Abstract
Objective
Carotid intima–media thickness (cIMT) and carotid plaque (CP) are proposed biomarkers of subclinical atherosclerosis associated with stroke risk. Whether cIMT and CP are distinct phenotypes or single traits at different stages of atherosclerotic development is unclear. We explored the relationship between these markers in the population-based Northern Manhattan Study.
Methods
We used high-resolution ultrasound and validated imaging protocols to study the cross-sectional (N=1,788 stroke-free participants) and prospective relationship (N=768 with follow-up scan; mean years between examinations=3.5) between CP and cIMT measured in plaque-free areas.
Results
The mean age was 66±9 (40% male, 19% black, 17% white, 61% Hispanic). The mean baseline cIMT was 0.92±0.09mm, 0.94±0.09mm among the 58% with prevalent plaque, 0.90±0.08mm among the 42% without prevalent plaque (p<0.0001). Each 0.1mm increase in baseline cIMT was associated with a 1.72-fold increased odds of plaque presence (95%CI=1.50-1.97), increased plaque thickness (effect on the median=0.46mm, p<0.0001), and increased plaque area (effect on the median=3.45mm2, p<0.0001), adjusting for demographics and vascular risk factors. Elevated baseline cIMT was associated with an increased risk of new plaque in any location at follow-up, but after adjusting for demographics and vascular risk factors this association was no longer present. No association was observed in carotid segment-specific analyses.
Conclusion
Increased cIMT was associated with baseline prevalent plaque but did not predict incident plaque independent of other vascular risk factors. This finding suggests that increased cIMT is not an independent predictor of plaque development although these atherosclerotic phenotypes often coexist and share some common vascular determinants.
Keywords: carotid artery, carotid intima media thickness, carotid plaque, atherosclerosis, carotid ultrasound
Introduction
Carotid atherosclerosis plays a large role in the etiology of stroke and cardiovascular disease (CVD). B-mode carotid ultrasound has been widely used to detect subclinical carotid atherosclerosis by quantifying carotid intima–media thickness (cIMT) and carotid plaque (CP). Both cIMT and CP have been proposed surrogate imaging biomarkers of subclinical atherosclerosis [1,2] until recently, when it became increasingly clear that cIMT and CP may be genetically and biologically distinct atherosclerotic phenotypes with evidence of heterogeneous etiology [3,4]. In addition, carotid atherosclerotic plaque burden, defined as the two-dimensional total plaque area (TPA) or three-dimensional total plaque volume, may be a powerful non-invasive imaging tool for vascular risk estimation, and stronger predictor for future ischemic stroke (IS) than cIMT [5-8].
cIMT and CP have been associated with prevalent and incident atherosclerotic disease with variable effects [9-11]. Whether cIMT and CP are distinct phenotypes or represent a single trait at a different stage of atherosclerotic development is unclear. Recent studies have suggested that increased cIMT more likely represents adaptive changes to increased shear stress with aging and less likely atherosclerotic changes [12]. The biological mechanism by which increased arterial wall thickening initiates focal plaque formation is poorly understood. Therefore, a greater understanding of adaptive changes in the arterial wall with aging and of how these changes relate to the development of atherosclerosis in various populations is needed.
In the current study, we sought to examine the cross-sectional and prospective relationships between cIMT and carotid plaque phenotypes in a multi-ethnic population of northern Manhattan. We hypothesized that increased cIMT was not related to presence of carotid plaque and to development of new carotid plaque over time.
Material and Methods
Study Participants
Subjects were participants in the Northern Manhattan Study (NOMAS), an ongoing, prospective, population-based study of stroke incidence and vascular risk factors, and were concurrently enrolled in the Oral Infections and Vascular Disease Epidemiology Study (INVEST). The details of the NOMAS and INVEST designs, methods and populations have been described previously [13,14].
Eligible subjects were those who a) had never been diagnosed with ischemic stroke; b) were >40 years old; and c) resided in Northern Manhattan for ≥3 months, in a household with a telephone. Subjects were identified by random-digit dialing and interviews were conducted by trained bilingual research assistants. Subjects were recruited from the telephone sample (telephone response rate was 91%) to have an in-person baseline interview and assessment. The enrollment response rate was 75%, the overall participation rate was 69%, and a total of 3,298 subjects were enrolled with an average annual contact rate of 95%. Of the 3,298 subjects, ultrasound measurements of cIMT and CP were performed for 1,788, and of those, 768 had multiple ultrasound measurements over time as a part of INVEST [14]. NOMAS and INVEST are approved by the Institutional Review Boards of the Columbia University Medical Center and the University of Miami. All subjects signed written consent for participation.
