Introduction
Lung carcinomas that initially respond to tyrosine kinase inhibitors (TKIs) often harbor somatic gain of function mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) gene.1,2 These mutations are more frequently associated with adenocarcinoma histology, female gender, East Asian ethnicity and low or absent tobacco exposure.3 Despite initial response to TKI therapy, the tumors usually recur or progress by development of acquired resistance.
The major mechanism of acquired resistance is the presence of a second EGFR point mutation T790M in exon 20.4,5 T790M mutations usually occur with a common activating mutation, and it greatly increases the ATP-binding affinity of the initial mutation.4,6-9 T790M mutations may occur in treatment naïve tumors and cell lines10,11 and the mutant peaks in sequencing electropherograms are present in a minority of cells and may require sensitive detection methods. By contrast, the common activating mutations are often equal to or greater than the wild type peak, a phenomenon we have termed as mutant allele specific imbalance (MASI).12 T790M germline mutations are present in 1% of lung cancers undergoing EGFR gene sequencing with the mutant allele occurring in about equal proportion to the wild type allele.13
In this report we describe the development of lung cancer in a young woman with a germline T790M mutation, and we combined an extensive study of her family pedigree with a review of the existing literature.
Materials and methods
Literature search
We searched the PubMed database using combinations of the terms “Lung Neoplasms”, “Receptor, Epidermal Growth Factor” “Germ-Line” and “T790M”.
Investigation of a family with germline T790M mutation
Using an IRB approved protocol and after informed consent, we were able to track a germline T790M mutation in the EGFR gene present in the proband, as well as relevant history through five generations of her family. The family pedigree was constructed with the assistance of the proband and several family members. The smoking histories of deceased individuals were confirmed by at least two close relatives. Blood samples were obtained from 17 family members representing three generations. Genetic testing of the T790M mutation was performed by a CLIA approved laboratory utilizing EGFR exon 20-specific primers to sequence the region containing T790, using a standardized Sanger sequencing protocol (BigDye® Terminator v3.1 Cycle Sequencing Kit Protocol, Applied Biosystems, 2002).
Detection of EGFR and germline T790M mutations in Japanese patients
Paired tumor and non-malignant lung samples from 629 Japanese patients with resected lung cancers were examined for EGFR mutations using previously published methods.14-16 The study received local IRB permission and all patients gave informed consent for mutation testing. Testing was performed for therapy selection. Two patients were excluded because they had neuroendocrine tumors, leaving 627 patients with NSCLC.
Results
Literature search
A search of the PubMed database yielded five references for germline T790M mutations.13,17-20
The proband
The proband was a 29-year-old female with a total tobacco exposure of 0.1 pack years. She presented with a 4.4 cm. left upper lobe adenocarcinoma and biopsy proven bilateral preneoplastic and preinvasive lesions (Supplemental Fig. 2). Analysis of tumor DNA for EGFR exons 18-21 revealed an L858R mutation in exon 21 (minor peak in comparison to the wild type peak), and a T790M mutation (equivalent in height to the wild type peak) (Fig. 1). Mutation analysis of her blood mononuclear cells indicated a T790M mutation, with equivalent heights of the mutant and wild type peaks, confirming the presence of a germline T790M mutation. No L858R mutation was detected in the mononuclear cells.
Figure 1.
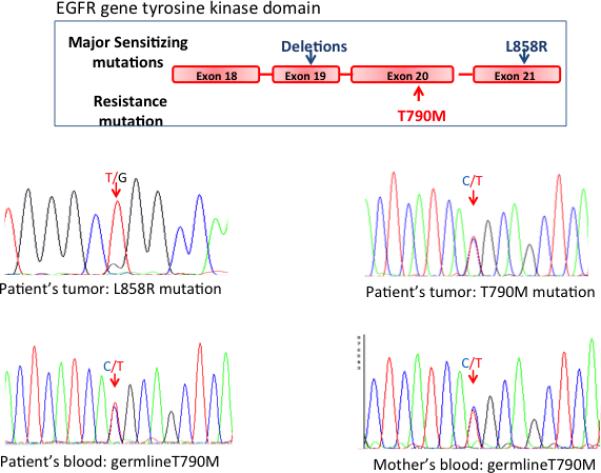
Sequencing of the EGFR gene in the proband's adenocarcinoma revealed an activating mutation (L858R) in the EGFR gene (minor peak) (A) and a prominent T790M mutation, equal in size to the wild type peak (B). The T790M mutation substitutes methionine for threonine at position 790 (nucleotide c.2369). The finding in a lung cancer of a T790M mutant band equal in size to the wild type band prior to TKI therapy suggests a germline mutation. Examination of the proband's blood cells confirmed a germline T790M mutation in the patient (C) and in her mother (D). A cartoon of the location of the common mutations in the tyrosine kinase domain of the EGFR gene is shown in the upper part of the figure.
