;76:153. doi: 10.1016/0300-483x(92)90162-8. [DOI] [PubMed] [Google Scholar]
- 12.Perkins MA, Osborne R, Rana FR, Ghassemi A, Robinson MK. Toxicol Sci. 1999;48:218. doi: 10.1093/toxsci/48.2.218. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Acosta D. Toxicol Lett. 1994;70:309. doi: 10.1016/0378-4274(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira R, Garcia H, Sousa AF, Guerreiro M, Duarte FJS, Freire CSR, Calhorda MJ, Silvestre AJD, Kunz W, Rebelo LPN, Pereira CS. RSC Adv. 2014;4:2993–3002. [Google Scholar]
- 15.Durand M, Zhu Y, Molinier V, Feron T, Aubry JM. J Surfactants Deterg. 2009;12:371. [Google Scholar]
- 16.Lide DR. CRC – Handbook of Chemistry and Physics. CR Press; Boca Raton, USA: 2004. [Google Scholar]
- 17.Evans HC. J Chem Soc. 1956:579. [Google Scholar]
- 18.Fleming R. J Chem Soc. 1960:4914. [Google Scholar]
- 19.Harkins WD, Jordan HF. J Am Chem Soc. 1930;52:1751. [Google Scholar]
- 20.Mosmann T. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Vlachy N, Jagoda-Cwiklik B, Vacha R, Touraud D, Jungwirth P, Kunz W. Adv Colloid Interface Sci. 2009;146:42. doi: 10.1016/j.cis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Weaver KD, Van Vorst MP, Vijayaraghavan R, Macfarlane DR, Elliott GD. Biochim Biophys Acta. 2013;1828:1856. doi: 10.1016/j.bbamem.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughlin RG. The Aqueous Phase Behavior of Surfactants. Academic Press; San Diego: 1994. [Google Scholar]
- 24.Evans DF, Wennerström H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet. Wiley-VCH; New York: 1999. [Google Scholar]
- 25.Klein R, Touraud D, Kunz W. Green Chem. 2008;10:433. [Google Scholar]
- 26.Bauduin P, Touraud D, Kunz W. Langmuir. 2005;21:8138. doi: 10.1021/la0509652. [DOI] [PubMed] [Google Scholar]
- 27.Ho PC. J Chem Eng Data. 1985;30:88. [Google Scholar]
- 28.Mukerjee P, Mysels KJ. Critical Micellar Concentrations of Aqueous Surfactant Systems. Washington D.C.: 1971. (NSRDS-NBS 36). [Google Scholar]
- 29.Rosen MJ. Surfactants and Interfacial Phenomena. 2. John Wiley & Sons, Inc.; New York: 1989. [Google Scholar]
- 30.Collins KD. Methods. 2004;34:300. doi: 10.1016/j.ymeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Collins KD, Neilson GW, Enderby JE. Biophys Chem. 2007;128:95. doi: 10.1016/j.bpc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Mukerjee P, Mysels KJ. Critical Micelle Concentrations of Aqueous Surfactant Systems. U.S. Government Printing Office; Washington, DC: 1971. [Google Scholar]
- 33.European Commission SCCS/1237/09. 2009 [Google Scholar]
- 34.Roguet R. Cell Biol Toxicol. 1999;15:63. doi: 10.1023/a:1007506824183. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez L, Mitjans M, Infante M, Vinardell M. Pharm Res. 2004;21:1637. doi: 10.1023/b:pham.0000041459.63362.6f. [DOI] [PubMed] [Google Scholar]
- 36.Rengstl D, Diat O, Klein R, Kunz W. Langmuir. 2013;29:2506. doi: 10.1021/la304431c. [DOI] [PubMed] [Google Scholar]
- 37.Blanco E, Gonzalez-Perez A, Ruso JM, Pedrido R, Prieto G, Sarmiento F. J Colloid Interface Sci. 2005;288:247. doi: 10.1016/j.jcis.2005.02.085. [DOI] [PubMed] [Google Scholar]
- 38.González-Pérez A, Prieto G, Ruso JM, Sarmiento F. Mol Phys. 2003;101:3185. [Google Scholar]
- 39.McBain JW, Vold RD, Frick M. J Phys Chem. 1940;44:1013. [Google Scholar]
- 40.Stolte S, Arning J, Bottin-Weber U, Matzke M, Stock F, Thiele K, Uerdingen M, Welz-Biermann U, Jastorff B, Ranke J. Green Chem. 2006;8:621. [Google Scholar]
- 41.Maugras M, Infante MR, Gerardin C, Selve C, Vinardell MP. Comp Biochem Physiol C Toxicol Pharmacol. 2001;128:541. doi: 10.1016/s1532-0456(01)00174-0. [DOI] [PubMed] [Google Scholar]
- 42.Stolte S, Matzke M, Arning J, Boschen A, Pitner WR, Welz-Biermann U, Jastorff B, Ranke J. Green Chem. 2007;9:1170. [Google Scholar]
- 43.Roberts DW, Costello JF. QSARComb Sci. 2003;22:226. [Google Scholar]
- 44.Roberts DW, Costello JF. QSAR Comb Sci. 2003;22:220. [Google Scholar]
- 45.Pham TP, Cho CW, Min J, Yun YS. J Biosci Bioeng. 2008;105:425. doi: 10.1263/jbb.105.425. [DOI] [PubMed] [Google Scholar]
- 46.Weaver KD, Kim HJ, Sun JZ, MacFarlane DR, Elliott GD. Green Chem. 2010;12:507. [Google Scholar]
- 47.Ogino K, Ichikawa Y. Bull Chem Soc Jpn. 1976;49:2683. [Google Scholar]
- 48.Prottey C, Ferguson TFM. Food Cosmet Toxicol. 1976;14:425. doi: 10.1016/s0015-6264(76)80180-0. [DOI] [PubMed] [Google Scholar]
- 49.Klein R, Tiddy GJT, Maurer E, Touraud D, Esquena J, Tache O, Kunz W. Soft Matter. 2011;7:6973. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


