Abstract
Introduction
During aging, advanced glycation end products (AGEs) accumulate in articular cartilage. In this study we determined whether AGEs induce endoplasmic reticulum (ER) stress and studied the ER stress-activated pathways that stimulate cyclooxygenase-2 (COX-2) expression in human chondrocytes.
Methods
Chondrocytes were stimulated with AGE-BSA. Gene expression was determined by quantitative PCR and protein expression was studied by immunoblotting. Studies to elucidate involved pathways were executed using siRNAs and specific inhibitors of eukaryotic initiation factor-2α (eIF2α), MAPKs and NF-κB.
Results
AGE-BSA induced expression of GRP78 with concomitant increase in COX-2 expression was observed in human chondrocytes. In addition, expression of Bag-1, an ER stress marker was also increased by AGE-BSA. RAGE knockdown inhibited AGE-BSA-induced expression of GRP78 and COX-2. Treatment with eIF2α inhibitor or eIF2α knockdown inhibited AGE-BSA-induced expression of GRP78 and COX-2 with decreased PGE2 production. Treatment with SB202190 inhibited AGE-BSA-induced expression of GRP78 and COX-2, while treatment with PD98051 inhibited AGE-BSA-induced GRP78 protein expression but had no effect on COX-2 protein expression. SP600125 had no effect on either GRP78 or COX-2 protein expression. Bay 11-7082 suppressed AGE-BSA-induced GRP78 and COX-2 expression. AGE-BSA-induced activation of NF-κB was inhibited by treatment with SB202190 and by eIF2α knockdown, but was not inhibited when chondrocytes were treated with SP600125 or PD98059.
Conclusion
This study demonstrates that AGEs induce ER stress and stimulate the expression of COX-2 through eIF2α, p38-MAPK and NF-κB pathways in human chondrocytes. Our results provide important insights into cartilage degradation in osteoarthritis associated with latent ER stress.
Keywords: ER stress, Chondrocyte, OA, eIF2-α, p38-MAPK, NF-κB
1. Introduction
In the endoplasmic reticulum (ER), secretory and transmembrane proteins fold into their native conformation and undergo posttranslational modifications important for their activity and structure [1]. Interference with proper protein folding activity results in the accumulation of misfolded proteins in ER, a phenomenon commonly referred to as ER stress [1,2]. To help proteins fold properly, ER contains a master molecular chaperone GRP78 (BiP). When protein folding is disturbed, GRP78 synthesis is increased and GRP78 subsequently binds to misfolded proteins to prevent them from forming aggregates and assists them to refold properly [3]. Bag-1 has recently been shown to be a downstream regulator of ER stress in chondrocytes [4]. It is now well established that overexpression of Bag-1 protects chondrocytes from ER stress [4,5]. Therefore elevated expressions of GRP78 and Bag-1 are considered to be reliable markers of ER stress [3,4]. The ER stress response is suggested to contribute in several human diseases, including osteoarthritis (OA) and rheumatoid arthritis (RA) [2].
OA, the most common forms of arthritis, is a progressive degenerative joint disease that has a major impact on joint function and the patient’s quality of life [6]. The most important risk factor for OA besides female sex, obesity, and joint trauma is aging [6,7]. However, it is relatively unknown how aging contributes to the onset and progression of OA. Advanced glycation end products (AGEs) are produced by the non-enzymatic glycation of macromolecules and accumulate in a number of different tissues [8]. In articular cartilage, accumulation of AGEs makes the collagen network brittle thus increasing the risk for the development of OA [8,9]. The biological activities of AGEs are thought to be mediated by specific receptors for AGE (RAGE) and activation of RAGE engages critical signaling pathways linked to pro-inflammatory responses and activation of various inflammatory genes [10]. Chondrocytes are secretory cells and are the only resident cell type found in cartilage which are responsible for synthesis and turnover of extracellular matrix (ECM) [11]. In OA, activation of RAGE has been shown to stimulate chondrocytes, resulting in increased production of cartilage degrading enzymes through the activation of intracellular signaling pathways comprising of MAPKS, and NF-κB [12,13]. Despite studies on AGEs induced cartilage destruction in OA, ER stress related cartilage degradation remains nebulous.
Signal transduction from the ER to the cell nucleus could be mediated by a signaling cascade similar to the plasma membrane-initiated cell signaling. ER stress, for example, is coupled to the activation of stress-activated protein kinases such as MAP kinases [15,16]. Members of the MAPK family phosphorylate a number of transcription factors including NF-κB, with subsequent induction of inflammatory genes expression [14]. p38-MAPK, in particular, is activated by a diverse array of ER stress inducing agents such as tunicamycin [15] and its activation is associated with inflammation and cartilage and bone destruction [17] as it regulates cytokine production through a variety of transcriptional and translational mechanisms [18]. In a rodent model of inflammatory arthritis, p38-MAPK inhibition suppressed the inflammation and bone destruction [19]. Phosphorylation of the subunit of eukaryotic initiation factor-2 (eIF2) is another important mechanism involved in the regulation of protein synthesis during ER stress [extensively reviewed in [20] and is required for the activation of NF-κB [21]. Activation of NF-κB by ER stress leads to induction of many cellular genes that are largely anti-apoptotic in function [22]. Now, it is well established that under the influence of inflammation, COX-2 is upregulated, which results in a disturbed cartilage matrix turnover [23–25]. It is also reported that selective COX-2 inhibition prevents proinflammatory cytokine-induced cartilage damage [23,24]. Furthermore, over expression of COX-2 mediated PGE2 production has been found in OA and was linked to OA progression and drug resistance [25,26]. Chondrocytes are thought to be the major source of PGE2 production in OA joints and suppression of COX-2 expression and PGE2 production was found to be chondroprotective [25].
Although AGEs have been shown to induce the inflammatory response via activation of NF-κB and MAPKs in various cell types including chondrocytes, but whether they induce the ER stress in chondrocytes has not yet been reported. In this report, we demonstrate that AGEs induce ER stress which leads to increased expression of COX-2 in human chondrocytes. Our results also showed that in human chondrocytes with ER stress induction of COX-2 was mediated through eIF2α, p38-MAPK and NF-κB pathways.
2. Patients, materials and methods
2.1. Cartilage selection and chondrocytes preparation
With Institutional Review Board (IRB) approval, discarded cartilage samples were obtained from the knee or hip joints of 44 OA patients aged 38–69 years (32 female and 12 male Caucasian; mean age, 48 ± 26 years) who underwent joint replacement surgery at Metrohealth Medical Center, Cleveland, OH. The macroscopic cartilage degeneration was determined by staining of femoral head samples with India ink [27], and the cartilage with smooth articular surface (unaffected cartilage) was resected and used to prepare chondrocytes by enzymatic digestion as previously described [14,28,29]. Histological analysis of the unaffected cartilage samples was performed on 5-μm-thick sections stained with H & E and Safranin O and graded using the Mankin score [30]. Grading of the histology slides revealed that all of the cartilage pieces taken from the unaffected area had a Mankin score <2 for structure and a Mankin score of 1 for cellularity. Normal cartilage samples were obtained from trauma patients (n=2) with no known history of OA or RA. Isolated chondrocytes were plated at a density of 1.2×106/ml in 35-mm tissue culture dishes (0.125×106 cells/cm2) in complete DMEM/Ham’s F-12 medium as previously described [14,28]. Only primary human OA chondrocytes were used in studies described here.