History and mutation status of Proband's family
Information about the proband's family pedigree is presented in Fig. 2 and Supplemental Table 1. Eight of 17 family members tested were positive for the mutation, including the proband's mother and brother. All family members tested received genetic counseling prior to testing and after the results were completed. Two of the family members were tested to establish lineage (IV:12, III:13), and were subsequently discarded from further analysis. Eight of 15 maternally related family members tested were mutation positive (53.3%), consistent for Mendelian inheritance of an autosomal gene, As documented in Figure 2 there are 14 family members that are tested, obligate or assumed carriers of the T790M mutation. Four of these 14 (including the proband) developed lung cancer. CT scans, were available on 5 mutation carriers. All scans showed one or multiple small pulmonary lesions of uncertain diagnosis (Supplemental data).
Figure 2.
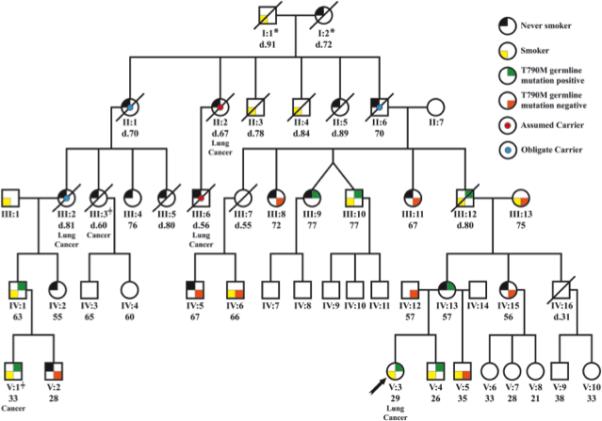
Pedigree of family with germline T790M mutation. Age, smoking history, mutation status and other cancer history are recorded.
*In addition to the three obligate carriers identified in the pedigree, either individual I:1 or I:2 is a obligate carrier based on pedigree analysis. Individual III:3 is reported to have died from bladder cancer. She had lung cancer, but it is uncertain if this was a primary cancer or a metastasis. She was not considered to be a lung cancer case. Individual V:1 had a lung carcinoid tumor. There are two never smokers that developed lung cancer in the family (II:2 and III:6), given the low likelihood that they represent two sporadic cases in this family we have assumed that they are also mutation carriers, thus for a total of 14 known, obligate or assumed mutation carriers. There are two never smokers that developed lung cancer in the family (II:2 and III:6), given the low likelihood that they represent two sporadic cases in this family we have assumed that they are also mutation carriers, thus for a total of 14 known, obligate or assumed mutation carriers.
Germline T790M mutations in lung cancer cases and controls
From our proband's family pedigree and the five reports in the literature there are a total of 29 mutation carriers. Lung cancer developed in 19 of these carriers (referred to as lung cancer cases (Table 1). The remaining 10 T790M germline cases without lung cancer are referred to as controls. However, the gender and smoking status of some of the cases and controls from the literature is lacking or ambiguous. Thus subgroup analysis contains varying number of cases and controls. (Table 2). It should be noted that, as with other germline mutation analyses, some of the controls may ultimately developed cancer.
Table 1.
