Abstract
Ultraviolet light B (UVB) exposure induces cutaneous squamous cell carcinoma (cSCC), one of the most prevalent human cancers. Reoccurrence of cSCC in high-risk patients is prevented by oral retinoids. But oral retinoid treatment causes significant side effects; and patients develop retinoid resistance. Exactly how retinoids prevent UVB-induced cSCC is currently not well understood. Retinoid resistance blocks mechanistic studies in the leading mouse model of cSCC, the UVB exposed SKH-1 hairless mouse. To begin to understand the role of retinoids in UVB-induced cSCC we first examined the localization pattern of key retinoid metabolism proteins by immunohistochemistry 48 hours after UVB treatment of female SKH-1 mice. We next inhibited retinoic acid (RA) synthesis immediately after UVB exposure. Acute UVB increased RA synthesis, signaling, and degradation proteins in the stratum granulosum. Some of these proteins changed their localization; while other proteins just increased in intensity. In contrast, acute UVB reduced the retinoid storage protein lectin:retinol acyltransferase (LRAT) in the epidermis. Inhibiting RA synthesis disrupted the epidermis and impaired differentiation. These data suggests that repair of the epidermis after acute UVB exposure requires endogenous RA synthesis.
Graphical Abstract
INTRODUCTION
UVB has a wavelength between 280-315 nm and causes sunburns (erythema) (1, 2). Acute UVB induces an inflammatory response characterized by increased neutrophils, monocytes, COX-2 activity, and PGE2 (3, 4). Long-term exposure to UVB results in: photoaging; immunosuppression; and skin cancers, such as basal cell carcinoma (BCC), squamous cell carcinoma (cSCC), and melanoma (2). UVB causes: DNA damage; reactive oxygen species (ROS) formation; and induction of p53 (1, 2, 5). During acute UVB exposure, p53 arrests the cell cycle such that cyclobutane-pyrimidine and thymine dimers can be repaired (1, 2). When DNA cannot be repaired, p53 induces apoptosis (6). Aberrant DNA repair or mutations in p53 that fail to induce apoptosis lead to permanent DNA damage and cancer, especially cSCC (1). UVB exposure also increases proliferation and alters the expression and localization of differentiation markers (7, 8). Altered differentiation peaks 48 hours after UVB and is restored within 3-7 days. Very high doses of UVB also impair barrier function (9).
Retinoids are a class of natural compounds and synthetic drugs derived from vitamin A. Retinoids include: retinol; all-trans retinoic acid (atRA and the drug Retin A); 13-cis RA (isotretinoin); etretinate; and acretin. Exogenous retinoids produce variable effects on cSCC due to retinoid resistance. Both oral and topical RA reduce cSCC in the chemical carcinogenesis mouse model where 7,12-dimethylbenz[a]anthracene (DMBA) initiates and 12-O-tetradecanoylphorbol-13-acetate (TPA) promotes tumorigenesis (10, 11). These chemicals cause defects in the RAS signaling pathway that account for only 21% of cSCC in humans (12). Retinoid treatments produced variable effects in the photocarcinogenesis model of cSCC using UVB or UVA&B treated hairless mice. Topical RA accelerated (13), inhibited (14), or had no effect (15) on photocarcinogenesis. These differential effects could be due to either the dose of RA or differences in the background strain. Both oral retinol and etretinate also failed to alter photocarcinogenesis when given at two high doses (16). In humans, low doses of oral retinoids prevent the reoccurrence of carcinomas in high-risk patients; but significant prevention by oral retinoids only occurs for a few years and relapse occurs upon stopping treatment (17-20). Topical RA failed to prevent carcinogenesis in humans (21-23). Therefore RA has the potential for chemoprevention; but something is blocking RA's effectiveness.
Regulation of retinoid metabolism maintains precise levels of RA. Retinol enters the cell by either passive diffusion or the transport protein referred to as stimulated by RA6 (STRA6) (24), and binds cellular retinol binding protein (RBP1) within the cell (25). The majority of retinol that enters the keratinocyte is stored by the action of lectin:retinol acyltransferase (LRAT) (26, 27). The remaining retinol is reversibly oxidized to retinal by retinol dehydrogenases (28, 29). Retinal is subsequently oxidized to RA by retinal dehydrogenases 1-3 (ALDH1A1, 2, and 3)(30). RA then binds either Cellular RA binding protein 1 or 2 (CRABP1, CRABP2). CRABP1 directs RA to degradation enzymes cytochrome P450 26A1, B1, and C1 (CYP26A1, B1, and C1)(31). In contrast, CRABP2 protects RA from degradation by CYP26 enzymes and directs the transport of cytosolic RA to the nucleus where it binds to its receptors (RARA, RARB and RARG) (32). These receptors are RA inducible transcription factors of the nuclear hormone family (33, 34) that regulate the expression of >500 genes involved in: differentiation; cell cycle control; and apoptosis (35). RA also regulates its own metabolism and function by directly inducing LRAT, CRABP2, CYP26A1, and RARB (36-43).
UVA and UVB cause retinol and retinyl esters to degrade, leading to reduced concentration of these retinoids in the skin (44-46). In vitro, UVA/UVB causes retinoids to photoexcite and form reactive oxygen species (ROS) (47). But these effects are not seen in vivo. In cultured keratinocytes, UVB exposure also increases dehydroretinol synthesis (48). Thus, UVB exposure alters the ratio of retinol to dehydroretinol and RA to dehydroRA. UVB and UVA also change the skin expression of retinoid metabolism proteins. UVA treatment decreases RXRA, increases B-carotene dioxygenase activity, and increases CRBP1 (44, 46). Sun exposed skin and the precursor lesion actinic keratosis (AK) express higher levels of CYP26A1 (49). SCC cell lines contain reduced retinyl esters and LRAT activity (50-52). Patients with cSCC express less message levels of Rara, Rarg, and Rarb1’ than patients with basal cell carcinoma (53).
To better understand retinoid resistance in UVB induced cSCC, we examined the effects of acute UVB exposure on key retinoid metabolism proteins. In the stratum granulosum, acute UVB reduced LRAT, and increased CRBP1, DHRS9, ALDH1A2, CRABP2 and CYP26A1. To better understand the role of this UVB-induced RA, we inhibited RA synthesis immediately after UVB exposure. Reducing RA damaged the suprabasal epidermis in a dose dependent manner. These results suggest that UVB-induced RA is important for the repair process after acute UVB exposure. In addition, some of these changes in retinoid metabolism and signalling proteins may contribute to retinoid resistance well before cancers arise in the UVB exposed skin.