Baseline Evaluation
Data were collected through interviews with trained bilingual research assistants in English or Spanish. Physical and neurological examinations were conducted by study neurologists. Race-ethnicity was based upon self-identification through a series of questions modeled after the US census and conforming to standard definitions outlined by Directive 15 [15]. Standardized questions were adapted from the Behavioral Risk Factor Surveillance System by the Centers for Disease Control regarding hypertension, diabetes, smoking, and cardiac conditions [16]. Blood pressure (BP) was measured with mercury sphygmomanometers and appropriately-sized cuffs. Hypertension was defined as a BP ≥140/90 mmHg (based on the average of two measurements during one sitting), the patient's self-reported hypertension, or use of anti-hypertensive medications. Diabetes mellitus was defined by the patient's self-reported diabetes, use of insulin or oral anti-diabetic medications, or fasting glucose ≥126 mg/dl. The fasting lipid profile was measured at enrollment. Body mass index (BMI) was calculated in kg/m2.
Carotid Ultrasound
High-resolution B-mode ultrasound imaging (GE LogIQ 700, 9- to 13-MHz linear-array transducer) was performed by trained and certified sonographers as previously described [17-19]. Presence of plaque was defined as a focal wall thickening or protrusion in the lumen more than 50% greater than the surrounding thickness. Carotid plaque area (mm2) and maximum thickness (mm) were measured with the automated computerized edge tracking software program M'Ath (M'Ath Inc, Paris, France) [20]. TPA was defined as the sum of all plaque areas measured in any of the carotid artery segments within an individual. cIMT was measured in areas without plaque. cIMT was calculated as a composite measure of the near and the far walls of the common carotid artery (CCA) IMT, bifurcation (bif) IMT, and internal carotid artery (ICA) IMT of both sides of the neck, and examined continuously as a mean of the maximum measurements of the 12 carotid sites. We also examined cIMT in the Bifurcation and ICA exclusively and cIMT in the CCA exclusively. Likewise, we examined plaque phenotypes in the Bifurcation and ICA exclusively. Figure 1 is a representation of cIMT and carotid plaque using high-resolution B-mode ultrasound.
Figure 1.
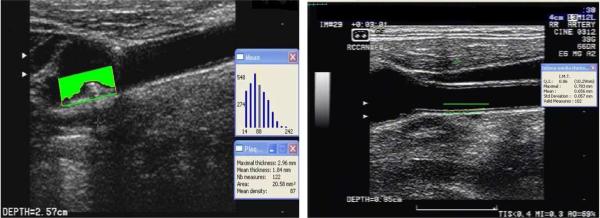
The left side is a representation of a left bifurcation carotid artery plaque measurement, and the right side is a representation of right common carotid artery cIMT measurement using high-resolution B-model ultrasound.
Statistical Analysis
The cross-sectional association between cIMT and plaque presence was examined with logistic regression models, where cIMT was the independent variable and plaque presence was the dependent variable. Due to the non-normal distribution of plaque thickness and area with a large percentage of the study population having no plaque, we used quantile regression to examine plaque thickness and area as continuous outcomes. For individuals without plaque, a value of 0 was assigned for plaque thickness and area. We chose the median (50th percentile) and 75th percentile as our outcome variables of interest. A three model sequence was constructed: univariate (model 1); adjusted for demographics (age, sex, race-ethnicity; model 2); and adjusted for demographics and systolic blood pressure, diastolic blood pressure, antihypertensive medication use, diabetes, low-density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, statin use, and BMI (model 3). A subset of the study population had data on left ventricular mass measured using transthoracic echocardiography, and a subset had data on diastolic intraluminal CCA diameter measured using M mode ultrasound with M'Ath software. Sensitivity analyses were conducted within these subsamples adding these two variables separately to model 3.
Next, we performed segment specific analyses as secondary exploratory analyses. We examined cIMT in the Bifurcation and ICA only in relation to plaque presence, thickness, and area in these segments only. For the latter analysis the left and right sides were examined separately. We examined cIMT in the CCA only in relation to plaque presence in any location.
Next, we used logistic regression to conduct a prospective analysis of baseline cIMT as a predictor of incident plaque from baseline to follow-up carotid ultrasound. Incident plaque was new plaque found at follow up in any of the carotid segments, which was not present at baseline at the same location. We constructed the same sequence of multivariable-adjusted models, and also controlled for the time span between the baseline and follow-up ultrasound imaging in all three models. In addition, these prospective analyses were repeated in a subpopulation restricted only to individuals who did not have carotid plaque at baseline. For each analysis, we ran exploratory models to examine any potential effects of race-ethnicity by including interaction terms between baseline cIMT and race-ethnicity while controlling for all covariates included in model 3. We used logistic regression to conduct an analysis of the change in IMT from baseline to follow up as a predictor of incident plaque in the full prospective sample as well as in those without plaque at baseline, using the same series of models described above and adjusted for the time spam between the baseline and follow up measurements.