Summary of literature regarding lung cancer patients with germline T790M mutations and family member with lung cancer (mutation status known and assumed).
| Reference | Case# | Family# | Other family members with lung cancer/Relationship to proband | Ethnicity | Age | Sex | Smoker | Tumor | T790M germline mutation | 2nd EGFR gene mutation | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bell et al21 | 1 | 1 | Proband | White | 50 | M | S | 5 ADCs | Yes | 5 tumors L858R in 2/5 delL747-T751 in 1/5 | Multiple bilateral nodules |
| Bell et al21 | 2 | 1 | Yes, Brother | White | 55 | M | ? | ADC | Yes | G719A | Widespread metastases |
| Bell et al21 | 3 | 1 | Yes, Mother | White | 62 | F | ? | ADC | Mutation assumed | N/A | |
| Bell et al21 | 4 | 1 | Yes, Grandfather | White | 72 | M | ? | ADC | Mutationassumed | N/A | |
| Girard et al18 | 5 | 2 | Proband | E. Indian | 66 | F | NS | ADC | yes | L858R | Multiple bilateral nodules |
| Girard et al18 | 6 | 2 | Yes, Father | E. Indian | 41 | F | NS | N/A | Mutation assumed | N/A | |
| Girard et al18 | 7 | 3 | Proband | White | 56 | M | NS | ADC | Yes | L858R | Widespread metastases |
| Girard et al18 | 8 | 3 | Yes, Either father or mother | White | 72/80 | M/F | S | N/A | Mutation Assumed | N/A | |
| Prudkin19 | 9 | 4 | Proband | ? | 72 | F | NS | 2 ADCs 1 LCNEC | yes | None in 3 tumors | |
| Prudkin | 10 | 4 | Yes, sister | ? | ? | F | ? | ADC | Mutation unknown | N/A | |
| Oxnard et al14 | 11-12 | 5,6 | One case had a sibling with cancer | ? | 44/73 | F/M | NS/S | ADC | Yes | Both had exon 19 deletions | |
| Oxnard et al14 | 13 | 7 | No | ? | 44-73 | F | NS | ADC | Yes | 6 Nodules- 4 had L858R, 2 exon 19 deletions. | |
| Tibaldi et al20 | 14 | 8 | Proband | White | 72 | F | NS | ADC | yes | Del; E746-A750 in exon 19 | Bilateral pulmonary lesions |
| Tibaldi et al20 | 15 | 8 | Yes, Sister | White | 74 | F | NS | NSCLC | yes | None | |
| UTSW V-3 | 16 | 9 | Proband | White | 29 | F | S | ADC | yes | L858R | |
| UTSW II-2 | 17 | 9 | Yes, great- great aunt of proband | White | 67 | F | NS | N/A | Mutation assumed | unknown | |
| UTSW III-6 | 18 | 9 | Yes, son of #17 | White | 56 | M | NS | N/A | Mutation assumed | unknown | |
| UTSW III-2 | 19 | 9 | Yes, distant aunt of proband | White | 81 | F | NS | N/A | Obligate carrier | unknown |
Under “Family history of lung cancer”, the number of family members with lung cancer are indicated in parentheses. The Oxnard reference only provides summary information about 5 germline mutation cases, two of which were previously reported by Girard, thus only recorded once. There is a both a paternal and maternal history of lung cancer in case #7- Girard so the lineage is unknown, however we would assume that one of the parents is a mutation carrier. Please see Supplemental Data for discussion on potential problems with the report by Tibaldi et al.20 ADC = pulmonary adenocarcinoma, LCNEC = large cell neuroendocrine carcinoma, NSCLC = non-small cell lung carcinoma, not otherwise specified
Table 2.
Distribution of germline T790M mutation carriers stratified by smoking status, gender, and lung cancer
Case = Lung Cancer arising in germline T790M mutation carrier, Control = Germline mutation carrier that has not developed lung cancer
Out of 19 lung cancer cases, there are two cases with unknown sex and four cases with unknown smoking status; overall, there are 13 cases with both sex and smoking status known (see Table 1). Athough there are 10 mutation carriers, we do not know whether the proband inherited the mutation from I:1 or I:2 (see Figure 2), so we can confirm the smoking status and gender in only 9 controls.