MATERIALS AND METHODS
Animals
Pilot experiment
RA reporter mice that contain a retinoic acid response element (RARE) linked to beta galactosidase-(Tg(RARE-Hspa1b/lacZ)12Jrt/J) were wax stripped and 3 days later topically treated with acetone or 2 mg/g BW of disulfiram daily for 3 days and sacrificed 24 hours after the last dose. Skin was fixed in Feketes acid-alcohol-formalin fixative (61% ethanol, 3.2% formaldehyde, 0.75N acetic acid) overnight, routinely processed, embedded in paraffin and sectioned at 5-6 μm, and placed on microscope slides (Superfrost/Plus Fisherbrand, Pittsburgh, PA). The Ohio State University Institutional Animal Care and Use Committee approved all procedures.
Main experiment
Female SKH-1 mice (6-8 weeks old, Charles River Laboratories, Wilmington, MA) were housed in the vivarium at The Ohio State University according to the requirements established by the American Association for Accreditation of Laboratory Animal Care and fed Harlen Teklad diet #7912 (Harlen Teklad, Indianapolis, IN) ad libitum. Mice were exposed to (N=10) or not exposed to (N=3-4) one minimal erythemic dose of UVB (2240 J/m2 as determined by a UVR meter; (UVP Inc., Upland, CA) emitted by Phillips FS40 UVB lamps (American Ultraviolet Company, Lebanon, IN) that were fitted with TA422 Kodacel filters (Eastman Kodak, Rochester, NY) to ensure the exclusion of UVC light and emission primarily of UVB light (290–320 nm). This was immediately followed by topical application of 1.5 or 2.0 mg disulfiram per g body weight or vehicle (acetone). Mice were sacrificed 48 hours later and dorsal skin was harvested and fixed in Feketes acid-alcohol-formalin fixative (61% ethanol, 3.2% formaldehyde, 0.75N acetic acid) overnight, routinely processed, embedded in paraffin and sectioned at 5-6 μm, and placed on microscope slides (Superfrost/Plus Fisherbrand, Pittsburgh, PA). The Ohio State University Institutional Animal Care and Use Committee approved all procedures.
Histological analysis
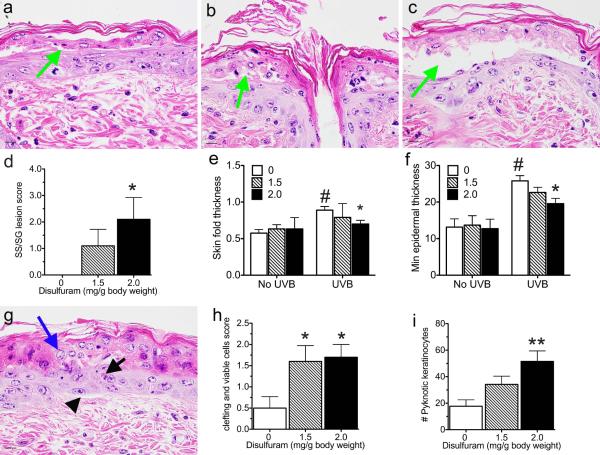
Hematoxylin and eosin (H&E) stained sections of skin (2-3/slide) were scored for lesions after consultation with a board-certified veterinary pathologist (Krista La Perle, DVM, PhD, DACVP) by methods modified from several published protocols (54-58). Clefting lesions between the stratum spinosum (SS) and stratum granulosum (SG) layers were scored for severity on a 3 point scale: 1 = thin, barely detectable cleft between the SG and SS (Figure 3a, green arrow); 2 = wider and taller cleft between the SG and SS containing cell debris (Figure 3b, green arrow); and 3 = very long and wide cleft +/− cell debris (Figure 3c, green arrow). A 6 point scale, incorporating the percent of the skin sections affected, was then created: 1 = ≤ 5% severity 1; 2 = > 5% severity 1; 3 = ≤ 5% severity 2; 4 = > 5% severity 2; 5 = ≤ 5% severity 3; 6 = > 5% severity 3. Lesions characterized by clefting with retention of viable keratinocytes within the SG and SS were semi-quantitatively scored as none (0), mild (1= ≤ 5%), moderate (2= 6-20%), or severe (3= > 20%) (Figure 3g, blue arrow). The presence of parakeratotic hyperkeratosis within the stratum corneum was noted but not analyzed as this lesion was simply increased by the UVB treatment as expected. Hypereosinophilic keratinocytes with pyknotic nuclei were counted in subcorneal layers (Figure 3g, black arrow). Sub-basilar clefting (Figure 3g, arrowhead) was scored on a three-point scale similar to the clefting between the SS and SG. The percentage of the skin sections in which intracellular edema was present was also recorded. Skin fold thickness, measured using digital calipers on whole skin, was confirmed by measuring epidermal thickness between the outer surface of the stratum granulosum and the inner edge of the stratum basale in digital photomicrographs. Photomicrographs were taken of 6 random 200× fields with an Olympus BX51 microscope and attached DP71 camera (Olympus, Tokyo, Japan). The minimum and maximum thickness in each image was measured with cellSens standard version 1.11 (Olympus, Tokyo, Japan) and averaged. The number of keratinocyte cell layers were also counted in both the minimum and maximum regions from each image and averaged.
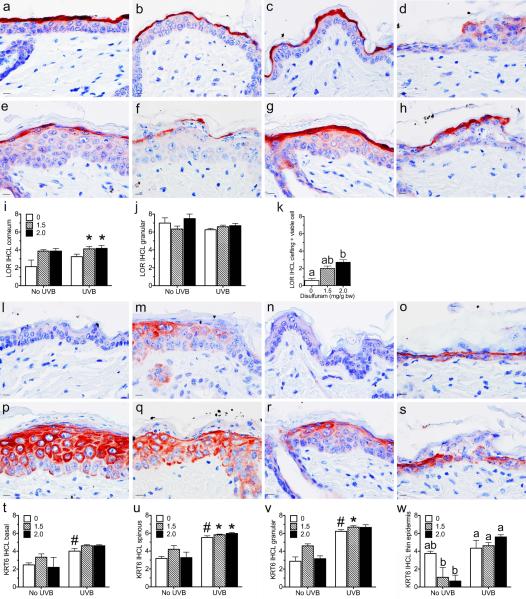
Figure 3. Reducing RA synthesis damages the epidermis.