Lastly, we ran secondary models to examine the segment-specific association between IMT and incident plaque during follow up. We examined CCA IMT at baseline as well as change in CCA IMT from baseline to follow up in relation to incident plaque in any segment. In addition, we examined ICA and bifurcation IMT at baseline as well as change in ICA and bifurcation IMT in relation to incident plaque in the ICA and bifurcation only. For the latter analysis the left and right sides were examined separately. Each of these analyses were repeated in the subsample restricted to those without plaque at baseline, and the same sequence of three models was run as described above.
Results
The characteristics of the study population broken down by plaque presence are reported in Table 1. Among the 1,787 participants (mean age of 66±9 years, 40% male, 61% Hispanics, 19% non-Hispanic black, and 17% non-Hispanic white), 57% had plaque (N=1026), and 37% had thick plaque >1.9mm (N=657). The mean of the maximum cIMT at baseline was 0.92±0.09 mm (range=0.62-1.41 mm). The mean cIMT among those with plaque was 0.94±0.09 mm (0.92±0.10 mm for left ICA and bif, 0.93±0.10 mm for right ICA and bif, 0.96±0.10 mm for the CCA), and among those without plaque was 0.90±0.08 mm (0.89±0.09 mm for left ICA and bif, 0.89±0.09 mm for right ICA and bif, 0.91±0.11 mm for the CCA), p<0.0001. Among those with plaque, the median plaque thickness was 1.57 mm (2.00 mm for left ICA and bif, 1.91 mm for right ICA and bif), and the plaque thickness at the 75th percentile was 2.17 mm (2.42 mm for left ICA and bif, 2.35 mm for right ICA and bif). The median total plaque area (TPA) was 4.69 mm2 (8.80 mm2 for left ICA and bif, 8.99 mm2 for right ICA and bif), and TPA at the 75th percentile was 16.21 mm2 (15.13 mm2 for left ICA and bif, 14.70 mm2 for right ICA and bif). Significant independent predictors of plaque presence in this population at baseline included older age, male sex, white race, hypertension, diabetes, and elevated LDL cholesterol, while significant independent predictors of cIMT were older age, male sex, diabetes, elevated LDL, and elevated BMI (data not shown).
Table 1.
Characteristics of the study population
| Overall (N=1787) | Plaque (N=1026) | No plaque (N=761) | |
|---|---|---|---|
| Age (years), mean ± SD | 66±9 | 68±9 | 63±8 |
| Male sex, % | 40 | 42 | 37 |
| White, % | 17 | 22 | 12 |
| Black, % | 19 | 20 | 17 |
| Hispanic, % | 61 | 56 | 69 |
| Hypertension, % | 70 | 74 | 65 |
| Diabetes, % | 19 | 23 | 15 |
| Statin use, % | 11 | 12 | 10 |
| LDL, mean±SD | 128.17±35.05 | 129.81±36.27 | 125.99±33.26 |
| HDL, mean±SD | 46.65±14.43 | 46.43±14.42 | 46.94±14.46 |
| Triglycerides, mean±SD | 134.79±79.20 | 135.90±75.80 | 133.31±83.54 |
| BMI, mean±SD | 28.2±5.0 | 28.0±4.9 | 28.4±5.2 |
| Baseline cIMT (mm), mean ± SD | 0.92±0.09 | 0.94±0.09 | 0.90±0.08 |
Abbreviations:
BMI (body mass index), cIMT (carotid intima-media thickness), LDL (Low-density lipoproteins), HDL (High-density lipoproteins)
Table 2 shows the cross-sectional association between baseline measurements of cIMT and plaque phenotypes. In all three models, cIMT was positively associated with plaque presence, plaque thickness, and TPA. Adjustment for demographics and vascular risk factors only attenuated the associations slightly. The associations remained consistent in sensitivity analyses adding left ventricular mass and diastolic CCA diameter to model 3 (data not shown). In model 3, the prevalence of plaque was increased by 72% for each 0.1mm increase in cIMT. In addition, cIMT in the Bifurcation and ICA was also positively associated with plaque presence, thickness, and TPA in the Bifurcation and ICA, and cIMT in the CCA was positively associated with plaque presence, thickness, and TPA in all segments.
Table 2.