2.2. AGE-BSA preparation
AGE-BSA was prepared by reacting BSA (Sigma Chemical Co., St Louis, MO, USA) with glycoaldehyde (Sigma), according to the method described by Valencia and colleagues [31] with slight modifications. Briefly, endotoxin-free BSA (2 mg/ml) was incubated under nonreducing conditions with 70 mM fresh glycoaldehyde in PBS (pH 7.4) without calcium chloride and magnesium chloride for 3 days at 37 °C. The reaction was terminated by removing nonreacted glycoaldehyde by dialyzing extensively against PBS.
Endotoxicity of glycoaldehyde was also tested on chondrocytes by incubating with freshly prepared glycoaldehyde (1–50 μM) and was found to be non-toxic for tested chondrocytes.
2.3. Treatment of chondrocytes with AGE-BSA and inhibitors for MAPKs, eIF2α and NF-κB
Human OA chondrocytes (1.2×106/ml) were plated in 35 mm culture dishes (0.125×106 cells/cm2) in complete DMEM/Hams-F12 medium and serum-starved for 12 h/overnight and then treated with 5–100 μg/ml of AGE-BSA for 0–24 h. Primary chondrocytes were treated with MAPKs, eIF2α or NF-κB inhibitors for 1 h prior to stimulation with AGE-BSA. Chondrocytes cultured without AGE-BSA or cultured with native BSA served as controls. Inhibitors used were SB202190 (catalog #1264, R&D Systems Inc., MN), SP600125 (catalog #40119, Calbiochem, MA); PD98059 (catalog #513000, Calbiochem); 2 Aminopurine (catalog #A3509, Sigma, St. Louis, MO), Bay 11–7082 (catalog #B5556, Sigma). The concentration of SB202190, SP600125, PD98059, 2 Aminopurine (2AP), Bay 11–7082 used in the study was 100 μM, 10 μM, 50 μM, 10 μM, 10 μM, respectively.
2.4. Knockdown of RAGE and eIF2α by transfection with small interfering RNA (siRNA)
Human OA chondrocytes were transfected with human RAGE-specific siRNA (On-Target SMART Pool, catalog #L00362500, Dharmacon RNA Technologies, Lafayette, CO), eIF2α-specific siRNA (catalog #sc-35272, Santa Cruz Biotechnology, CA) or with a negative human GAPDH control siRNA (catalog #D-0018301020, Dharmacon) using Amaxa Human Chondrocytes Nucleofector Kit (catalogue #VPF-1001, Lonza, Walkersville, MD). Transfection efficiency was monitored with red fluorescent siRNA oligonucleotides (siGLO red indicator, catalogue #D0016300220, Dharmacon). Approximately 70–80% of the chondrocytes emitted red fluorescence signal when transfected with siGLO.
2.5. Cell viability assays
Human OA chondrocytes viability after nucleofection was examined using the CytoTox-Glo™ Luminescent Cell Viability Assay Kit (Promega, Madison, WI). The viability of the transfected cell was 70% in comparison to non-transfected cells. Whereas AGE-BSA or inhibitors used in the study have no cytotoxic effect on human OA chondrocytes.
2.6. Real time-PCR
Real time quantitative polymerase chain reaction (qRT-PCR) was used to quantify the expression of mRNAs for GRP78 and COX-2 with expression of GAPDH as endogenous control. Primers used for PCR assisted amplification were listed in Table 1. PCR amplification was carried out using the core kit for SYBR Green or Taqman (Applied Biosystem, Foster City, CA) and the Step One Real Time PCR System (Applied Biosystems). Typical profile times used were initial step, 95 °C for 10 minutes, followed by a second step at 95 °C for 15 sec and 60 °C for 60 sec for 40 cycles with melting curve analysis. The level of target mRNA was normalized to the level of GAPDH and compared to control (untreated sample). Data was analyzed using comparative ΔΔCT method [32].
Table 1.
Sequences of primers used in gene expression studies.
| Gene | Accession number |
Forward primer | Backward primer |
|---|---|---|---|
| GRP78 | NM_005347 | 5′-TCC TGC GTC GGC GTG T-3′ |
5′-GTT GCC CTG ATC GTT GGC-3′ |
| COX-2 | NM_000963 | 5′-CAA ATC CTT GCT GTT CCC ACC CAT-3′ |
5′-GTG CAC TGT GTT TGG AGT GGG TTT-3′ |
| GAPDH | NM_002046 | 5′-TCG ACA GTC AGC CGC ATC TTC TTT-3′ |
5′-ACC AAA TCC GTT GAC TCC GAC CTT-3′ |
| COL2A1 | NM_001844 | 5′-ACG TGA AAG ACT GCC TCA GC-3′ |
5′-TTT CAT CAA ATC CTC CAG CC-3′ |
| COL10A1 | NM_000493 | 5′-TGA TCC TGG AGT TGG AGG AC-3′ |
5′-GAG ATC GAT GAT GGC ACT CC-3′ |
| ACAN | NM_013227 | 5′-GAC CTG CAA GGA GAC AGA GG-3′ |
5′-CCA CTG GTA GTC CTT GGG CAT-3′ |
| SOX-9 | NM_011448 | 5′-GAT TTT TCA CGC AGC CCT AA-3′ |
5′-ATA CAG TCC AGG CAG ACC CA-3′ |
2.7. Western immunoblotting
Stimulated and control human OA chondrocytes were washed with cold PBS and lysed using the cell lysis RIPA buffer (50 mM Tris:HCl, pH 7.5; 150 mM NaCl; 1% IGEPAL, 4 mM EDTA, 0.1% sodium deoxycholate; 10 mM Na4P2O7, 10 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin). Cytoplasmic and nuclear fractions were prepared as previously described [33]. Blots were analyzed as described previously [33,34] and images were captured using AFP-Imaging System (Minimedical Series, Elms Ford, NY).
2.8. Enzyme-linked immunosorbent assay (ELISA)
Human OA chondrocytes were stimulated with or without AGE-BSA (100 μg/ml) for 0–24 h. PGE2 produced in the culture medium was quantified using commercially available ELISA kit (Cayman Chemical Company, Ann Arbor, MI). Plates were read using Synergy HT microplate reader (Biotek Instrument, Winooski, VT).
2.9. NF-κB activity assays
Activation of NF-κBp65 in AGE-BSA stimulated human OA chondrocytes was determined using a highly sensitive Transcription Factor ELISA Kit (Assay Designs, Ann Arbor, MI). Activation of NF-κB p50 and Rel B in AGE-BSA stimulated chondrocytes was determined by Western immunoblotting using specific antibodies against NF-κBp50 and Rel B (Cell signaling technology, Beverly, MA). Upregulation of NF-κBp50 by AGE-BSA was further verified by Sandwich ELISA established in our laboratory. Briefly, flat bottomed 96-well microtiter plates (polystryrene polysorp immunoplates, Nunc-Immuno™ MicroWell, Sigma) were coated with anti-NF-κBp50 (N-19) polyclonal antibodies (catalog #sc-1191, Santa Cruz Biotechnology, CA; diluted 1:100 in coating buffer) overnight at 4 °C. The plates were washed with TBS-Tween 20 and the non-specific binding sites were blocked with TBS containing 1% BSA (Sigma) at room temperature for 1 h. After washing extensively with TBS-Tween 20, 100 μl of nuclear cell extracts (10 μg/ml) were added to duplicate wells of the coated plate and incubated at room temperature for 2 h and overnight at 4 °C. The plates were washed five times with TBS-Tween 20 and 100 μl of anti-NF-κBp50 (D-6) monoclonal IgG (catalog #sc-166588, Santa Cruz Biotechnology; diluted 1:100) was incubated at room temperature for 2 h. The plates were washed extensively and 100 μl of anti-human IgG-horseradish peroxidase (catalog #sc2769, Santa Cruz Biotechnology) was added and incubated for 2 h. After washing, 100 μl of 3,3′,5,5′-Tetramethylbenzidine peroxidase substrate (TMB, catalog #206697A, Santa Cruz Biotechnology) was added to each well. The reaction was stopped after 10 minutes by adding 100 μl of stop solution (2 M H2SO4) and OD was read at 450 nm on an automatic microplate reader (Anthos Zenyth 3100 Multimode Detectors, Salzburg, Austria).