SKH-1 mice were unexposed or exposed to 1 MED of UVB light, treated with disulfiram, and samples collected 48 hours later. Clefting (green arrows) between the spinous and granular layers was scored as 1 (a), 2 (b), or 3 (c) and a 6 point scale was developed based on the amount of involvement (d). Skin fold thickness was measured on whole mice (e), while epidermal thickness was measured from photomicrographs (f). Retention of viable keratinocytes associated with clefts (g blue arrow) were scored on a 3 point scale based on skin involvement (h). Pyknotic keratinocytes (g black arrow) were counted (i). Arrowhead, sub-basilar clefts. Bar = 10.1 uM. * p < 0.05, ** p < 0.005 disulfiram verses control within UVB treatment group. # p < 0.001 all UVB treatment groups verses control groups.
Immunohistochemistry
Immunohistochemistry (IHC) was performed as per Everts et al (2007) (59). Antibodies against STRA6, CRBP, DHRS9, ALDH1A1, ALDH1A2, CRABP2, and LRAT were made in David Ong's laboratory, while antibodies against CYP26A1 (Alpha Diagnostics Intl,San Antonio, TX), β-galactosidase (Abcam, Cambridge, MA), RARA (Santa Cruz Biotechnology, Dallas, TX), Loricrin (LOR), and Keratin 6 (KRT6, Biolegend, Dedham, MA) were purchased. Biotinylated anti-rabbit secondary antibody was purchased from Jackson Immunoresearch Laboratories, Inc. (West Grove, PA) while a horseradish peroxidase conjugated anti-biotin tertiary antibody was purchased from Bethyl Laboratories (Montgomery, TX). All antibodies were mixed in blocking solution consisting of 3% Bovine Serum Albumin (Fraction V, Fisher BioReagents #BP1600, Pittsburg, PA) in 100mM TBS and 1.28% normal goat serum (Vector, Burlingame, CA). The red 3-amino-9-ethylcarbazole plus enhancers (AEC+) substrate chromogen and aquamount were purchased from Dako (Carpinteria, CA). Gils Hematoxylin III was purchased from Poly Scientific (Bay Shore, NY). A nonspecific IgG, produced during affinity purification, was used as a negative control. Immunoreactivity in the epidermis was semi-quantitatively scored in a blinded fashion on a 4-point scale for both intensity (negative (0), weak (1), moderate (2), strong (3), or very strong (4)) and percent of cells (0 = 0, 1-25 = 1, 26-50 = 2, 51-75 = 3, 76-100 = 4). An IHC level (IHCL) was calculated as the sum of intensity plus percent of cells as previously validated (60, 61). An IHCL of 8 is the maximum. Lesions and abnormally thin areas of the epidermis were scored on a similar scale of 0-8 for IHCL.
Statistics
Effects of UVB on retinoid metabolism
Mann Whitney tests were performed with GraphPad Prism version 5.0d (GraphPad Software, Inc., La Jolla, CA) or SPSS, version v21 (IBM; Armonk, NY). A p value less than 0.05 was considered significant.
Disulfiram study
Skin fold thickness, epidermal thickness, number of keratinocyte layers, Loricrin and Krt 6 expression were analysed by a 2 × 3 analysis of variance, followed by Tukey post hoc tests when appropriate using SPSS, version v22 (IBM; Armonk, NY). When a significant interaction occurred this was followed by a One-Way ANOVA and Tukey post hoc test. Lesions were analyzed in only the UVB treated mice by Kruskal-Wallis analysis, followed by Tukey post hoc tests when appropriate using SPSS, version v22 (IBM; Armonk, NY). Independent T-tests were performed to compare the IHCL in the lesions to normal skin in disulfiram treated samples for KRT6 and LOR using SPSS, version v22 (IBM; Armonk, NY).
RESULTS
Acute UVB exposure increased proteins involved in RA synthesis, signaling, and degradation; while reducing storage within the upper epidermis
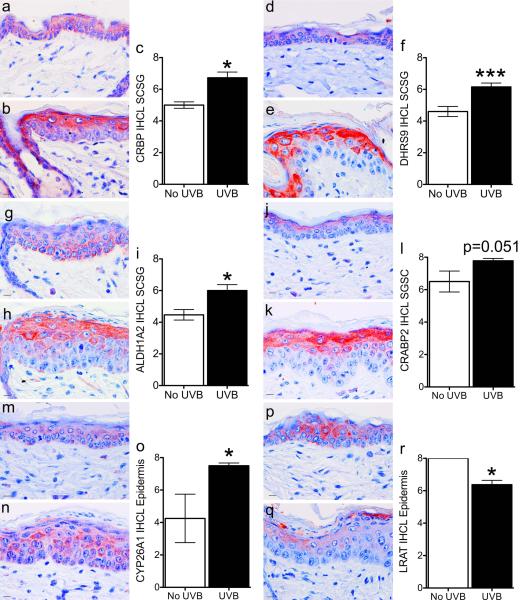
SHK-1 hairless mice begin to lose hair after the first growth cycle (3). The hair follicles then become deformed and utriculus and dermal cysts form, leading to a very heterogeneous tissue. We found that retinoid metabolism proteins localized to specific cell layers of the hair follicle and epidermis that changed throughout the hair cycle (59). Because of these issues we chose IHC to examine the expression and localization of retinoid metabolism proteins 48 hours after UVB exposure. Immunoreactivity was then semi-quantitatively scored. In the upper epidermis (stratum corneum and stratum granulosum) acute UVB significantly increased: STRA6; CRBP (RBP1); DHRS9; ALDH1A2; CRABP2; RARA; and CYP26A1 (Figure 1a-q). STRA6, CRBP, DHRS9, ALDH1A2, CRABP2, and RARA localized closer to the stratum corneum. In contrast, CYP26A1 localized lower in the granulosum layer and extended into the stratum spinosum. In addition, UVB treatment reduced the expression of LRAT throughout the epidermis (Figure 1p-r).
Figure 1. Acute UVB exposure localizes retinoid metabolism proteins to the upper epidermis.
SKH-1 mice were unexposed (a, d, g, j, m, p) or exposed (b, e, h, k, n, q) to 1 MED of UVB light and samples collected 48 hours later. Immunohistochemistry (IHC) was performed with antibodies against CRBP (a-c), DHRS9 (d-f), ALDH1A2 (g-i), CRABP2 (j-l), CYP26A1 (m-o), or LRAT (p-r). An IHC level (IHCL) was determined as the sum of intensity (0-4) plus percent of cells (0-4) for a maximum IHCL of 8. Bar = 10.1 uM. * p < 0.05, ** p < 0.01, *** p < 0.005.