Cross-sectional association between carotid IMT, and carotid plaque prevalence, thickness and area at baseline
| Plaque presence (N=1026) OR (95% CI) | Plaque thickness (mm) 50% effect, p-value | Plaque thickness (mm) 75% effect, p-value | Plaque area (mm2) 50% effect, p-value | Plaque area (mm2) 75% effect, p-value | |
|---|---|---|---|---|---|
| cIMT per 0.1mm | |||||
| Model 1 | 1.87 (1.65-2.12) | 0.56, <0.0001 | 0.32, <0.0001 | 4.48, <0.0001 | 9.11, <0.0001 |
| Model 2 | 1.68 (1.47-1.91) | 0.46, <0.0001 | 0.26, <0.0001 | 3.31, <0.0001 | 7.21, <0.0001 |
| Model 3 | 1.72 (1.50-1.97) | 0.46, <0.0001 | 0.26, <0.0001 | 3.45, <0.0001 | 7.08, <0.0001 |
| cIMT in left Bif and ICA per 0.1mm in relation to plaque in the left Bif and ICA (N=776 with plaque) | |||||
| Model 1 | 1.48 (1.34-1.64) | 0.25, <0.0001 | 0.27, <0.0001 | 0.85, <0.0001 | 2.85, <0.0001 |
| Model 2 | 1.41 (1.27-1.57) | 0.25, <0.0001 | 0.20, <0.0001 | 0.85, <0.0001 | 2.28, <0.0001 |
| Model 3 | 1.44 (1.29-1.60) | 0.28, <0.0001 | 0.23, <0.0001 | 0.96, <0.0001 | 2.59, <0.0001 |
| cIMT in right Bif and ICA per 0.1mm in relation to plaque in the right Bif and ICA (N=777 with plaque) | |||||
| Model 1 | 1.48 (1.34-1.63) | 0.26, <0.0001 | 0.23, <0.0001 | 0.85, <0.0001 | 2.67, <0.0001 |
| Model 2 | 1.46 (1.31-1.62) | 0.26, <0.0001 | 0.22, <0.0001 | 0.85, <0.0001 | 2.45, <0.0001 |
| Model 3 | 1.46 (1.31-1.63) | 0.24, <0.0001 | 0.24, <0.0001 | 0.93, <0.0001 | 2.47, <0.0001 |
| cIMT in CCA per 0.1mm in relation to any plaque | |||||
| Model 1 | 1.68 (1.52-1.86) | 0.52, <0.0001 | 0.28, <0.0001 | 3.52, <0.0001 | 7.07, <0.0001 |
| Model 2 | 1.46 (1.31-1.62) | 0.36, <0.0001 | 0.20, <0.0001 | 2.35, <0.0001 | 5.25, <0.0001 |
| Model 3 | 1.51 (1.35-1.69) | 0.36, <0.0001 | 0.18, <0.0001 | 2.54, <0.0001 | 4.67, <0.0001 |
Model 1: univariate
Model 2: controlling for age, sex, race/ethnicity
Model 3: controlling for age, sex, race/ethnicity, systolic blood pressure, diastolic blood pressure, anti-hypertensive medication use, diabetes, LDL, HDL, triglycerides, BMI, statin use
The mean time-span between the two carotid measurements among those who had multiple scans was 3.5 years (range: 1.9-7.3; N=768), and 43% of participants developed a new plaque during follow-up (N=334, N=161 new plaque in left bifurcation and ICA, N=160 in the right bifurcation and ICA). Of the 324 participants without plaque at baseline, 40% developed a new plaque during follow-up (N=130, N=100 new plaque in left bifurcation and ICA, N=100 in the right bifurcation and ICA).
Table 3 shows the association between cIMT at baseline and incident plaque during follow-up. In the univariate model 1, elevated cIMT at baseline was associated with an increased risk of incident plaque during follow-up in the full subpopulation as well as in the group without plaque at baseline. However, after adjustment for demographics and vascular risk factors the association was no longer significant. Findings remained consistent in sensitivity analyses including left ventricular mass and diastolic CCA diameter in the fully adjusted model, conducted in subsamples with available data for these variables (data not shown). This lack of the relationship between cIMT and incident plaque was observed across all race-ethnic groups (no significant interactions were found). The mean longitudinal change in cIMT was 0.11±0.16 mm among all participants, and 0.09±0.15 and 0.12±0.15 mm among those with and without plaque, respectively (p=0.01). There was a marginally statistically significant trend towards a positive association between increasing IMT from baseline to follow up and incident plaque among the full prospective study sample (p=0.06) while there was no apparent relationship among those without plaque at baseline.
Table 3.