2.10. Statistical analysis
All measurements were performed in triplicates and repeated at least three times using chondrocytes prepared from different OA patients. Data sets were analyzed by analysis of variance followed by Tukey’s post hoc multiple comparison tests using Graph Pad Prism version 5.0 (Graph Pad Software Inc., San Diego, CA) or Origin software package version 5.0 (Origin Lab Corporation, Northampton, MA). A p value of <0.05 was considered statistically significant. Values shown are mean with 95% CI unless stated otherwise.
3. Results
3.1. Human chondrocytes in monolayers maintain their chondrogenic phenotype
We determined whether primary human chondrocytes used in these studies maintained their phenotype - by analyzing the expression of COL2A1, ACAN, and SOX-9 mRNA, which are considered to be signatures of the chondrogenic phenotype [35]. PCR primers are listed in Table 1. Our results show that primary human chondrocytes in monolayer culture maintained their phenotype, when they were plated (1.2×106/ml) in 35-mm culture dishes (0.125×106 cells/cm2) in complete DMEM with 10% FBS and were allowed to grow at 37 °C and 5% CO2 in a tissue culture incubator, as judged by the continued expression of COL2A1, ACAN, and SOX-9 mRNAs, whereas COL10A1 mRNA was not expressed (data not given). Based on these data, human chondrocytes were used within 72 h after plating to avoid possible dedifferentiation.
3.2. AGE-BSA induce ER stress and the expression of COX-2 in human chondrocytes
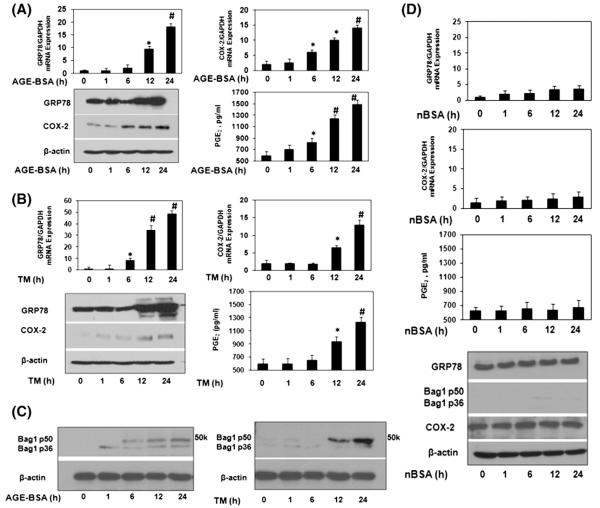
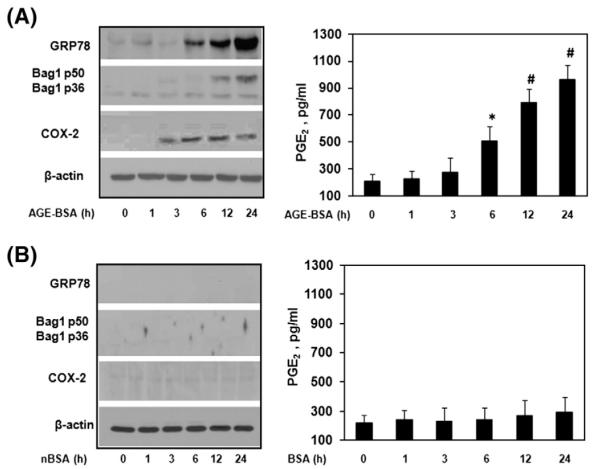
This study comprised of 44 OA patients, out of which 24 OA patients primary chondrocytes showed up-regulation of mRNA and protein expression of ER stress marker GRP78, and COX-2 upon AGE-BSA stimulation (p<0.05, Fig. 1A). Treatment of OA chondrocytes with a well known ER stress inducer drug tunicamycin (TM), used as a positive control for ER stress, also increased the gene/protein expression of GRP78 and COX-2 (Fig. 1B). Similarly, enhanced mRNA and protein expression of COX-2 by AGE-BSA or by TM, subsequently increased the production of PGE2 in the culture medium (Fig. 1A, B). To further strengthen that AGE-BSA induced ER stress, we estimated the protein expression of another ER stress marker protein Bag-1 [4,5] in AGE-BSA stimulated human OA chondrocytes. Our results showed that AGE-BSA time dependently induced the protein expression of Bag-1 (p50/p36) in human OA chondrocytes (Fig. 1C). These finding were further re-confirmed by well known ER stress inducer tunicamycin. Tunicamycin treated human OA chondrocytes also increased the protein expression of Bag-1 (Fig. 1C). Similarly, human OA chondrocytes were treated with native BSA (nBSA) and expression of GRP78, Bag1, COX-2 was determined. Our data showed that nBSA was not involved in up-regulation of GRP78, Bag1, COX-2 or PGE2 production in human OA chondrocytes (Fig. 1D). We have also tested the role of AGE-BSA or nBSA on chondrocytes obtained from normal cartilage. Normal human chondrocytes were stimulated with either AGE-BSA or nBSA (100 μg/ml) for 0–24 h and levels of GRP78, Bag1 and COX-2 was quantified by immunoblotting and compared with the levels in untreated normal chondrocytes. Our results showed that AGE-BSA increased the protein expression of GRP78, Bag1, COX-2 or PGE2 production in a time dependent manner (Fig. 2A). Treatment of normal human chondrocytes with nBSA for the same time points showed no increase in GRP78, Bag1 and COX-2 expressions or PGE2 production (Fig. 2B).
Fig. 1.
Elevated expression of GRP-78, Bag-1 and COX-2 in AGE-BSA stimulated human chondrocytes. (A) Human OA chondrocytes (70–80% confluent) were treated with AGE-BSA (100 μg/ml) for 0, 1, 6, 12 and 24 h. Gene expression of GRP78 and COX-2 was determined by real time quantitative PCR and normalized to GAPDH and compared to the levels present in untreated OA chondrocytes using comparative ΔΔCT method. Cell lyastes were analyzed by immunoblotting with antibodies specific for GRP78 (cat. #3183, Cell Signaling Tech), COX-2 (cat. #4842, Cell Signaling Tech) and β-actin (cat. #sc-47778, Santa Cruz Biotech). β-actin was used as a loading control. Production of PGE2 in the culture medium was quantified by ELISA. (B) Human OA chondrocytes were treated with tunicamycin, TM (2.5 μM) for 0, 1, 6, 12 and 24 h. Gene or protein expressions were determined as described in section A. (C) Bag-1 expression in human OA chondrocytes stimulated by AGE-BSA or TM. Cell lyastes were analyzed by immunoblotting with antibodies specific for Bag-1 (cat. #sc-135844, Santa Cruz Biotech). (D) Human OA chondrocytes were treated with native (n)BSA (100 μg/ml) for 0, 1, 6, 12 and 24 h. Gene or protein expressions were determined as described in section A. Western blots are from single chondrocytes sample, but are representative of three chondrocytes samples. Bars show the mean with 95% confidence interval (CI) results of three independent assays, each of which run in triplicate experiments. *p<0.05 versus control; #p<0.0001 versus control.