Reducing RA synthesis altered differentiation and damaged the upper epidermis 48 hours after acute UVB treatment
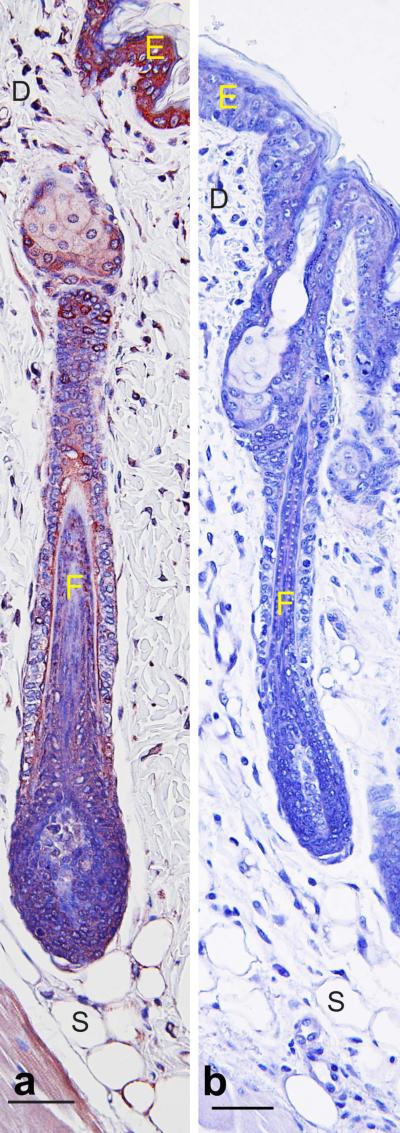
A pilot study was performed first in RA reporter mice to confirm that a retinal dehydrogenase inhibitor (disulfiram) is effective at reducing RA synthesis in vivo when applied topically. Figure 2 shows that disulfiram reduced RA synthesis in these mice to undetectable levels, as indicated by reduced β-galactosidase expression. To determine the role of UVB-induced RA, SKH-1 mice were topically treated with two doses of disulfiram immediately after the UVB exposure. Disulfiram treatment caused focal clefting of the epidermis between the spinous and granular layers in a dose dependent manner (Figure 3a-d, green arrow). Other areas of the epidermis were reduced to a single layer of keratinocytes. Disulfiram significantly reduced skin fold thickness and the minimal epidermal thickness (Figure 3e-f). The minimal number of keratinocyte layers ranged from 1-5, but the average of all 6 sites only trended to decrease with disulfiram (p = 0.074, data not shown). Disulfiram also did not alter the maximum epidermal thickness or number of cell layers, which ranged from 2-8 (data not shown). UVB treatment significantly increased all measures of epidermal thickness and cell layers (p < 0.01, Figure 3e-f and data not shown). UVB treatment also significantly increased clefting between the basal layer and the dermis (Figure 3g, arrowhead) and intracellular edema (p < 0.001); but disulfiram did not alter either of these lesions (data not shown). Disulfiram also dose dependently increased the number of hypereosinophilic keratinocytes with pyknotic nuclei (Figure 3g, black arrow, i). Both doses of disulfiram caused clefting with retention of viable keratinocytes subjacent to the stratum corneum (Figure 3g-h, blue arrow). LOR expression significantly increased with dislufiram dose in the stratum corneum (Figure 4a-i); but LOR was significantly reduced within regions containing viable keratinocytes compared to normal cells in both the stratum corneum and granulosum (p < 0.05; Figure 4d, k). UVB exposure significantly increased LOR IHCL in the spinous and basal layers (Figure 4a-c, e-g). KRT6 expression was used to assess epidermal hyperplasia (62). In epidermis thick enough to define all four layers, disulfiram and UVB treatments increased KRT6 IHCL in the granular and spinous layers (Figure 4j, k, m-t). UVB also increased KRT6 IHCL in the basal layer (data not shown). In thin epidermis, disulfiram trended to decrease KRT6 in skin unexposed to UVB (Control verse high dose, p = 0.062); but KRT6 remained high in UVB exposed skin regardless of disulfiram (Figure 4l).
Figure 2. Disulfiram inhibits RA synthesis in RA reporter mice.
RA reporter mice that contain a retinoic acid response element (RARE) linked to beta galactosidase-(Tg(RARE-Hspa1b/lacZ)12Jrt/J) were wax stripped and 3 days later topically treated with acetone (a, control), or 2 mg/g BW of disulfiram (b) daily for 3 days and sacrificed 24 hours after the last dose. Skin was fixed, routinely processed, paraffin embedded, and sectioned. IHC was performed with an antibody against beta-galactosidase using a red chromagen. Bar = 50 μM, n = 5. E=epidermis, D=dermis, F= hair follicle, S=subcutaneous fat.
Figure 4. Reducing RA synthesis and UVB light altered LOR and KRT6 expression.
SKH-1 mice were unexposed (a-c, l-n) or exposed (d-h, o-s) to 1 MED of UVB light, treated with 0 (a, e, l, p) 1.5 (b, f, m, q), or 2.0 (c, d, g, h, n, o, r, s) mg disulfiram per g body weight, and samples collected 48 hours later. Immunohistochemistry (IHC) was performed with antibodies against LOR (a-k), or KRT6 (l-w). An IHC level (IHCL) was determined as the sum of intensity (0-4) plus percent of cells (0-4) for a maximum IHCL of 8. Bar = 10.1 uM. * p < 0.05 disulfiram in both exposed and unexposed skin verses control groups. # p < 0.001 all UVB treatment groups verses control groups. Different letters are significantly different p < 0.05.
DISCUSSION
This study highlights the role of endogenous RA synthesis in UVB exposed skin. Acute UVB exposure changed the localization patterns and/or intensity of all proteins tested. Forty-eight hours after UVB exposure RA synthesis and signaling proteins localized to the upper stratum granulosum with increased intensity. Acute UVB increased CYP26A1 further down in the epidermis. Acute UVB also reduced retinoid storage by reducing LRAT in the epidermis. Inhibiting RA synthesis caused epidermal hyperplasia and cell death in the suprabasal cells; but left some live cells just under the stratum corneum. These results suggest that endogenously synthesized RA is important in epidermal differentiation and repair following UVB exposure.