Characteristics of Lung cancers arising in T790M germline carriers
As demonstrated in Table 1, there are 19 cancer cases known to have arisen in germline carriers including our pedigree. All but one of the probands had a family history of lung cancer, and the germline mutation status of 11 was confirmed by sequencing, one was an obligate carrier, and seven family members are assumed to be carriers. In one case (case 8) both parents had lung cancer, and the parental inheritance could not be determined. Twelve of the 14 patients with known ethnicity were Caucasian, and two were East Indian. Their ages ranged from 29-81, and there were 13 females, five males, and one of unknown gender. The median age of the cancer cases was about 63 years, with our proband at 29 years being the youngest case identified. Tumor histology was available for 14 patients, all of who had NSCLC, and all of who had one or more adenocarcinomas (case 9 also had a large cell neuroendocrine carcinoma). Three patients had multiple pathology documented lung cancers, and three had documented invasive cancers as well as multiple bilateral nodules (biopsy proven to be microinvasive cancers in one of the cases).21
Of the 22 apparently individual tumors arising in 11 patients that were tested for EGFR gene mutations and 16 (73%) had a second activating mutation in addition to the T790M germline mutation.
A logistic regression model was used to test the association between smoking status and lung cancer among the germline T790M carriers adjusting for sex, as there are more female cases whereas more males are represented in the mutation carriers that have not developed lung cancer (controls) (Table 2). Without adjusting for sex, the odds ratio for a 2-by-2 table can be calculated as ad/bc, which is 2*4 / (5*11) = 0.15. After taking gender into consideration, it became 0.31 (95% confidence interval (CI):((0.04, 2.25)). Although this OR itself was not significant (P=0.34) given the small sample size, it is significantly smaller (P=6.0E-05 by a two-sample mean test) than the OR of 40.4 (95% CI: (21.8,79.6)) estimated from the general US population.22 There was an excess of never smokers in lung cancer cases arising in germline T790M carriers. The proportions of smokers in all lung cancer cases were estimated to be 0.91 in males and 0.81 in females.23 In contrast, only 2 out of 13 germline T790M derived cases (with both sex and smoking status known) were ever smokers, which was significantly smaller (P=6.2E-07) than the expectation - a conservative estimate 0.81-in the general lung cancer cases. The p-value remained significant (P=7.4E-06) even if the two cases of smokers with uncertain gender were taken into account, i.e., 4 out of 15 cases were ever smokers.
Prevalence of T790M germline mutations in lung cancer patients and the general population
We estimated the overall penetrance of the germline mutation and its penetrance in both ever smokers (ES) and never smokers (NS) without regard to age by modeling the conditional probability of family phenotypes and genotypes given the proband's phenotype. The overall penetrance was 0.23, and 0.15 in ESs and 0.31 in NSs.
We examined 627 resected Japanese NSCLC lung cancers for the presence of EGFR mutations prior to the onset of TKI or other therapies. Of these cases, 553 were from frozen tissues (552 tumors, one malignant pleural effusion), and 74 from formalin fixed, paraffin embedded materials of resected tumors. EGFR mutations were detected in 209/627 samples (33.3%). One patient harbored both L858R and an acquired T790M mutation. We compared the reported prevalence of germline T790M mutations in lung cancer patients in the USA (5/503)13 with the prevalence in our Japanese cases (0/627) (Fisher's exact test p-value = 0.017).
As germline T790M mutations predispose to the development of lung cancer, the prevalence of germline mutations in lung cancer cases may exceed the prevalence in the general population The T790M mutation was absent in the 1000 Genomes Project database22 and in the genomes of 6503 individuals from the NHLBI GO Exome Sequencing Project ( http://evs.gs.washington.edu/EVS/). Thus, without adjusting for ethnicity, we estimate that the prevalence of germline T790M mutations in the general population is less than 1 in 7500 subjects. Only one T790M germline mutation has been identified during non-EGFR gene targeted sequencing - in a patient with an unrelated malignancy.23
Discussion
As the rare germline T790M mutations predispose to lung cancer, they represent a rare but interesting lung cancer familial predisposition gene. Based on the data learned from our family, germline T790M is a major cancer predisposition gene, with an estimated 31% risk for lung cancer in never smoker carriers. We report the investigation of several cases from a single family, with the 29 year old proband inheriting the mutation maternally. As we obtained relevant family history from several family members for five generations, and tested for the germline mutation in members from three generations, our family represents the most extensively studied pedigree reported. We combined the family data with the scant published literature data to summarize our current knowledge of this familial form of lung cancer. Our review of the literature was complicated by double reporting of some cases,13,19 deliberate alteration of some patient data for confidentiality reasons,13 and by incorrect or difficult to interpret data from another report (see Supplemental data).20
A comparison of lung cancers arising in patients with sporadic activating mutations in the EGFR gene versus cancers arising in T790M carriers demonstrates similarities and differences. Germline T790M mutations are rare, and, with the exception of family member testing, are almost always detected during analysis of lung cancers for EGFR mutations. As germline inheritance predisposes to lung cancer, the prevalence in lung cancer cases is likely to be higher than in controls without cancer. Vikis et al sequenced the probands of 237 families with predisposition to lung cancer, but did not identify any germline T790M mutations, confirming that these mutations are very rare, even in families with a genetic predisposition for lung cancer.8 While about 1% of NSCLC cases have heterozygous germline mutations, an analysis of public databases indicated that the prevalence in the general population was probably less than one in 7500. While EGFR mutations are more frequent in lung cancers arising in East Asian ethnicities, our data from 627 Japanese cases indicated that the prevalence of germline mutations in lung cancer cases in Japan was lower than that reported for USA cases. To date, not a single case of germline T790M mutation has been reported in East Asians.