Fig. 2.
Elevated expression of GRP78, Bag1 and COX-2 in AGE-BSA stimulated normal human chondrocytes. Normal human chondrocytes (70–80% confluent) were treated with AGE-BSA (100 μg/ml) (A) or with nBSA (100 μg/ml) (B) for 0, 1, 6, 12 and 24 h. Cell lyastes were analyzed by immunoblotting with antibodies specific for GRP78, COX-2. β-actin was used as a loading control. Production of PGE2 in the culture medium was quantified by ELISA. Normal chondrocytes were obtained from trauma patients. Immunoblots are from single chondrocytes sample, but are representative of two chondrocytes samples. Bars show the mean with 95% CI results of two independent assays, each of which run in triplicate experiments. *p<0.05 versus control; #p<0.0001 versus control.
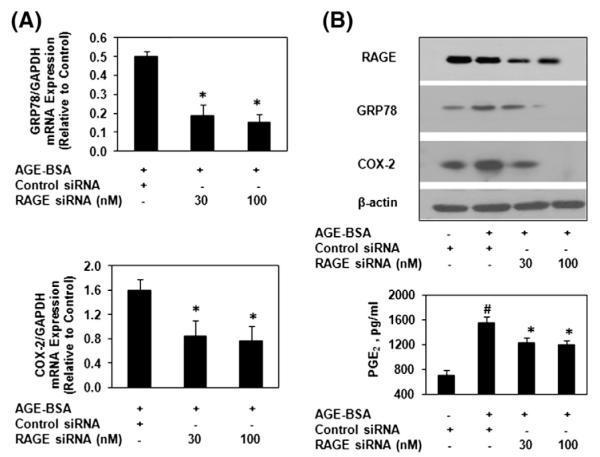
3.3. Necessity of RAGE for AGE-BSA stimulation of COX-2 during ER stress in human chondrocytes
To determine whether AGE-BSA-induced expression of GRP78 and COX-2 in primary human OA chondrocytes was mediated via binding to RAGE, we used siRNA-mediated knockdown of RAGE expression in OA chondrocytes. Transfection of human OA chondrocytes with RAGE-specific siRNAs significantly knocked down the expression of RAGE and inhibited the AGE-BSA-induced GRP78 and COX-2 expression and PGE2 production (Fig. 3A and B). Taken together these findings provide strong evidence of the involvement of RAGE in AGE-BSA-mediated induction of ER stress and the expression of COX-2 in human OA chondrocytes.
Fig. 3.
AGE-BSA induced ER stress is mediated through RAGE in human chondrocytes. (A) RAGE-specific siRNA transfection on AGE-BSA-induced expression of GRP78 and COX-2 in OA chondrocytes. OA chondrocytes were transfected with RAGE-siRNA or control siRNA and then stimulated with AGE-BSA for 24 h. (A) Expression of GRP78 and COX-2 mRNA was determined as described under Fig. 1. (B) Protein expression of RAGE, GRP78 and COX-2 was determined by Immunoblot analysis. β-actin was used as a loading control. Production of PGE2 was quantified by ELISA. Immunoblots are from single chondrocytes sample, but are representative of five chondrocytes samples. Bars show the mean with 95% CI results of five independent assays, each of which run in triplicate experiments. #p<0.05 versus chondrocytes treated with AGE-BSA+control siRNA; *p<0.001 versus chondrocytes transfected with control siRNA alone.
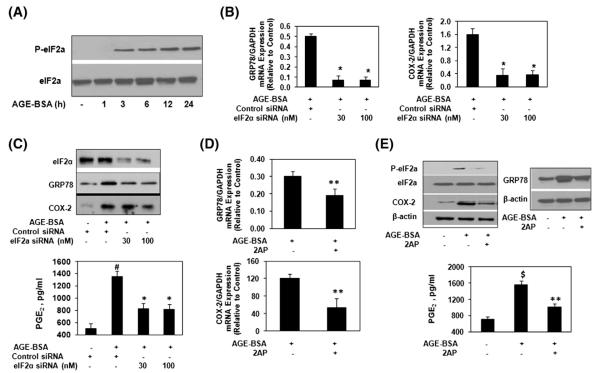
3.4. Activation of eIF2α is required for ER stress stimulated expression of COX-2 in human OA chondrocytes
Treatment of primary human OA chondrocytes with AGE-BSA, induced eIF2α phosphorylation in a time-dependent manner, and the maximum increase over the basal levels was observed 24 h after the treatment (Fig. 4A). To determine whether AGE-BSA-induced phosphorylation of eIF2α is required for the elevated expression of COX-2, human chondrocytes were transfected with eIF2α-specific siRNAs and then stimulated with AGE-BSA for 24 h. Total RNA was isolated and then subjected to real time PCR. Our results showed that eIF2α knockdown significantly inhibited the AGE-BSA-induced mRNA expression of GRP78 and COX-2 (p<0.05; Fig. 4B). To determine whether mRNA inhibition of GRP78 and COX-2 by eIF2α knockdown also affects the protein levels and the production of PGE2, chondrocyte lysates were analyzed by Western immunoblotting for GRP78 and COX-2 proteins and cell culture supernatants were assayed for PGE2 production using ELISA. As shown in Fig. 4C, knockdown of eIF2α significantly inhibited the AGE-BSA-induced protein expression of GRP78 and COX-2 or production of PGE2 (p<0.05) in OA chondrocytes. Furthermore, treatment of human OA chondrocytes with 2-aminopurine (2-AP), an inhibitor of eIF2α phosphorylation attenuated the AGE-BSA-induced mRNA and protein expression of GRP78, COX-2 and PGE2 production (Fig. 4D and E). Taken together, these results clearly indicate that activating eIF2α is essential for the AGE-BSA-induced ER stress and expression of COX-2 in human OA chondrocytes.
Fig. 4.
AGE-BSA-induced stimulation of GRP78 and COX-2 is mediated through α subunit of eIF2 in human chondrocytes. (A) Human OA chondrocytes were treated with AGE-BSA for the indicated times, the phosphorylation of eIF2α was determined by immunobloting. (B) eIF2α-specific siRNA transfection and gene expression of GRP78 and COX-2 in AGE-BSA-stimulated OA chondrocytes. (C) eIF2α-specific siRNA transfection and protein expression of GRP78 and COX-2 in AGE-BSA-stimulated OA chondrocytes. (D) eIF2α-phosphorylation inhibitor 2AP and gene expression of GRP78 and COX-2. OA chondrocytes were pretreated with 2AP (10 μM) for 2 h and stimulated with AGE-BSA for 24 h. (E) 2AP and protein expression of eIF2α, GRP78 and COX-2 or PGE2 production. OA chondrocytes were pretreated with 2AP for 2 h and stimulated with AGE-BSA for 24 h. See Fig. 1 for details. Western immunoblots are from single chondrocytes sample, but are representative of three chondrocytes samples. Bars show the mean with 95% CI results of three independent experiments, each of which run in triplicate experiments. *p<0.001 versus chondrocytes treated with AGE-BSA+control siRNA; #p<0.05 versus chondrocytes transfected with control siRNA; **p<0.05 versus chondrocytes treated with AGE-BSA alone; $p<0.0001 versus untreated chondrocytes.