To our knowledge, this is the first study to examine the effects of acute UVB on the localization of the complete system for retinoid metabolism. Sorg et. al. (2002)(44) found that CRBP expression increased in the epidermis upon UVA treatment. The same group found a non-significant decrease in CRBP expression with UVB treatment (45). This study, however, used homogenized epidermis and protein extractions, which do not account for the differences in expression between the skin layers. Here, we used IHC and were able to evaluate protein expression in the various epidermal layers. Our finding of increased CYP26A1 in the epidermis is consistent with reports in humans of greater expression of CYP26A1 in areas of skin exposed to sun and in actinic keratosis (49). The drop in LRAT expression is also consistent with previous findings of reduced retinyl esters with UVA and UVB exposure (44-46) and reduced LRAT expression and activity in SCC (50-52). It has been argued that a decrease in retinyl esters may contribute to retinoid deficiency in cancer cells, and LRAT expression may reduce cancer cell survival (51, 63). But by also showing that RA synthesis enzymes and binding proteins are increased we paint a different picture. CRABP2 and CYP26A1 are both induced by retinoic acid in the skin, and their increased expression in this study is an indication of increased RA synthesis and signaling with acute UVB exposure (42, 64, 65). Taken together, the changes in these key vitamin A metabolism proteins indicate that acute UVB exposure increased RA synthesis and signaling in the upper epidermis.
The epidermal lesions seen with disulfiram treatment are consistent with a role for this endogenously synthesized RA in epidermal differentiation during the repair of UVB exposed skin. The role of endogenous RA on epidermal differentiation has been elusive due to mixed results in genetically modified mice (66-72) and opposite results of exogenous RA in studies performed in vivo verses in vitro (73). The reduction of the suprabasal layers seen at the higher dose of disulfiram were also seen in two studies with dominant-negative RARA mice (68, 72). In addition, the current short-term study was able to show patches of skin with active apoptosis/necrosis of these layers in addition to patches with just the thin epidermis. This suggests that RA is involved in the maintenance of these layers after UVB treatment. The lack of histological abnormalities with disulfiram treatment in skin not exposed to UVB is consistent with results in RARA/G double null mice (67). LOR is a marker of terminal differentiation and is normally expressed in the granular layer (74). The reduction of LOR in the viable cells just under the stratum corneum suggests that these cells are not terminally differentiated. Similar cells were also seen in skin specific conditional CYP26B1 null mice (71), suggesting that both RA synthesis and degradation are important for differentiation and formation of the cornified envelop. The overall increase of LOR throughout the corneum with disulfiram is also consistent with skin specific conditional CYP26B1 null mice (71).
Increased KRT6 was also seen in two studies with dominant-negative RARA mice (70, 72), in mice lacking CYP26B1 (71), and with exogenous RA treatment in vivo (73). KRT6 is a marker of epidermal hyperproliferation (62). However, Imakado et al (1995)(70) argue that increased KRT6 is also associated with impaired barrier function, as they saw an increase in KRT6 in the absence of increased proliferation in their dominant-negative RARA transgenic mice. In both their study and in mice lacking CYP26B1 (71) Krt6 is also increased after embryonic day 17.5, when the cornified envelope replaces the periderm in maintaining barrier function. Repair after UVB treatment may be another time when the cornified envelope needs to be reestablished. The drop in KRT6 seen in the thin skin in the absence of UVB exposure is consistent with a reduction of RA by disulfiram; as KRT6 is argued to be a RA target gene (73). The lack of such a drop in the thin skin of UVB exposed skin suggests that epidermal hyperplasia and potential effects due to defective barrier function are dominant over RA in the regulation of KRT6. Retinoid metabolism message levels are also increased during spontaneously regressing cutaneous keratoacanthoma (KA) in mice; and pharmacological doses of RA induced differentiation and led to regression of KAs (75). Thus, RA may prevent tumor formation by assisting in the repair of UVB exposed skin.
In conclusion, this study suggests that UVB exposure causes significant changes in retinoid metabolism throughout the epidermis, which impacts epidermal differentiation. Future studies are needed to investigate the role retinoids may be playing in the repair of UVB damage and restoration of barrier function. Promoting the differentiation of the epidermis and repairing UVB damaged skin are essential to prevent skin cancer.
ACKNOWLEDGMENTS
This work was supported by the Department of Human Sciences (Human Nutrition), The Molecular Carcinogenesis and Chemoprevention Program and Comparative Pathology & Mouse Phenotyping Shared Resource of The Ohio State University Comprehensive Cancer Center (grant P30 CA16058, National Cancer Institute) and the Center for Advanced Functional Foods Research and Entrepreneurship (CAFREE). The authors thank Keith Fluegge and the members of the Oberyszyn laboratory for their assistance in treating and sacrificing the mice.
Footnotes
Part of this work was presented at the 2nd International Conference on Retinoids, June 1-6, 2014, Itasca, Illinois.