Almost all of the lung cancers having acquired EGFR mutations as well as those arising in T790M carriers have adenocarcinoma histology. While, some sporadic adenocarcinomas of the lung arise as multiple apparently independent tumors and may be associated with multiple preinvasive lesions,21,24-26 these findings are characteristic of many inherited cancer syndromes.27-30 We obtained CT scans of six of the germline mutation carriers, including the proband and five unaffected carriers. The proband had multiple bilateral ground glass and solid nodular lesions, histologically proven to represent the entire spectrum of preneoplastic, preinvasive and microinvasive lesions associated with peripheral adenocarcinomas.21 One or more subcentimeter solid or ground glass nodules of uncertain etiology were identified in all the unaffected carriers (Supplemental Table 2).
Females were over-represented in the germline cases. Of the 22 lung tumors arising in 11 patients whose tumors had EGFR gene sequencing, 16 (73%) had a second activating mutation. Thus, the important gain-of-function properties of these double mutants may explain their frequent presence in lung cancers arising in T790M carriers. The median age of the cancer cases was about 63 years, with our proband at 29 years being the youngest case identified. The median age for the T790M cases is considerably higher than for breast cancer development in BRCA1 carriers (median age 40 years), although the age ranges are similar.31 Female predominance is present in both lung cancers with sporadic EGFR mutations and for germline T790M cases.
Perhaps the most interesting and unexpected finding of our study was the effect of smoking status on the appearance of lung cancer in germline carriers. The distribution between smoking status in the germline carriers did not deviate from the expected in the US population. However, the distribution of smoking status among lung cancer cases indicated a considerable, highly significant over representation of never smokers (p = 3,2E-07), based on the expectation that 86% of lung cancers in the USA arise in ever smokers.32 The calculations are based on our proband being classified as an ever smoker, even though her lifetime tobacco exposure was 0.1 pack years and was highly unlikely to have contributed to the development of her lung cancer. We estimated the overall penetrance to be 0.23, and 0.15 in ever smokers and 0.31 in ever smokers. However, as we were unable to adjust for age and given the small sample size, our estimates must be interpreted with caution.
These findings are puzzling, as smoking is universally accepted as the major risk factor for lung cancer. Our hypothetical explanation is illustrated in Fig. 3. While the T790M mutation is a weak oncogene by itself, when combined with a common activating EGFR mutation such as L858R, the oncogenic potential of both mutations is greatly enhanced, and 73% of lung cancers arising in germline carriers contained a second activating mutation. Multiple studies have demonstrated that sporadic activating EGFR mutations favor never smoking status and female gender.3,33 KRAS mutations, a major driver mutation in adenocarcinomas arising in ever smokers, are mutually exclusive with EGFR mutations.3 Because somatic mutations of EGFR and KRAS are mutually exclusive, germline T790M mutations and KRAS mutations in ever smoker carriers may also be mutually exclusive. Thus second activating EGFR mutations in germline carriers may arise more frequently in never smokers and women and predispose to cancer development. Of interest, both lung cancers with sporadic EGFR mutations and the germline T790M cases favor female gender, adenocarcinoma histology and never smoking status. However one notable difference is the absence of reported T790M germline mutations in those of East Asian ethnicity.
Figure 3.