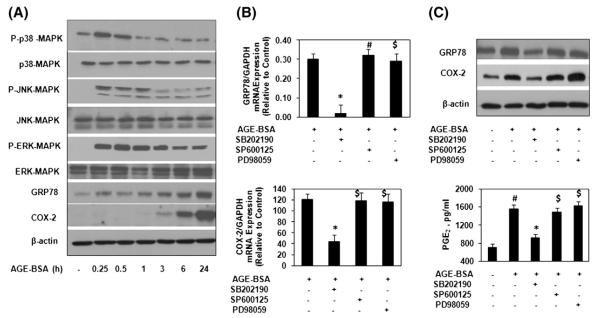
3.5. MAPKs activation in human OA chondrocytes is required for the AGE-BSA-induced COX-2 expression during ER stress
As the expression of COX-2 is regulated through the MAPKs pathways [15], we investigated whether these pathways played a role in the induction of COX-2 during ER stress in AGEs stimulated OA chondrocytes. Primary human OA chondrocytes were treated with AGE-BSA for 0–24 h and the phosphorylation of p38-MAPK, JNK and ERK was examined by Western immunoblotting. All three MAPKs were phosphorylated in AGE-BSA-stimulated OA chondrocytes but the duration of phosphorylation was different with phosphorylated JNK being detectable for 1 h, p38-MAPK for 15 minutes while ERK was found to be phosphorylated at all the time points analyzed (Fig. 5A). To determine the relation of MAPKs p38, JNK, ERK activation and GRP78 or COX-2 expressions in AGE-BSA-stimulated human OA chondrocytes, we used pharmacological agents that inhibit activation of p38-MAPK (SB202190), JNK (SP600125) and ERK (PD89059). Our results showed that pretreatment with SB202190 caused significant decrease in mRNA and protein expression of GRP78 (p<0.05) but in contrast, treatment with the PD98059 and SP600125 had no significant effect on AGE-BSA induced GRP78 mRNA or protein expressions compared to controls (Fig. 5A and B). Expression of COX-2 mRNA or protein and PGE2 production in the culture medium was significantly inhibited with SB202190 treatment (p<0.05), whereas treatment with SP600125 and PD98059 had no effect on AGE-BSA-induced COX-2 expression and PGE2 production in AGE-BSA-stimulated OA chondrocytes (p<0.05; Fig. 5 B&C). Taken together, our results demonstrated that p38-MAPK is the only MAPK involved in AGE-BSA-induced induction of ER stress characterized by the expression of GRP78 and COX-2 in OA chondrocytes (Fig. 5B and C).
Fig. 5.
AGE-BSA-induced activation of p38-MAPKs is essential for COX-2 induction during ER stress in human chondrocytes. (A) Human OA chondrocytes were treated with AGE-BSA for the times indicated and the activation of MAPKs was determined by Immunoblot analysis. GRP78 and COX-2 were indicators for ER stress. (B) Effect of specific inhibitors for MAPKs on the gene expression of GRP78 and COX-2 in AGE-BSA-stimulated OA chondrocytes. (C) Effect of specific inhibitors for MAPKs on the protein expression of GRP78 and COX-2, and production of PGE2 in AGE-BSA-stimulated OA chondrocytes. OA chondrocytes were pretreated with inhibitors of p38 (SB202190), JNK (SP600125) and ERK (PD98059) for 1 h and then stimulated with AGE-BSA for 24 h. The concentration of SB202190, SP600125 and PD98059 used in these studies was 100 μM, 10 μM and 50 μM, respectively. Western immunoblots are from single chondrocytes sample, but are representative of five chondrocytes samples. Bars show the mean with 95% CI results of five independent assays, each of which run in triplicate experiments. *p<0.001 versus chondrocytes treated with AGE-BSA alone; #p<0.0001 versus untreated chondrocytes; $p>0.05 versus chondrocytes treated with AGE-BSA alone.
3.6. Induction of NF-κB is dependent on the activation of p38-MAPK and eIF2α in AGE-BSA-stimulated human OA chondrocytes
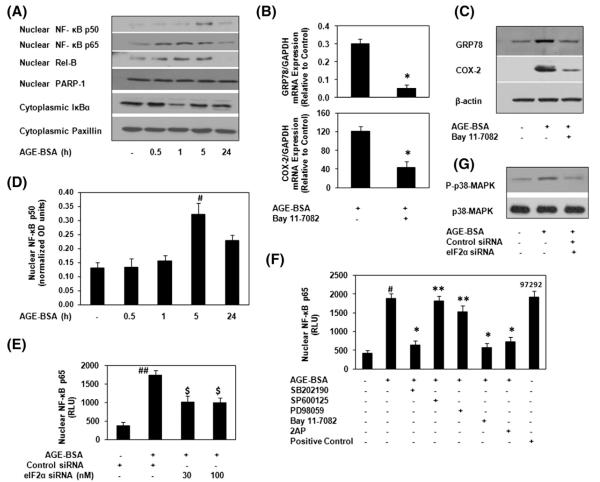
To evaluate the mechanism of AGE-BSA induced expression of COX-2 in OA chondrocytes with ER stress, NF-κB activation was studied in detailed. Stimulation of OA chondrocytes with AGE-BSA induced the degradation of cytoplasmic IκBα and increased the nuclear level of NF-κB subunits p65 and Rel-B (Fig. 6A). Our results also showed that after 5 h of AGE-BSA treatment, the level of nuclear NF-κBp50 was enhanced (Fig. 6A). These observations were further verified by NF-κBp50 specific ELISA and the results are shown in Fig. 6D. The co-relation of NF-κB activation and the expression of GRP78 and COX-2 in our studies, were investigated by the use of pharmacological agent, Bay 11-7082, a known inhibitor of NF-κB (IKKα/β). Treatment of primary human OA chondrocytes with Bay 11-7082, significantly blocked the AGE-BSA-induced gene/protein expression of GRP78 or COX-2 (p<0.05) in human OA chondrocytes (Fig. 6B and C).
Fig. 6.
AGE-BSA-induced activation of NF-κB is mediated through eIF2α or p38-MAPK in human chondrocytes during ER stress. (A) OA chondrocytes were incubated with AGE-BSA for the time indicated and NF-κB subunits were analyzed by Immunoblotting. PARP-1 and Paxillin were used as internal markers for nuclear and cytoplasmic proteins. (B–C) NF-κB inhibitor (BAY 11-7082) inhibited the AGE-BSA-induced GRP78 and COX-2. Chondrocytes were pretreated with Bay 11-7082 (10 μM) for 1 h and then stimulated with AGE-BSA for 24 h. (D) Activation of NF-κBp50 was further verified by NF-κBp50 specific ELISA using nuclear extracts at indicated time intervals. (E) eIF2α-knockdown and NF-κBp65 activation in AGE-BSA-stimulated OA chondrocytes. eIF2α-knockdown chondrocytes were incubated with AGE-BSA for 45 minutes, and activated NF-κBp65 was determined by ELISA. (F) p38-MAPK and eIF2α inhibitors inhibited the AGE-BSA-induced activation of NF-κBp65 in chondrocytes. Chondrocytes were pretreated with SB202190, SP600125 and PD98059, 2AP for 1 h and then stimulated with AGE-BSA for 45 minutes and activated NF-κBp65 was determined ELISA. TNF-α treated HeLa cell extract and Bay 11-7082/AGE-BSA treated chondrocytes extract were used as positive controls. (G) AGE-BSA-induced phosphorylation of p38-MAPK was inhibited by eIF2α-knockdown. eIF2α-knockdown chondrocytes were incubated with AGE-BSA for 45 minutes. Immunoblots are from single chondrocytes sample, but are representative of five chondrocytes samples. Bars show the mean with 95% CI results of five independent assays, each of which run in triplicate experiments. *p<0.0001 versus chondrocytes treated with AGE-BSA alone; #p<0.0001 versus untreated chondrocytes; ##p<0.001 versus chondrocytes transfected with control siRNA alone; $p<0.05 versus chondrocytes treated with AGE-BSA+control siRNA; **p>0.05 versus chondrocytes treated with AGE-BSA alone.