REFERENCES
- 1.Polefka TG, Meyer TA, Agin PP, Bianchini RJ. Effects of solar radiation on the skin. J Cosmet Dermatol. 2012;11:134–143. doi: 10.1111/j.1473-2165.2012.00614.x. [DOI] [PubMed] [Google Scholar]
- 2.Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci. 2013;12:54–64. doi: 10.1039/c2pp25152c. [DOI] [PubMed] [Google Scholar]
- 3.Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. J Dermatological Science. 2009;53:10–18. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilgus TA, Koki AT, Zweifel BS, Kusewitt DF, Rubal PA, Oberyszyn TM. Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Molecular Carcinogenesis. 2003;38:49–58. doi: 10.1002/mc.10141. [DOI] [PubMed] [Google Scholar]
- 5.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular-response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 6.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 7.Del Bino S, Vioux C, Rossio-Pasquier P, Jomard A, Demarchez M, Asselineau D, Bernerd F. Ultraviolet B induces hyperproliferation and modification of epidermal differentiation in normal human skin grafted on to nude mice. Br J Dermatol. 2004;150:658–667. doi: 10.1111/j.0007-0963.2004.05886.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, An HT, Chung JH, Kim KH, Eun HC, Cho KH. Acute effects of UVB radiation on the proliferation and differentiation of keratinocytes. Photodermatol Photoimmunol & Photomed. 2002;18:253–261. doi: 10.1034/j.1600-0781.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 9.Haratake A, Uchida Y, Schmuth M, Tanno O, Yasuda R, Epstein JH, Elias PM, Holleran WM. UVB-induced alterations in permeability barrier function: Roles for epidermal hyperproliferation and thymocyte-mediated response. Journal of Investigative Dermatology. 1997;108:769–775. doi: 10.1111/1523-1747.ep12292163. [DOI] [PubMed] [Google Scholar]
- 10.Verma AK, Shapas BG, Rice HM, Boutwell RK. Correlation of the inhibition by retinoids of tumor promotion-induced mouse epidermal ornithine decarboxylase activity and of skin tumor promotion. Cancer Res. 1979;39:419–425. [PubMed] [Google Scholar]
- 11.Chen LC, Sly L, De Luca LM. High dietary retinoic acid prevents malignant conversion of skin papillomas induced by a 2-stage carcinogenesis protocol in female SENCAR mice. Carcinogenesis. 1994;15:2383–2386. doi: 10.1093/carcin/15.10.2383. [DOI] [PubMed] [Google Scholar]
- 12.Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest. 2012;122:464–472. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliday GM, Robertson BO, Barnetson RS. Topical retinoic acid enhances, and a dark tan protects, from subedemal solar-simulated photocarcinogenesis. J Invest Dermatol. 2000;114:923–7. doi: 10.1046/j.1523-1747.2000.00861.x. [DOI] [PubMed] [Google Scholar]
- 14.Kligman LH, Crosby MJ. Topical tretinoin enhances corticosteroid-induced inhibition of tumorigenesis in hairless mice previously exposed to solar simulating radiation. Cancer Letters. 1996;107:217–222. doi: 10.1016/0304-3835(96)04377-7. [DOI] [PubMed] [Google Scholar]
- 15.Kligman LH, Kligman AM. Lack of enhancement of experimental photocarcinogenesis by topical retinoic acid. Arch Dermatol Res. 1981;270:453–462. doi: 10.1007/BF00403790. [DOI] [PubMed] [Google Scholar]
- 16.Kelly GE, Meikle WD, Sheil AGR. Effects of oral retinoid (vitamin-A and etretinate) therapy on photocarcinogenesis in hairless mice. Photochemistry and Photobiology. 1989;50:213–215. doi: 10.1111/j.1751-1097.1989.tb04150.x. [DOI] [PubMed] [Google Scholar]
- 17.Harwood CA, Leedham-Green M, Leigh IM, Proby CM. Low-dose Retinoids in the prevention of cutaneous squamous cell carcinomas in organ transplant recipients - A 16-year retrospective study. Arch Dermatol. 2005;141:456–464. doi: 10.1001/archderm.141.4.456. [DOI] [PubMed] [Google Scholar]
- 18.Brewster AM, Lee JJ, Clayman GL, Clifford JL, Reyes M, Zhou X, Sabichi AL, Strom SS, Collins R, Meyers CA, Lippman SM. Randomized trial of adjuvant 13-cis-retinoic acid and interferon alpha for patients with aggressive skin squamous cell carcinoma. J Clin Oncol. 2007;25:1974–1978. doi: 10.1200/JCO.2006.05.9873. [DOI] [PubMed] [Google Scholar]
- 19.Kadakia KC, Barton DL, Loprinzi CL, Sloan JA, Otley CC, Diekmann BB, Novotny PJ, Alberts SR, Limburg PJ, Pittelkow MR. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer. 2012;118:2128–2137. doi: 10.1002/cncr.26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu PA, Stern RS. Topical tretinoin, another failure in the pursuit of practical chemoprevention for non-melanoma skin cancer. J Invest Dermatol. 2012;132:1532–1535. doi: 10.1038/jid.2012.136. [DOI] [PubMed] [Google Scholar]
- 21.Weinstock MA, Bingham SF, DiGiovanna JJ, Rizzo AE, Marcolivio K, Hall R, Eilers D, Naylor M, Kirsner R, Kalivas J, Cole G, Vertrees JE, T. Vet Affairs Topical Tretinoin and the Prevention of Keratinocyte Carcinoma (Basal and Squamous Cell Carcinoma of the Skin): A Veterans Affairs Randomized Chemoprevention Trial. J Invest Dermatol. 2012;132:1583–1590. doi: 10.1038/jid.2011.483. [DOI] [PubMed] [Google Scholar]
- 22.Stuttgen G. Historical perspectives of tretinoin. J Am Acad Dermatol. 1986;15:735–40. doi: 10.1016/s0190-9622(86)70228-4. [DOI] [PubMed] [Google Scholar]
- 23.Weinstock MA, Bingham SF, Lew RA, Hall R, Eilers D, Kirsner R, Naylor M, Kalivas J, Cole G, Marcolivio K, Collins J, DiGiovanna JJ, Vertrees JE, Grp VT. Topical tretinoin therapy and all-cause mortality. Arch Dermatol. 2009;145:18–24. doi: 10.1001/archdermatol.2008.542. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi R, Yu JM, Honda J, Hu J, Whitelegge J, Ping PP, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 25.Ong DE. Cellular transport and metabolism of vitamin A: roles of the cellular retinoid-binding proteins. Nutr Rev. 1994;52:S24–31. doi: 10.1111/j.1753-4887.1994.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald PN, Ong DE. A lecithin:retinol acyltransferase activity in human and rat liver. Biochem Biophys Res Commun. 1988;156:157–63. doi: 10.1016/s0006-291x(88)80818-0. [DOI] [PubMed] [Google Scholar]