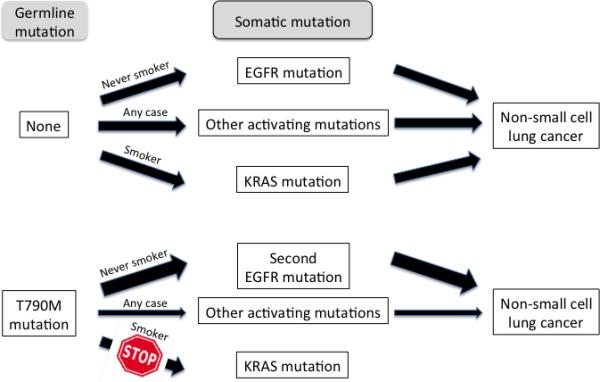
Hypothetical mutation driven pathways to NSCLC development in patients with and without germline T790M mutations. In subjects without the germline mutation, the major pathways are via EGFR mutations (in never smokers, NS) or via KRAS mutations (in ever smokers, ES), although other driver mutations may occur in either group. Subjects with germline T790M mutations, a weak driver mutation, usually require one or more other driver mutations for NSCLC development. In NS the second mutation is usually another EGFR mutation although other pathways may occasionally be activated. In ES, the presence of the germline EGFR gene mutation as well as the smoking status decrease or preclude the possibility of developing a KRAS mutation, although alternative driver mutations may occasionally occur.
The management and prevention of lung cancers in germline carriers presents unusual and controversial options. The presence of the T790M mutation (with or without being accompanied by a more typical activating mutation at presentation predicts for resistance to the standard TKIs. The only germline cases reported to have received TKI therapy were the two sisters reported by Tibaldi et al,20 who had partial responses. Thus in the absence of another known or suspected molecular target, conventional chemotherapy appears to be the preferred first line therapy option. Several newer third and even fourth generation TKIs have entered clinical trial, and some were designed to overcome T790M mediated resistance.34,35 and offer the possibility of effective targeted therapy in the future. Regular CT scans are one method for the early detection of lung cancers developing in carriers, and the carriers in the family of our proband are being offered this option. Clearly, optimal management and prevention strategies will require more information obtained from observation, prevention and therapy of many more carriers and cases. For these reasons The Dana-Farber Cancer Institute has initiated the “INHERIT EGFR study: Investigating the hereditary risk from T790M” (http://clinicaltrials.gov/ct2/show/NCT01754025).
Conclusions
Germline T790M mutations result in a very rare and unique lung cancer hereditary syndrome that targets female never smokers. The risk of lung cancer development in never smoking carriers is greater than the risk of heavy smokers with or without the mutation. Unaffected carriers with this mutation are at increased risk for developing lung cancer irrespective of their smoking status, and should be followed by increased surveillance including low dose CT scans. The resultant cancers share several features with lung cancers containing sporadic EGFR mutations, as well as known or suspected differences.
Supplementary Material
Table 3.
Comparison of lung cancers associated with sporadic and germline mutations of EGFR gene.
| Feature | Sporadic | Germline |
|---|---|---|
| Frequency | Relatively common | Exceedingly rare |
| Lung cancer type | Mostly adenocarcinoma | Mostly adenocarcinoma |
| Ethnic preference | East Asian | *White |
| Gender preference | Female | Female |
| Smoking status | More frequent in never smokers | More frequent in never smokers |
| Field cancerization effects | Occasional | *Occasional |
| Presence of T790M | Rare at initial diagnosis | Present at initial diagnosis (by definition) |
| Presence of second activating EGFR mutation | Rare at initial diagnosis | Frequent at initial diagnosis. |
| Inheritance | Not applicable | Dominant |
| Penetrance | Not applicable | *May vary with smoke exposure |
| Response to TKIs | Frequent initial response | *Predicted to be resistant |
Given the exceedingly rare prevalence of germline T790M mutations, several of the observations (indicated by astericks) are tentative and need confirmation by study of further cases.
Acknowledgments
Funding was provided by the Texas Specialized Program of Research Excellence in Lung Cancer (P50CA70907), with additional support from the Harold C. Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas, TX
Role of the funding source: The sponsors of the study had no role in study design, data collection, analysis or interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Authors’ disclosures of potential conflicts of interest: The authors have no relevant disclosures to report
Authors contributions:
All authors contributed to writing of the manuscript and to collection and reporting of clinical findings (JHS, LR, KK, ST, JS, DO, LW, WT, AFG), the family pedigree (LR, DO) or data analysis (CX, YX, LR, AFG).