Phosphorylation of the α subunit of eIF2α is required for the activation of NF-κB in response to ER stress [21]; therefore we examined whether inhibition of eIF2α-activation by 2-AP treatment or eIF2α-siRNA knockdown affects the activation of NF-κB subunits p50 or p65 in AGE-BSA-stimulated human OA chondrocytes. Treatment of human OA chondrocytes with 2-AP or knockdown of eIF2α expression using specific siRNAs significantly inhibited the activation and DNA binding activity of NF-κBp65 (p<0.05) (Fig. 6E and F) and also inhibited the translocation of nuclear NF-κBp50 (data not shown). Similarly, treatment with p38-MAPK inhibitor SB202190 caused a significant decrease in the DNA binding activity of NF-κBp65 (Fig. 6F) in AGE-BSA stimulated OA chondrocytes (p<0.05). In contrast, treatment with JNK inhibitor SP600125 or ERK inhibitor PD98059 had no significant effect on AGE-BSA induced activation of NF-κBp65 (Fig. 6F, p>0.05). Together these results indicate that activation of NF-κB is dependent on the activation of p38-MAPK and eIF2α during ER stress in AGE-BSA-stimulated human OA chondrocytes.
3.7. eIF2α is required to activate the stress-activated p38-MAPK in OA chondrocytes
Transfection with eIF2α-specific siRNA (30 nM) significantly inhibited the AGE-BSA-induced phosphorylation of p38-MAPK in OA chondrocytes (Fig. 6G), indicating that activation eIF2α is upstream of p38-MAPK and is required for its activation during ER stress in AGE-BSA-stimulated OA chondrocytes.
4. Discussion
This report shows AGEs induce ER stress in human chondrocytes, a finding in agreement with previous reports showing that AGEs mediated ER stress in other cell types [36,37]. Iwawaki et al. [38] used a transgenic mouse model to monitor ER stress in vivo, and demonstrated that ER stress occurred in a variety of organs such as liver, spleen, brain, and kidney. However, little is known about the role of ER stress in cartilage but ER stress likely plays an important role in cartilage biology as the expression of GRP78 and Bag-1, ER stress marker proteins, were found to be upregulated several folds in human as well as in mouse model of arthritis [4,5,39,40]. In addition, recent reports suggest that multiple outcomes exist following induction of ER stress in chondrocytes [2,17,40,41]. Despite these advances, additional studies are needed to fully understand the action of various inducers of ER stress on chondrocytes and the consequences of ER stress.
Accumulation of AGEs in articular cartilage has been proposed as a mechanism for the age-related development of OA [8–10]. The age-related accumulation of AGEs results in crosslinking of cartilage matrix components and suggests a putative molecular mechanism for the development of OA [9]. Therefore, the levels of AGEs might predict susceptibility for developing OA. In contrast, it is recently reported that In end stage osteoarthritis, cartilage tissue pentosidine levels are inversely related to parameters of cartilage damage [42]. This is likely due to the ongoing (ineffective) increased turnover of cartilage matrix protein even in end stage disease. Moreover, elevation of cartilage AGEs does not accelerate initiation of canine experimental OA upon mild surgical damage [43]. These data further support the diminished cartilage turnover in OA, but only a tendency towards enhanced cartilage damage in AGEs accumulated articular cartilage. However, in OA chondrocytes, elevated levels of AGEs may also trigger the activation of catabolic pathways through RAGE (receptor for AGEs) [10,12–14,26]. The activation of RAGE stimulates critical signaling pathways linked to the activation and expression of various inflammatory genes [12–14,26]. Studies have shown that abnormally high levels of COX-2-PGE2 are present in the synovial fluid, synovium and cartilage tissues of arthritic patients [24,25]. In this study, using primary human OA chondrocytes, we have demonstrated that in-vitro generated AGE-BSA activates a RAGE-mediated signaling cascade that results in the induction of ER stress which leads to increased expression of COX-2 and PGE2 production. We used AGE-BSA as a model for AGEs, which is basically a complex that includes N3-carboxymethyllysine (CML), pentosidine, and other AGEs, produced by reacting BSA with glycoaldehyde under sterile conditions, followed by extensive dialysis as previously described [31]. Several lines of evidence support the notion of an interaction of AGE-BSA with RAGE. Knockdown of RAGE by siRNAs significantly inhibited the AGE-BSA-induced GRP78 and COX-2 expression (p<0.05), confirming that AGE-BSA-induced ER stress related stimulation of COX-2 expression requires signaling through RAGE. ER stress and its associated affects on COX-2 expression are well established in other cell types [15]. Tunicamycin is a well known ER stress inducer drug [15,17]. Studies have shown that tunicamycin in various cell types enhanced COX-2 expression [15,26]. In addition, similar stimulation of COX-2 expression was observed by another ER stress inducer brefeldin A [15]. These studies clearly indicate that COX-2 is a part of ER stress. Furthermore, ER stress induced by expression of hepatitis B virus surface protein also stimulates the expression of COX-2 in-vitro [15,22] and in-vivo [26]. These studies further support that COX-2 is a part of ER stress. In the present study, we have established a relation between ER stress and COX-2 in human chondrocytes for the first time. Treatment of human chondrocytes with tunicamycin, significantly increased the expression of COX-2. This again indicating that COX-2 is a part of ER stress.
ER stress can activate multiple signal transduction pathways including the eIF2α, MAPK and NF-κB [2,15–17]. These pathways may cross-talk with each other or converge on common downstream effectors [15,16]. Here we demonstrated that AGE-BSA-induced expression of COX-2 and PGE2 production required the phosphorylation of eIF2α during ER stress in human OA chondrocytes. These are novel findings and have not been reported previously. In addition, we also demonstrated that treatment of OA chondrocytes with SB202190 (p38 inhibitor) significantly down regulated the AGE-BSA-stimulated GRP78 and COX-2 expression and PGE2 production. Whereas, PD98059 (ERK inhibitor) or SP600125 (JNK inhibitor) had no effect on AGE-BSA-induced GRP78 and COX-2 expressions and PGE2 production. But this is in contrast to the previous study that showed all MAPK inhibitors (SB202190, SP600125, PD98059) inhibited AGE-BSA induction of COX-2 expression and PGE2 production in human OA chondrocytes [26]. Although we could not find how much inhibitors concentrations authors were used in this study [26], but we cannot rule out of SP600126 or PD98059 in AGE-BSA stimulated expression of COX-2 and PGE2 production. But our findings in the present study demonstrate that AGE-BSA-stimulated expression of COX-2 and PGE2 production was mediated via p38-MAPK activation in OA chondrocytes during ER stress.