Prospective association between IMT at baseline and plaque presence at follow up
| Incident plaque, OR (95% CI) | |
|---|---|
| cIMT per 0.1mm (N=768) | N=334 new plaque |
| Model 1 | 1.13 (1.02-1.27) |
| Model 2 | 1.06 (0.95-1.19) |
| Model 3 | 1.06 (0.94-1.19) |
| cIMT per 0.1mm, among those without plaque at baseline (N=324) | N=130 with incident plaque |
| Model 1 | 1.24 (1.03-1.50) |
| Model 2 | 1.19 (0.97-1.46) |
| Model 3 | 1.19 (0.96-1.48) |
| cIMT change from baseline to follow up per 0.1mm (N=768) | |
| Model 1 | 1.09 (0.99-1.20) |
| Model 2 | 1.10 (0.99-1.21) |
| Model 3 | 1.10 (1.00-1.22) |
| cIMT change from baseline to follow up per 0.1mm, among those without plaque at baseline (N=324) | |
| Model 1 | 1.08 (0.93-1.25) |
| Model 2 | 1.08 (0.93-1.26) |
| Model 3 | 1.07 (0.91-1.26) |
Model 1: controlling for time between measurements
Model 2: controlling for time between measurements, age at baseline, sex, race/ethnicity
Model 3: controlling for time between measurements, age at baseline, sex, race/ethnicity, systolic blood pressure, diastolic blood pressure, anti-hypertensive medication use, diabetes, LDL, HDL, triglycerides, statin use, BMI
Table 4 shows the segment-specific relationship between baseline IMT and incident plaque in the full prospective sample, and among those without plaque at baseline. CCA IMT was positively associated with developing a new plaque in any segment during follow up, but this association was attenuated and no longer significant in multivariable-adjusted models. Among all participants, the mean longitudinal change in bifurcation and ICA cIMT was 0.11±0.18 mm for the left side (0.07±0.20 and 0.11±0.18 mm among those with and without any plaque, respectively, p=0.01) and 0.11±0.18 mm for the right side (0.08±0.21 and 0.12±0.18 mm among those with and without any plaque, respectively, p=0.01). The mean longitudinal change in CCA cIMT was 0.12±0.19 mm among all participants, and 0.12±0.18 and 0.13±0.20 mm among those with and without plaque, respectively (p=0.23). Progression in CCA IMT from baseline to follow up was positively associated with the presence of incident plaque in any location in the full sample, but the association was attenuated and no longer significant in the sample restricted to those without plaque at baseline. Baseline and progression of cIMT in the left and right ICA and bifurcation did not predict incident plaque in the respective segments.
Table 4.
Prospective association between segment-specific IMT at baseline and plaque presence at follow up
| Incident plaque, OR (95% CI) | |
|---|---|
| cIMT in the CCA per 0.1mm (N=768) | N=334 new plaque in any segment |
| Model 1 | 1.10 (1.01-1.20) |
| Model 2 | 1.05 (0.96-1.15) |
| Model 3 | 1.03 (0.94-1.14) |
| cIMT in the CCA per 0.1mm, among those without plaque at baseline (N=324) | N=130 with incident plaque in any segment |
| Model 1 | 1.16 (1.01-1.34) |
| Model 2 | 1.13 (0.97-1.32) |
| Model 3 | 1.14 (0.96-1.35) |
| Left cIMT in the ICA and Bifurcation per 0.1mm (N=768) | N=161 new plaque in the left ICA and bifurcation |
| Model 1 | 0.96 (0.85-1.08) |
| Model 2 | 0.93 (0.83-1.05) |
| Model 3 | 0.94 (0.83-1.06) |
| Left cIMT in the ICA and Bifurcation per 0.1mm, among those without plaque at baseline (N=324) | N=100 with incident plaque in the left ICA and bifurcation |
| Model 1 | 1.11 (0.93-1.32) |
| Model 2 | 1.07 (0.89-1.29) |
| Model 3 | 1.10 (0.90-1.33) |
| Right cIMT in the ICA and Bifurcation per 0.1mm (N=768) | N=160 new plaque in the right ICA and bifurcation |
| Model 1 | 0.96 (0.86-1.07) |
| Model 2 | 0.93 (0.83-1.04) |
| Model 3 | 0.92 (0.81-1.03) |
| Right cIMT in the ICA and Bifurcation per 0.1mm, among those without plaque at baseline (N=324) | N=100 with incident plaque in the right ICA and bifurcation |
| Model 1 | 1.19 (1.00-1.41) |
| Model 2 | 1.15 (0.96-1.38) |
| Model 3 | 1.12 (0.93-1.35) |
| CCA cIMT change from baseline to follow up per 0.1mm (N=768) | |
| Model 1 | 1.09 (1.01-1.18) |
| Model 2 | 1.09 (1.00-1.18) |
| Model 3 | 1.10 (1.01-1.20) |
| CCA cIMT change from baseline to follow up per 0.1mm, among those without plaque at baseline (N=324) | |
| Model 1 | 1.07 (0.95-1.20) |
| Model 2 | 1.06 (0.94-1.19) |
| Model 3 | 1.05 (0.92-1.20) |
| Left ICA and bifurcation cIMT change from baseline to follow up per 0.1mm (N=768) | |
| Model 1 | 1.01 (0.92-1.10) |
| Model 2 | 1.01 (0.91-1.10) |
| Model 3 | 1.01 (0.91-1.11) |
| Left ICA and bifurcation cIMT change from baseline to follow up per 0.1mm, among those without plaque at baseline (N=324) | |
| Model 1 | 1.00 (0.88-1.15) |
| Model 2 | 1.03 (0.90-1.19) |
| Model 3 | 1.01 (0.87-1.18) |
| Right ICA and bifurcation cIMT change from baseline to follow up per 0.1mm (N=768) | |
| Model 1 | 1.05 (0.96-1.15) |
| Model 2 | 1.05 (0.96-1.16) |
| Model 3 | 1.06 (0.97-1.17) |
| Right ICA and bifurcation cIMT change from baseline to follow up per 0.1mm, among those without plaque at baseline (N=324) | |
| Model 1 | 1.00 (0.88-1.15) |
| Model 2 | 0.99 (0.86-1.14) |
| Model 3 | 0.99 (0.86-1.15) |
Model 1: controlling for time between measurements
Model 2: controlling for time between measurements, age at baseline, sex, race/ethnicity
Model 3: controlling for time between measurements, age at baseline, sex, race/ethnicity, systolic blood pressure, diastolic blood pressure, anti-hypertensive medication use, diabetes, LDL, HDL, triglycerides, statin use, BMI
Discussion
Accumulating evidence suggests that cIMT and CP may be distinct phenotypes rather than a manifestation of the same phenotype at different stages or phases in the progression of atherosclerosis [21-23]. Though our study design did not evaluate this hypothesis directly, the results may provide some modest indirect support. We observed a positive association between cIMT and prevalent carotid plaque phenotypes in a cross-sectional analysis, but not between cIMT and incident plaque in a prospective analysis after accounting for vascular risk factors. cIMT and carotid plaque seem to be distinct manifestation of an arterial wall thickening process although they often coexist. Carotid plaque therefore may not be a simple result of progressive intima-media thickening, but rather a “de novo” event.