- 27.Kurlandsky SB, Xiao JH, Duell EA, Voorhees JJ, Fisher GJ. Biological activity of all-trans retinol requires metabolic conversion to all-trans retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. J Biol Chem. 1994;269:32821–7. [PubMed] [Google Scholar]
- 28.Rexer BN, Ong DE. A novel short-chain alcohol dehydrogenase from rats with retinol dehydrogenase activity, cyclically expressed in uterine epithelium. Biol Reprod. 2002;67:1555–1564. doi: 10.1095/biolreprod.102.007021. [DOI] [PubMed] [Google Scholar]
- 29.Wu BX, Chen YM, Chen Y, Fan C, Rohrer B, Crouch RK, Ma JX. Cloning and characterization of a novel all-trans retinol short-chain dehydrogenase/reductase from the RPE. Investigative Ophthalmology & Visual Science. 2002;43:3365–3372. [PubMed] [Google Scholar]
- 30.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 31.Boylan JF, Gudas LJ. The level of Crabp-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem-cells. J Biol Chem. 1992;267:21486–21491. [PubMed] [Google Scholar]
- 32.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 33.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 34.Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- 35.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 36.Lorie EP, Chamcheu JC, Vahlquist A, Torma H. Both all-trans retinoic acid and cytochrome P450 (CYP26) inhibitors affect the expression of vitamin A metabolizing enzymes and retinoid biomarkers in organotypic epidermis. Arch Dermatol Res. 2009;301:475–485. doi: 10.1007/s00403-009-0937-7. [DOI] [PubMed] [Google Scholar]
- 37.Lorie EP, Cools M, Borgers M, Wouters L, Shroot B, Hagforsen E, Torma H, Vahlquist A. Topical treatment with CYP26 inhibitor talarozole (R115866) dose dependently alters the expression of retinoid-regulated genes in normal human epidermis. Br J Dermatol. 2009;160:26–36. doi: 10.1111/j.1365-2133.2008.08895.x. [DOI] [PubMed] [Google Scholar]
- 38.Lorie EP, Li H, Vahlquist A, Torma H. The involvement of cytochrome p450 (CYP) 26 in the retinoic acid metabolism of human epidermal keratinocytes. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2009;1791:220–228. doi: 10.1016/j.bbalip.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Reijntjes S, Blentic A, Gale E, Maden M. The control of morphogen signalling: Regulation of the synthesis and catabolism of retinoic acid in the developing embryo. Dev Biol. 2005;285:224–237. doi: 10.1016/j.ydbio.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Matsuura T, Ross AC. Regulation of hepatic lecithin: retinol acyltransferase activity by retinoic acid. Arch Biochem Biophys. 1993;301:221–7. doi: 10.1006/abbi.1993.1137. [DOI] [PubMed] [Google Scholar]
- 41.Kurlandsky SB, Duell EA, Kang S, Voorhees JJ, Fisher GJ. Auto-regulation of retinoic acid biosynthesis through regulation of retinol esterification in human keratinocytes. J Biol Chem. 1996;271:15346–52. doi: 10.1074/jbc.271.26.15346. [DOI] [PubMed] [Google Scholar]
- 42.Giguere V, Lyn S, Yip P, Siu CH, Amin S. Molecular cloning of cDNA-encoding a 2nd cellular retinoic acid-binding protein. Proc Nat Acad Sci USA. 1990;87:6233–6237. doi: 10.1073/pnas.87.16.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dethe H, Marchio A, Tiollais P, Dejean A. Differential expression and ligand regulation of the retinoic acid receptor-alpha and receptor-beta genes. Embo J. 1989;8:429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorg O, Tran C, Carraux P, Didierjean L, Falson F, Saurat JH. Oxidative stress-independent depletion of epidermal vitamin a by UVA. J Invest Dermatol. 2002;118:513–518. doi: 10.1046/j.0022-202x.2002.01674.x. [DOI] [PubMed] [Google Scholar]
- 45.Sorg O, Tran C, Carraux P, Didierjean L, Saurat JH. Retinol and retinyl ester epidermal pools are not identically sensitive to UVB irradiation and anti-oxidant protective effect. Dermatology. 1999;199:302–307. doi: 10.1159/000018279. [DOI] [PubMed] [Google Scholar]
- 46.Takeda A, Morinobu T, Takitani K, Kimura M, Tamai H. Measurement of retinoids and beta-carotene 15,15′-dioxygenase activity in HR-1 hairless mouse skin with UV exposure. J Nutr Sci Vitaminol. 2003;49:69–72. doi: 10.3177/jnsv.49.69. [DOI] [PubMed] [Google Scholar]
- 47.Fu PP, Xia Q, Yin JJ, Cherng S-H, Yan J, Mei N, Chen T, Boudreau MD, Howard PC, Wamer WG. Photodecomposition of vitamin a and photobiological implications for the skin. Photochem Photobiol. 2007;83:409–424. doi: 10.1562/2006-10-23-IR-1065. [DOI] [PubMed] [Google Scholar]
- 48.Tafrova JI, Pinkas-Sarafova A, Stolarzewicz E, Parker KA, Simon M. UVA/B exposure promotes the biosynthesis of dehydroretinol in cultured human keratinocytes. Mol Cell Biochem. 2012;364:351–361. doi: 10.1007/s11010-012-1237-7. [DOI] [PubMed] [Google Scholar]
- 49.Osanai M, Lee GH. Enhanced expression of retinoic acid-metabolizing enzyme CYP26A1 in sunlight-damaged human skin. Medical Molecular Morphology. 2011;44:200–206. doi: 10.1007/s00795-010-0528-x. [DOI] [PubMed] [Google Scholar]
- 50.Jurukovski V, Simon M. Reduced lecithin:retinol acyl transferase activity in cultured squamous cell carcinoma lines results in increased substrate-driven retinoic acid synthesis. Biochim Biophys Acta. 1999;1436:479–490. doi: 10.1016/s0005-2760(98)00154-4. [DOI] [PubMed] [Google Scholar]
- 51.Guo X, Ruiz A, Rando RR, Bok D, Gudas LJ. Esterification of all-trans retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: reduced expression of lecithin:retinol acyltransferase in carcinoma lines. Carcinogenesis. 2000;21:1925–1933. doi: 10.1093/carcin/21.11.1925. [DOI] [PubMed] [Google Scholar]
- 52.Guo X, Gudas LJ. Metabolism of all-trans-retinol in normal human cell strains and squamous cell carcinoma (SCC) lines from the oral cavity and skin: reduced esterification of retinol in SCC lines. Cancer Res. 1998;58:166–76. [PubMed] [Google Scholar]
- 53.Hartmann F, Kosmidis M, Muhleisen B, French LE, Hofbauer GFL. Retinoic Acid Receptor Isoform mRNA Expression Differs Between BCC and SCC of the Skin. Archives of Dermatology. 2010;146:675–676. doi: 10.1001/archdermatol.2010.107. [DOI] [PubMed] [Google Scholar]