NF-κB is a transcription factor that participates in immunity and inflammation [44]. NF-κB proteins include p65 (Rel A), Rel B, c-Rel, p50/p105 and p52/p100, which normally bind to IκB in the cytoplasm usually in three different complexes [18,44]. It is well documented that NF-κB is involved in AGE-mediated effects of RAGE signaling [10,12–14,26] and during ER stress the expression of COX-2 is dependent on the activation of NF-κB [15]. In order to gain further insight into the mechanism, AGE-BSA induced IκBα degradation and nuclear translocation of NF-κB p65, p50 and Rel B have been studies in this report (Fig. 6 and 7). Direct effect of NF-κB on COX-2 expression during ER stress was investigated using the NF-κB (IKKα/β) inhibitor Bay 11-7082. Treatment of OA chondrocytes with Bay 11-7082, significantly blocked the AGE-BSA-induced gene/protein expression of GRP78 or COX-2 (p<0.05) which showed that AGE-BSA-induced ER stress stimulate the expression of COX-2 via activation of NF-κB.
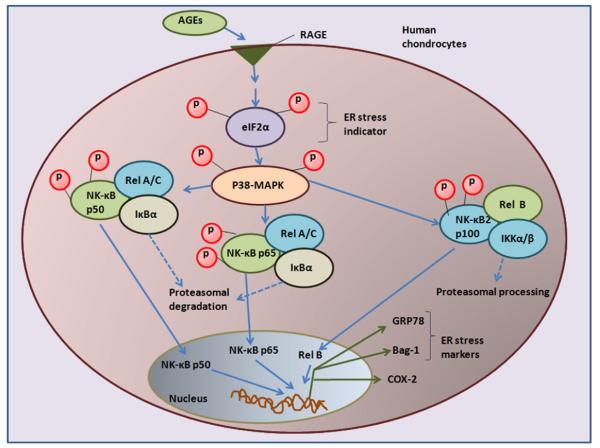
Fig. 7.
Signaling of endoplasmic stress to COX-2 in AGEs stimulated human chondrocytes. AGEs through RAGE activates eIF2α which relays ER stress signals to the nuclear transcription factor NF-κB via activation of p38-MARK. Different NF-κB complexes were demonstrated by NF-κBp50, NF-κBp65, Rel B and by the proteasomal degradation of IκBα. Intermediates indicated in blue were not demonstrated in this report. Abbreviations: ER stress: endoplasmic reticulum stress; AGEs: advanced glycation end products; RAGE; receptor for AGEs; eIF2-α: eukaryotic initiation factor α-2; p38-MAPK: p38-mitogen activated protein kinase; NF-κB; nuclear factor-Κb; COX-2: cyclooxygenase-2.
It has been reported that phosphorylation of eIF2α is required for the activation of NF-κB in response to ER stress [21]; therefore we examined whether inhibition of eIF2α-activation affects the activation of NF-κB in AGE-BSA-stimulated OA chondrocytes. Treatment of OA chondrocytes with 2-aminopurine (2AP, an eIF2α inhibitor) or eIF2α-knockdown by siRNAs significantly inhibited the activation and DNA binding activity of NF-κB (p<0.05) in AGE-BSA-stimulated OA chondrocytes. On the other hand, treatment with p38-MAPK inhibitor caused significant decrease in the DNA binding activity of NF-κB in AGE-BSA stimulated OA chondrocytes (p<0.05). In contrast, treatment with either JNK inhibitor or ERK inhibitor had no significant effect on AGE-BSA induced activation of NF-κB. These data indicate that activation of p38-MAPK appears to be essential for the activation of NF-κB in AGE-BSA-stimulated OA chondrocytes. We also investigated, whether eIF2α is required for the AGE-BSA-induced activation of p38-MAPK during ER stress. eIF2α knockdown by eIF2α-specific siRNAs inhibited the AGE-BSA-induced phosphorylation of p38-MAPK in OA chondrocytes, indicating that eIF2α is required for the ER stress-mediated activation of p38-MAPK in OA chondrocytes. All together these results suggest that activation of NF-κB is dependent on the phosphorylation of eIF2α and activation of p38-MAPK during ER stress in AGE-BSA-stimulated human chondrocytes (data summarized in Fig. 7). In short, our novel data strongly suggest that AGE-BSA-induced ER stress stimulates COX-2 expression and PGE2 production via activation of eIF2α, p38-MAPK, and NF-κB pathways in human chondrocytes.
5. Conclusions
This study demonstrates that AGEs stimulate ER stress via RAGE in human chondrocytes. Activation of RAGE by AGEs induce ER stress and trigger a cascade of signaling events, which includes phosphorylation of eIF2α and p38-MAP kinase which in turn activates NF-κB and increases the COX-2 expression and PGE2 production. Taken together, our data suggest that the stress induced by AGEs in the endoplasmic reticulum activates multiple signaling pathways that may play an important role in the degeneration of cartilage in OA. Additional work is needed to further define the role of AGEs induced ER stress in cartilage matrix turnover and to determine if RAGE mediated-ER stress inhibition could reduce the progression of cartilage damage in arthritis.
Acknowledgments
This work was supported by the USPHS/NIH grants RO1-AT-003267, RO1-AT-005520, R21-AT504615 to TMH and financial support from Metrohealth, Cleveland, Ohio is also gratefully acknowledged. We also thank Dr. Brendan Patterson (Department of Orthopedics, MHHC) for providing human cartilage samples.
Abbreviations
- ER stress
endoplasmic reticulum stress
- OA
osteoarthritis
- AGEs
advanced glycation end products
- RAGE
receptor for AGEs
- GRP78
glucose regulated protein-78 (bip)
- Bag-1
Bcl-2 associated athanogene 1
- eIF2-α
eukaryotic initiation factor α-2
- COX-2
cyclooxygenase-2
- PGE2
prostaglandin E2
- p38-MAPK
p38-mitogen activated protein kinase
- NF-κB
nuclear factor-κb
Footnotes
Authors’ contributions
ZR carried out the experimental work, data collection, interpretation and manuscript drafting. TMH conceived of the study, its design, coordinated, data interpretation and manuscript drafting.
Conflict of interest statement
The authors declare that they have no conflict of interests.
