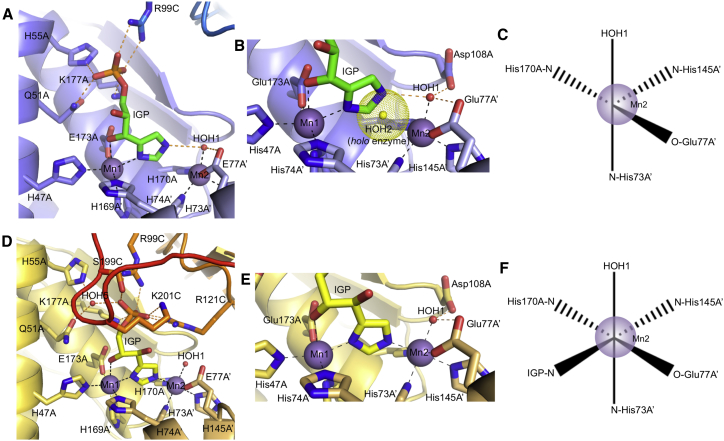
Figure 3.
Comparison of the Mode of Binding of IGP in the Form A and Form B Complexes
(A) An overview of the mode of binding of IGP in the form A complex. The protein backbone and side-chain carbon atoms are shown in a different shade of blue for each chain, and the substrate carbon atoms are shown in green. The non-carbon atoms, water molecules, metal ions, and metal and hydrogen bonding interactions are all drawn, labeled, and colored as in Figure 2. See also Figure S3.
(B) Detail around the metal binding site in the form A complex, including the superimposed position of HOH2 from the holoenzyme complex (yellow sphere with dots representing the van der Waals radius). The binding of the neutral IGP-imidazole displaces HOH2 from the coordination sphere of Mn2, leaving the metal ion five-coordinate. Figure drawn as in (A).
(C) A schematic drawing depicting the five ligands that coordinate Mn2 in the form A complex. The manganese ion is represented as a purple sphere.
(D) An overview of the mode of binding of IGP in the form B complex. The protein backbone and side-chain carbon atoms are shown in a different shade of orange for each chain, the substrate carbon atoms are yellow, and the C loop is highlighted in red. The non-carbon atoms, water molecules, metal ions, and metal and hydrogen bonding interactions are all drawn, labeled, and colored as in Figure 2. See also Figure S4.
(E) Detail around the metal binding site in the form B complex showing that the IGP-imidazolate is bound between the two Mn(II) ions, both of which are octahedrally coordinated. Figure drawn as in (D).
(F) A schematic drawing depicting the octahedral metal coordination around Mn2 in the form B structure. The manganese ion is represented as a purple sphere.