- 54.Boyd AS, Stasko T, Cameron GS, Russell M, King LE. Histologic features of actinic keratoses in solid organ transplant recipients and healthy controls. J Am Acad Dermatol. 2001;45:217–221. doi: 10.1067/mjd.2001.114740. [DOI] [PubMed] [Google Scholar]
- 55.Dhong E-S, Hwang N-H, Kim D-W, Rajashekhar G, Johnstone BH, March KL. Morphologic Changes in Photodamaged Organotypic Human Skin Culture After Treatment of Autologous Adipose-Derived Stromal Cells. J Craniofacial Surgery. 2012;23:805–811. doi: 10.1097/SCS.0b013e31824e6c87. [DOI] [PubMed] [Google Scholar]
- 56.Gambichler T, Boms S, Stucker M, Moussa G, Kreuter A, Sand M, Sand D, Altmeyer P, Hoffmann K. Acute skin alterations following ultraviolet radiation investigated by optical coherence tomography and histology. Arch Dermatol Res. 2005;297:218–225. doi: 10.1007/s00403-005-0604-6. [DOI] [PubMed] [Google Scholar]
- 57.Goettsch W, Garssen J, de Gruijl FR, Dortant P, van Loveren H. Methods for exposure of laboratory animals to ultraviolet radiation. Laboratory Animals. 1999;33:58–67. doi: 10.1258/002367799780578507. [DOI] [PubMed] [Google Scholar]
- 58.Nissen JB, Avrach WW, Hansen ES, Stengaard-Pedersen K, Kragballe K. Increased levels of enkephalin following natural sunlight (combined with salt water bathing at the Dead Sea) and ultraviolet A irradiation. Br J Dermatol. 1998;139:1012–1019. doi: 10.1046/j.1365-2133.1998.02557.x. [DOI] [PubMed] [Google Scholar]
- 59.Everts HB, Sundberg JP, King LE, Jr., Ong DE. Immunolocalization of enzymes, binding proteins, and receptors sufficient for retinoic acid synthesis and signaling during the hair cycle. J Invest Dermatol. 2007;127:1593–1604. doi: 10.1038/sj.jid.5700753. [DOI] [PubMed] [Google Scholar]
- 60.Shamonki MI, Kligman I, Shamonki JM, Schattman GL, Hyjek E, Spandorfer SD, Zaninovic N, Rosenwaks Z. Immunohistochemical expression of endometrial L-selectin ligand is higher in donor egg recipients with embryonic implantation. Fertil Steril. 2006;86:1365–1375. doi: 10.1016/j.fertnstert.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 61.Peng C, Zhang X, Wang Y, Li L, Wang Q, Zheng J. Expression and prognostic significance of wnt7a in human endometrial carcinoma. Obstet Gynecol Int. 2012;2012:134962. doi: 10.1155/2012/134962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss RA, Eichner R, Sun TT. Monoclonal-antibody analysis of keratin expression in epidermal diseases-a 48-and 56-Kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984;98:1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassel JC, Amann PM, Schadendorf D, Eichmueller SB, Nagler M, Bazhin AV. Lecithin retinol acyltransferase as a potential prognostic marker for malignant melanoma. Exp Dermatol. 2013;22:757–759. doi: 10.1111/exd.12236. [DOI] [PubMed] [Google Scholar]
- 64.Elder JT, Astrom A, Pettersson U, Tavakkol A, Griffiths CEM, Krust A, Kastner P, Chambon P, Voorhees JJ. Differential Regulation of Retinoic Acid Receptors and Binding- Proteins in Human Skin. Journal of Investigative Dermatology. 1992;98:673–679. doi: 10.1111/1523-1747.ep12499896. [DOI] [PubMed] [Google Scholar]
- 65.Pavez Lorie E, Chamcheu JC, Vahlquist A, Torma H. Both all-trans retinoic acid and cytochrome P450 (CYP26) inhibitors affect the expression of vitamin A metabolizing enzymes and retinoid biomarkers in organotypic epidermis. Arch Dermatol Res. 2009;301:475–485. doi: 10.1007/s00403-009-0937-7. [DOI] [PubMed] [Google Scholar]
- 66.Calleja C, Messaddeq N, Chapellier B, Yang HY, Krezel W, Li M, Metzger D, Mascrez B, Ohta K, Kagechika H, Endo Y, Mark M, Ghyselinck NB, Chambon P. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev. 2006;20:1525–1538. doi: 10.1101/gad.368706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chapellier B, Mark M, Messaddeq N, Calleja C, Warot X, Brocard J, Gerard C, Li M, Metzger D, Ghyselinck NB, Chambon P. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402–3413. doi: 10.1093/emboj/cdf331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen CF, Lohnes D. Dominant-negative retinoic acid receptors elicit epidermal defects through a non-canonical pathway. J Biol Chem. 2005;280:3012–3021. doi: 10.1074/jbc.M411522200. [DOI] [PubMed] [Google Scholar]
- 69.Imakado S, Bickenbach J, Rothnagel JA, Bundman D, Walczak VR, Wisniewski S, Gordon JS, Heyman R, Roop DR. Targeted Expression of a Dominant-Negative Retinoic Acid Receptor Mutant in the Epidermis of Transgenic Mice Results in Loss of Barrier Function and Neonatal Lethality. J Invest Dermatol. 1994;102:547–547. [Google Scholar]
- 70.Imakado S, Bickenbach JR, Bundman DS, Rothnagel JA, Attar PS, Wang XJ, Walczak VR, Wisniewski S, Pote J, Gordon JS, Heyman RA, Evans RM, Roop DR. Targeting expression of a dominant-negative retinoic acid receptor mutant in the epidermis of transgenic mice results in loss of barrier function. Genes Dev. 1995;9:317–329. doi: 10.1101/gad.9.3.317. [DOI] [PubMed] [Google Scholar]
- 71.Okano J, Lichti U, Mamiya S, Aronova M, Zhang G, Yuspa SH, Hamada H, Sakai Y, Morasso MI. Increased retinoic acid levels through ablation of Cyp26b1 determine the processes of embryonic skin barrier formation and peridermal development. J Cell Science. 2012;125:1827–1836. doi: 10.1242/jcs.101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saitou M, Sugai S, Tanaka T, Shimouchi K, Fuchs E, Narumiya S, Kakizuka A. Inhibition of skin development by targeted expression of a dominant-negative retinole acid receptor. Nature. 1995;374:159–162. doi: 10.1038/374159a0. [DOI] [PubMed] [Google Scholar]
- 73.Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. Faseb J. 1996;10:1002–1013. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 74.Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochim Et Biophy Acta-Mol Cell Res. 2013;1833:3471–3480. doi: 10.1016/j.bbamcr.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 75.Zito G, Saotome I, Liu ZZ, Ferro EG, Sun TY, Nguyen DX, Bilguvar K, Ko CJ, Greco V. Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk. Nat Commun. 2014;5:3543. doi: 10.1038/ncomms4543. [DOI] [PMC free article] [PubMed] [Google Scholar]