One particularly novel component to the current study was the examination of segment-specific relationships between cIMT and plaque. CCA cIMT predicted plaque presence overall, consistent with the findings of other studies [7, 8, 11, 24]. ICA and bifurcation cIMT was predictive of plaque in those segments on the same side in cross-sectional analyses, but not in longitudinal analyses, indicating that cIMT may not directly progress to plaque.
Atherosclerotic plaque formation represents a dynamic process involving a complex cascade of inflammatory events [25] while carotid intima-media thickening may be related to adaptive hypertrophy of the media layer and not a true representation of an atherosclerotic lesion [26]. Increased cIMT may be represented by “fatty streaks” composed of foamy macrophages which have a non-raised appearance in the arterial lumen and have been shown to regress rather than to progress to raised lesions even in the presence of risk factors [7]. In contrast, “pathologic intimal thickening” is increased cIMT, which represents the earliest manifestation of progressive atherosclerosis [7] and is rich in proteoglycans and lipids, lacks smooth-muscle cells and collagen, and may rapidly transform into plaque through mechanisms not entirely understood. Ultrasonographic measurement of cIMT however cannot distinguish these two different intima-media thickening processes. The strong relationship between cIMT and plaque in our cross-sectional analysis was most likely a result of “pathological intimal thickening” present among those individuals with atherosclerotic plaque, sharing a common pathological mechanism, which was not completely explained by the presence of traditional vascular risk factors. In the prospective analyses however, this association attenuated after adjustment for vascular risk factors, suggesting a distinct mechanism leading to formation of incident plaque, rather than a continuum of the development of plaque from intima-media thickening. Distinct mechanisms underlying the development of plaque and cIMT has been further suggested by evidence from genetic studies which demonstrated that variants in genes encoding proteins implicated in pathways leading to formation of carotid atherosclerosis (eg oxidative stress, inflammation, and diabetes) were differentially associated with cIMT and carotid plaque [21-23]. This suggests that these two phenotypes of carotid atherosclerosis, even if correlated, could be under different biological and genetic control. Although the two processes—cIMT and plaque formation—may share some common mechanisms, their overlap is partial, and their predictive power of cerebrovascular disease (CVD) risk differs [6, 27-29].
The relationship between cIMT and carotid plaque has been examined previously. The European Vascular Aging (EVA) Study demonstrated a significant association between increased cIMT measured in the CCA and both the presence and severity of atherosclerotic plaque [29]. In a longitudinal EVA study [30], the odds of having CP was 2.7-fold greater in subjects with intermediate baseline cIMT values, and 3. 7-fold greater among those with the highest baseline cIMT values compared to subjects with the lowest baseline cIMT values. Interestingly, adjustments for major vascular risk factors did not modify these values. We did not confirm these observations. Although our study had similar duration of follow up (3.5 years in our study, 4 years in EVA), discrepant results most likely are related to the differences in characteristics of the study populations, definitions of risk factors, and definition of plaque. Several studies conducted in predominantly Caucasian populations reported cross-sectional associations between cIMT and plaque [31-33]. In addition, five longitudinal studies, with time spans from four to twelve years, have shown positive associations between cIMT, particularly in the CCA, with the development of carotid plaque [24, 30, 34-36]. Our findings regarding CCA cIMT in relation to incident plaque are not inconsistent with the latter studies. However, differences in study design, including longer follow up in other studies [34, 35], categorization of cIMT [24, 30, 34] rather than a continuous examination, as well as differences in study sample characteristics and size may explain variability in findings. The other longitudinal studies did not examine lateral-specific ICA and bif cIMT in relation to incident plaque like we did.