Final approval of manuscript: All authors
Contributors: All authors contributed to writing of the manuscript and to collection and reporting of clinical findings (JHS, LR, KK, ST, JS, DO, LW, WT, AFG), the family pedigree (LR, DO) or data analysis (CX, YX, LR, AFG).
Contributor Information
Adi Gazdar, Hamon Center for Therapeutic Oncology Research and Department of Pathology, UT Southwestern Medical Center, Dallas, TX.
Linda Robinson, Cancer Genetics, Simmons Cancer Center, UT Southwestern Medical Center, Dallas, TX.
Dwight Oliver, Department of Pathology, UT Southwestern Medical Center, Dallas, TX.
Chao Xing, Department of Clinical Science and McDermott Center for Human Growth and Development, UT Southwestern Medical Center, Dallas, TX
William D Travis, Department of Pathology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Junichi Soh, Department of Thoracic Surgery, Graduate School of Medicin Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan.
Shinichi Toyooka, Department of Thoracic Surgery, Graduate School of Medicin Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan.
Lori Watumull, Department of Radiology, UT Southwestern Medical Center, Dallas, TX.
Yang Xie, Department of Clinical Science and Simmons Cancer Center, UT Southwestern Medical Center, Dallas, TX
Kemp Kernstine, Department of Cardiovascular and Thoracic Surgery, UT Southwestern Medical Center, Dallas, TX.
Joan H Schiller, Department of Medicine and Simmons Cancer Center, UT Southwestern Medical Center, Dallas, TX.
References
- 1.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 2.Linardou H, Dahabreh IJ, Bafaloukos D, et al. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009;6:352–66. doi: 10.1038/nrclinonc.2009.62. [DOI] [PubMed] [Google Scholar]
- 3.Shigematsu S, Lin L, Takahashi T, et al. Clinical and biological features associated with Epidermal Growth Factor Receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 4.Yu H, Arcila ME, Rekhtman N, et al. Analysis of Mechanisms of Acquired Resistance to EGFR TKI therapy in 155 patients with EGFR-mutant Lung Cancers. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godin-Heymann N, Bryant I, Rivera MN, et al. Oncogenic activity of epidermal growth factor receptor kinase mutant alleles is enhanced by the T790M drug resistance mutation. Cancer Res. 2007;67:7319–26. doi: 10.1158/0008-5472.CAN-06-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suda K, Onozato R, Yatabe Y, et al. EGFR T790M mutation: a double role in lung cancer cell survival? J Thorac Oncol. 2009;4:1–4. doi: 10.1097/JTO.0b013e3181913c9f. [DOI] [PubMed] [Google Scholar]
- 8.Vikis H, Sato M, James M, et al. EGFR-T790M is a rare lung cancer susceptibility allele with enhanced kinase activity. Cancer Res. 2007;67:4665–70. doi: 10.1158/0008-5472.CAN-07-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regales L, Balak MN, Gong Y, et al. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS One. 2007;2:e810. doi: 10.1371/journal.pone.0000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi J, Zhang J, Xie Y, et al. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS One. 2009;4:e4576. doi: 10.1371/journal.pone.0004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosell R, Moran T, Carcereny E, et al. Non-small-cell lung cancer harbouring mutations in the EGFR kinase domain. Clin Transl Oncol. 2010;12:75–80. doi: 10.1007/S12094-010-0473-0. [DOI] [PubMed] [Google Scholar]
- 12.Soh J, Okumura N, Lockwood WW, et al. Oncogene mutations, copy number gains and mutant allele specific imbalance (MASI) frequently occur together in tumor cells. PLoS One. 2009;4:e7464. doi: 10.1371/journal.pone.0007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxnard GR, Miller VA, Robson ME, et al. Screening for Germline EGFR T790M Mutations Through Lung Cancer Genotyping. J Thorac Oncol. 2012;7:1049–52. doi: 10.1097/JTO.0b013e318250ed9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asano H, Toyooka S, Tokumo M, et al. Detection of EGFR gene mutation in lung cancer by mutant-enriched polymerase chain reaction assay. Clin Cancer Res. 2006;12:43–8. doi: 10.1158/1078-0432.CCR-05-0934. [DOI] [PubMed] [Google Scholar]