References
- [1].Schroder M. Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang L, Carlson SG, McBurney D, Horton WE., Jr. Multiple signals induce endoplasmic reticulum stress in both primary and immortalized chondrocytes resulting in loss of differentiation, impaired cell growth, and apoptosis. J. Biol. Chem. 2005;280:31156–31165. doi: 10.1074/jbc.M501069200. [DOI] [PubMed] [Google Scholar]
- [3].Kohno K, Normington K, Sambrook J, Gething MJ, Mori K. The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol. Cell. Biol. 1993;13:877–890. doi: 10.1128/mcb.13.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nugent AE, Speicher DM, Gradisar I, McBurney DL, Baraga A, Doane KJ, Horton WE., Jr. Advanced osteoarthritis in humans is associated with altered collagen VI expression and upregulation of ER-stress markers Grp78 and bag-1. J. Histochem. Cytochem. 2009;57:923–931. doi: 10.1369/jhc.2009.953893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang L, McBurney D, Tang SC, Carlson SG, Horton WE., Jr. A novel role for Bcl-2 associated-athanogene-1 (Bag-1) in regulation of the endoplasmic reticulum stress response in mammalian chondrocytes. J. Cell. Biochem. 2007;102:786–800. doi: 10.1002/jcb.21328. [DOI] [PubMed] [Google Scholar]
- [6].Loeser RF. Age-Related Changes in the Musculoskeletal System and the Development of Osteoarthritis. Clin. Geriatr. Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention [review] Arthritis Rheum. 1998;41:1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [8].DeGroot J, Verzijl N, Wenting-van Wijk MJ, Jacobs KM, van El B, van Roermund PM, Bank RA, Bijlsma JW, TeKoppele JM, Lafeber FP. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50:1207–1215. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- [9].Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, Mizrahi J, Schalkwijk CG, Thorpe SR, Baynes JW, Bijlsma JW, Lafeber FP, TeKoppele JM. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [10].Steenvoorden MM, Huizinga TW, Verzijl N, Bank RA, Ronday HK, Luning HA, Lafeber FP, Toes RE, DeGroot J. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–263. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- [11].Akiyama H, Lefebvre V. Unraveling the transcriptional regulatory machinery in chondrogenesis. J. Bone Miner. Metab. 2011;29:390–395. doi: 10.1007/s00774-011-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, Bursch LS, Yan SD. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 2006;52:2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nah SS, Choi IY, Yoo B, Kim YG, Moon H, Lee C. Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett. 2007;581:1928–1932. doi: 10.1016/j.febslet.2007.03.090. [DOI] [PubMed] [Google Scholar]
- [14].Rasheed Z, Akhtar N, Haqqi TM. Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology (Oxford) 2011;50:838–851. doi: 10.1093/rheumatology/keq380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC, Lai MD. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp 38 mitogen-activated protein kinase. J. Biol. Chem. 2004;279:46384–46392. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- [16].Liang SH, Zhang W, McGrath BC, Zhang P, Cavener DR. PERK (eIF2alpha kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem. J. 2006;393:201–209. doi: 10.1042/BJ20050374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hamamura K, Goldring MB, Yokota H. Involvement of p38 MAPK in regulation of MMP13 mRNA in chondrocytes in response to surviving stress to endoplasmic reticulum. Arch. Oral Biol. 2009;54:279–286. doi: 10.1016/j.archoralbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rasheed Z, Haqqi TM. Update on targets of biologic therapies for rheumatoid arthritis. Curr. Rheumatol. Rev. 2008;4:246–253. doi: 10.2174/157339708786263915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McLay LM, Halley F, Souness JE, McKenna J, Benning V, Birrell M. The discovery of RPR 200765A, a p38 MAP kinase inhibitor displaying a good oral anti-arthritic efficacy. Bioorg. Med. Chem. 2001;9:537–554. doi: 10.1016/s0968-0896(00)00331-x. [DOI] [PubMed] [Google Scholar]
- [20].Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- [21].Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol. Cell. Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Waris G, Tardif KD, Siddiqui A. Endoplasmic reticulum (ER) stress: hepatitis C virus induces an ER-nucleus signal transduction pathway and activates NF-kappaB and STAT-3. Biochem. Pharmacol. 2002;64:1425–1430. doi: 10.1016/s0006-2952(02)01300-x. [DOI] [PubMed] [Google Scholar]
- [23].Mastbergen SC, Lafeber FP, Bijlsma JW. Selective COX-2 inhibition prevents proinflammatory cytokine-induced cartilage damage. Rheumatology (Oxford) 2002;41:801–808. doi: 10.1093/rheumatology/41.7.801. [DOI] [PubMed] [Google Scholar]
- [24].Mateos JL. Selective inhibitors of cyclooxygenase-2 (COX-2), celecoxib and parecoxib: a systematic review. Drugs Today. 2010;46:1–25. [PubMed] [Google Scholar]
- [25].de Boer TN, Huisman AM, Polak AA, Niehoff AG, van Rinsum AC, Saris D, Bijlsma JW, Lafeber FJ, Mastbergen SC. The chondroprotective effect of selective COX-2 inhibition in osteoarthritis: ex vivo evaluation of human cartilage tissue after in vivo treatment. Osteoarthr. Cartil. 2009;17:482–488. doi: 10.1016/j.joca.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [26].Nah SS, Choi IY, Lee CK, Oh JS, Kim YG, Moon HB, Yoo B. Effects of advanced glycation end products on the expression of COX-2, PGE2 and NO in human osteoarthritic chondrocytes. Rheumatology (Oxford) 2008;47:425–431. doi: 10.1093/rheumatology/kem376. [DOI] [PubMed] [Google Scholar]
- [27].Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J. Bone Joint Surg. Am. 1982;64:88–94. [PubMed] [Google Scholar]
- [28].Rasheed Z, Akhtar N, Haqqi TM. Pomegranate extract inhibits the interleukin-1β-induced activation of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res. Ther. 2010;12:R195. doi: 10.1186/ar3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss F, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end products-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res. Ther. 2009;11:R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 1971;53:523–537. [PubMed] [Google Scholar]
- [31].Valencia JV, Weldon SC, Quinn D, Kiers GH, DeGroot J, TeKoppele JM, Hughes TE. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal. Biochem. 2004;324:68–78. doi: 10.1016/j.ab.2003.09.013. [DOI] [PubMed] [Google Scholar]
- [32].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rasheed Z, Akhtar N, Anbazhagan AN, Ramamurthy S, Shukla M, Haqqi TM. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP kinases and NF-kappaB in human KU812 cells. J. Inflamm. (Lond.) 2009;6:1. doi: 10.1186/1476-9255-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rasheed Z, Akhtar N, Khan A, Khan KA, Haqqi TH. Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-kappaB in activated human mast cells: suppression of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8. J. Pharmacol. Exp. Ther. 2010;333:354–363. doi: 10.1124/jpet.109.165209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- [36].Loughlin DT, Artlett CM. Precursor of advanced glycation end products mediates ER-stress-induced caspase-3 activation of human dermal fibroblasts through NAD(P)H oxidase 4. PLoS One. 2010;5:e11093. doi: 10.1371/journal.pone.0011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev. Mol. Med. 2009;11:e9. doi: 10.1017/S146239940900101X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- [39].Brownlie RJ, Myers LK, Wooley PH, Corrigall VM, Bodman-Smith MD, Panayi GS, Thompson SJ. Treatment of murine collagen-induced arthritis by the stress protein BiP via interleukin-4-producing regulatory T cells: a novel function for an ancient protein. Arthritis Rheum. 2006;54:854–863. doi: 10.1002/art.21654. [DOI] [PubMed] [Google Scholar]
- [40].Nugent AE, McBurney DL, Horton WE., Jr. The presence of extracellular matrix alters the chondrocyte response to endoplasmic reticulum stress. J. Cell. Biochem. 2011;112:1118–1129. doi: 10.1002/jcb.23025. [DOI] [PubMed] [Google Scholar]
- [41].Price J, Zaidi AK, Bohensky J, Srinivas V, Shapiro IM, Ali H. Akt-1 mediates survival of chondrocytes from endoplasmic reticulum-induced stress. J. Cell. Physiol. 2010;222:502–508. doi: 10.1002/jcp.22001. [DOI] [PubMed] [Google Scholar]
- [42].Vos PA, Mastbergen SC, Huisman AM, de Boer TN, DeGroot J, Polak AA, Lafeber FP. In end stage osteoarthritis, cartilage tissue pentosidine levels are inversely related to parameters of cartilage damage. Osteoarthr. Cartil. 2012;20:233–240. doi: 10.1016/j.joca.2011.12.007. [DOI] [PubMed] [Google Scholar]
- [43].Vos PA, Degroot J, Barten-van Rijbroek AD, Zuurmond AM, Bijlsma JW, Mastbergen SC, Lafeber FP. Elevation of cartilage AGEs does not accelerate initiation of canine experimental osteoarthritis upon mild surgical damage. J. Orthop. Res. 2012;30:1398–1404. doi: 10.1002/jor.22092. [DOI] [PubMed] [Google Scholar]
- [44].Hayden MS, Ghosh S. Signaling to NF-κB. Gene Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]