In the present study, an association between cIMT and plaque at baseline was present in all race-ethnic groups, but a more robust association was found in Hispanics than in blacks and whites. The Atherosclerosis Risk in Communities (ARIC) Study showed no difference between whites and blacks in site-specific prevalence of carotid plaque, as well as a positive association between plaque and cIMT at baseline in both race-ethnicities [32]. Differences in carotid geometry, vascular risk factor profiles and differential predisposition to vascular remodeling and atherosclerotic development may be among possible factors explaining these race-ethnic variations [27, 37, 38].
Our results do not provide direct evidence to confirm the hypothesis that carotid plaque may be a biologically different atherosclerosis phenotype from cIMT, though some of our findings are consistent with this hypothsis. Although carotid plaque seems to be more strongly influenced by environmental factors [39] than cIMT, we have reported that traditional vascular risk factors explain about 21% of the variance in the total carotid plaque burden [20] and only 11% of the variance in cIMT [40]. This suggests that other unaccounted factors, both environment and genetic, play an important role in the determination of these phenotypes. The Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium showed different genetic loci associated with cIMT and carotid plaque [41]. Similarly, we observe no overlap in genetic variants associated with cIMT and CP in our family study [21, 22, 42-44].
Strengths of the current study include its multi-ethnic population-based design, a systematic collection of vascular risk factors, inclusion of both cross-sectional and prospective analyses, and the use of multiple plaque phenotypes for comparisons. However, our study has several limitations. Our population is an older cohort with high burden of risk factors and great proportion of carotid plaque at baseline. Our conclusions may not be generalizable to younger and healthier populations. Also, the population sample available for our cross-sectional analysis was much larger than that used in our prospective analysis, limiting the power of the longitudinal analysis. Further research with multiple follow-up ultrasound measurements and starting earlier in life is needed to fully elucidate the complex temporal relationship between intima-media thickening and plaque formation, and to determine how these atherosclerotic phenotypes interact to affect stroke risk in various populations.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Institutes of Health/National Institute of Neurological Diseases and Stroke K24 NS062737 (Dr. Rundek); R37 NS 29993 (Drs. Sacco, Elkind, Rundek, Dong, Gardener, and Cabral); R01 NS 065114 (Drs. Rundek, Tiozzo, Della-Morte, Gardener, Dong, Cabral, and Sacco); R01 DE-13094 (Drs. Desvarieux, Demmer, Rundek, Sacco); and a Chair in Chronic Disease, École des Hautes Études en Santé Publique, France (Dr. Desvarieux). The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spence JD, et al. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33(12):2916–22. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 2.Pignoli P, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399–406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 3.Spence JD. Measurement of intima-media thickness vs. carotid plaque: uses in patient care, genetic research and evaluation of new therapies. Int J Stroke. 2006;1(4):216–21. doi: 10.1111/j.1747-4949.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 4.Della-Morte D, et al. Genetics and genomics of ischemic tolerance: focus on cardiac and cerebral ischemic preconditioning. Pharmacogenomics. 2012;13(15):1741–57. doi: 10.2217/pgs.12.157. [DOI] [PubMed] [Google Scholar]
- 5.Gomez CR. Carotid plaque morphology and risk for stroke. Stroke. 1990;21(1):148–51. doi: 10.1161/01.str.21.1.148. [DOI] [PubMed] [Google Scholar]
- 6.Mathiesen EB, et al. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromso Study. Stroke. 2011;42(4):972–8. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 7.Wannarong T, et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke. 2013;44(7):1859–65. doi: 10.1161/STROKEAHA.113.001461. [DOI] [PubMed] [Google Scholar]
- 8.Nanayakkara ND, et al. Nonrigid registration of three-dimensional ultrasound and magnetic resonance images of the carotid arteries. Med Phys. 2009;36(2):373–85. doi: 10.1118/1.3056458. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz MW, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379(9831):2053–62. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rundek T, Salameh MJ. Carotid plaque assessment: a bumpy road to improved risk prediction. J Am Coll Cardiol. 2010;56(13):1069. doi: 10.1016/j.jacc.2010.04.051. author reply 1069-70. [DOI] [PubMed] [Google Scholar]
- 11.Den Ruijter HM, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308(8):796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 12.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30(2):177–81. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32(8):1725–31. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 14.Desvarieux M, et al. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005;111(5):576–82. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallman KK, Hodgdon J. Race and ethnic standards for Federal statistics and administrative reporting. Stat Report. 1977;(77-110):450–4. [PubMed] [Google Scholar]