- 15.Borras E, Jurado I, Hernan I, et al. Clinical pharmacogenomic testing of KRAS, BRAF and EGFR mutations by high resolution melting analysis and ultra-deep pyrosequencing. BMC Cancer. 2011;11:406. doi: 10.1186/1471-2407-11-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagai Y, Miyazawa H, Huqun, et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005;65:7276–82. doi: 10.1158/0008-5472.CAN-05-0331. [DOI] [PubMed] [Google Scholar]
- 17.Girard N, Lou E, Azzoli CG, et al. Analysis of genetic variants in never-smokers with lung cancer facilitated by an Internet-based blood collection protocol: a preliminary report. Clin Cancer Res. 2010;16:755–63. doi: 10.1158/1078-0432.CCR-09-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prudkin L, Tang X. Wistuba, II: Germ-line and somatic presentations of the EGFR T790M mutation in lung cancer. J Thorac Oncol. 2009;4:139–41. doi: 10.1097/JTO.0b013e3181915f92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nature genetics. 2005;37:1315–6. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 20.Tibaldi C, Giovannetti E, Vasile E, et al. Inherited germline T790M mutation and somatic epidermal growth factor receptor mutations in non-small cell lung cancer patients. J Thorac Oncol. 2011;6:395–6. doi: 10.1097/JTO.0b013e3182059a6f. [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genomes Project C. Abecasis GR, Auton A, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi JH, Huh J, Kim HJ, et al. Genome-wide single-nucleotide polymorphism array-based karyotyping in myelodysplastic syndrome and chronic myelomonocytic leukemia and its impact on treatment outcomes following decitabine treatment. Ann Hematol. 2013;92:459–69. doi: 10.1007/s00277-012-1635-7. [DOI] [PubMed] [Google Scholar]
- 24.Gazdar AF, Minna JD. Multifocal lung cancers--clonality vs field cancerization and does it matter? J Natl Cancer Inst. 2009;101:541–3. doi: 10.1093/jnci/djp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung JH, Choe G, Jheon S, et al. Epidermal growth factor receptor mutation and pathologicradiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol. 2009;4:1490–5. doi: 10.1097/JTO.0b013e3181bc9731. [DOI] [PubMed] [Google Scholar]
- 26.Flieder DB, Vazquez M, Carter D, et al. Pathologic findings of lung tumors diagnosed on baseline CT screening. Am J Surg Pathol. 2006;30:606–13. doi: 10.1097/01.pas.0000202040.51967.d0. [DOI] [PubMed] [Google Scholar]
- 27.Ashworth M. The pathology of preclinical medullary thyroid carcinoma. Endocr Pathol. 2004;15:227–31. doi: 10.1385/ep:15:3:227. [DOI] [PubMed] [Google Scholar]
- 28.Marsh DJ, Gimm O. Multiple endocrine neoplasia: types 1 and 2. Adv Otorhinolaryngol. 2011;70:84–90. doi: 10.1159/000322479. [DOI] [PubMed] [Google Scholar]
- 29.Lohmann D. Retinoblastoma. Adv Exp Med Biol. 2010;685:220–7. doi: 10.1007/978-1-4419-6448-9_21. [DOI] [PubMed] [Google Scholar]
- 30.Gatalica Z, Torlakovic E. Pathology of the hereditary colorectal carcinoma. Fam Cancer. 2008;7:15–26. doi: 10.1007/s10689-007-9146-8. [DOI] [PubMed] [Google Scholar]
- 31.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–90. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 32.Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25:472–8. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsao A, Tang X, Sabloff B, et al. Clinical-pathological characteristics of the EGFR gene mutation in non-small cell lung cancer. J Thorac Oncol. 2006;1:231–239. doi: 10.1016/s1556-0864(15)31573-2. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi K, Maruvka YE, Michor F, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013;31:1070–80. doi: 10.1200/JCO.2012.43.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch FR, Janne PA, Eberhardt WE, et al. Epidermal Growth Factor Receptor Inhibition in Lung Cancer: Status 2012. J Thorac Oncol. 2013;8:373–384. doi: 10.1097/JTO.0b013e31827ed0ff. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