- 16.Sacco RL, et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281(1):53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Rundek T, et al. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke. 2002;33(5):1420–3. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rundek T, et al. Endothelial dysfunction is associated with carotid plaque: a cross-sectional study from the population based Northern Manhattan Study. BMC Cardiovasc Disord. 2006;6:35. doi: 10.1186/1471-2261-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhakaran S, et al. Presence of calcified carotid plaque predicts vascular events: the Northern Manhattan Study. Atherosclerosis. 2007;195(1):e197–201. doi: 10.1016/j.atherosclerosis.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo F, et al. Traditional cardiovascular risk factors explain the minority of the variability in carotid plaque. Stroke. 2012;43(7):1755–60. doi: 10.1161/STROKEAHA.112.651059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong C, et al. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid plaque. PLoS One. 2011;6(11):e27157. doi: 10.1371/journal.pone.0027157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della-Morte D, et al. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid intima-media thickness. Transl Res. 2012;160(5):389–90. doi: 10.1016/j.trsl.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Shali KZ, et al. Genetic variation in PPARG encoding peroxisome proliferator-activated receptor gamma associated with carotid atherosclerosis. Stroke. 2004;35(9):2036–40. doi: 10.1161/01.STR.0000138784.68159.a5. [DOI] [PubMed] [Google Scholar]
- 24.von Sarnowski B, et al. Common carotid intima-media thickness and framingham risk score predict incident carotid atherosclerotic plaque formation: longitudinal results from the study of health in Pomerania. Stroke. 2010;41(10):2375–7. doi: 10.1161/STROKEAHA.110.593244. [DOI] [PubMed] [Google Scholar]
- 25.Fuster V, et al. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46(6):937–54. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 26.Touboul PJ, et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;18(4):346–9. doi: 10.1159/000081812. [DOI] [PubMed] [Google Scholar]
- 27.Rundek T, et al. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70(14):1200–7. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nambi V, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55(15):1600–7. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonithon-Kopp C, et al. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries. The Vascular Aging (EVA) Study. Arterioscler Thromb Vasc Biol. 1996;16(2):310–6. doi: 10.1161/01.atv.16.2.310. [DOI] [PubMed] [Google Scholar]
- 30.Zureik M, et al. Common carotid intima-media thickness predicts occurrence of carotid atherosclerotic plaques: longitudinal results from the Aging Vascular Study (EVA) study. Arterioscler Thromb Vasc Biol. 2000;20(6):1622–9. doi: 10.1161/01.atv.20.6.1622. [DOI] [PubMed] [Google Scholar]
- 31.Persson J, et al. Ultrasound-determined intima-media thickness and atherosclerosis. Direct and indirect validation. Arterioscler Thromb. 1994;14(2):261–4. doi: 10.1161/01.atv.14.2.261. [DOI] [PubMed] [Google Scholar]
- 32.Li R, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25(12):2377–83. doi: 10.1161/01.str.25.12.2377. [DOI] [PubMed] [Google Scholar]
- 33.Rosfors S, et al. Relationship between intima-media thickness in the common carotid artery and atherosclerosis in the carotid bifurcation. Stroke. 1998;29(7):1378–82. doi: 10.1161/01.str.29.7.1378. [DOI] [PubMed] [Google Scholar]
- 34.Prati P, et al. Determinants of carotid plaque occurrence. A long-term prospective population study: the San Daniele Project. Cerebrovasc Dis. 2006;22(5-6):416–22. doi: 10.1159/000094993. [DOI] [PubMed] [Google Scholar]
- 35.Eigenbrodt ML, et al. B-mode ultrasound common carotid artery intima-media thickness and external diameter: cross-sectional and longitudinal associations with carotid atherosclerosis in a large population sample. Cardiovasc Ultrasound. 2008;6:10. doi: 10.1186/1476-7120-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnsen SH, et al. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke. 2005;36(4):715–9. doi: 10.1161/01.STR.0000158909.07634.83. [DOI] [PubMed] [Google Scholar]
- 37.Markert MS, et al. Ethnic differences in carotid artery diameter and stiffness: the Northern Manhattan Study. Atherosclerosis. 2011;219(2):827–32. doi: 10.1016/j.atherosclerosis.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Wong KS. Racial distribution of intracranial and extracranial atherosclerosis. J Clin Neurosci. 2003;10(1):30–4. doi: 10.1016/s0967-5868(02)00264-3. [DOI] [PubMed] [Google Scholar]
- 39.Spence JD, et al. An approach to ascertain probands with a non-traditional risk factor for carotid atherosclerosis. Atherosclerosis. 1999;144(2):429–34. doi: 10.1016/s0021-9150(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 40.Rundek T, et al. Traditional risk factors are not major contributors to the variance in carotid intima-media thickness. Stroke. 2013;44(8):2101–8. doi: 10.1161/STROKEAHA.111.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bis JC, et al. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat Genet. 2011;43(10):940–7. doi: 10.1038/ng.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacco RL, et al. Heritability and linkage analysis for carotid intima-media thickness: the family study of stroke risk and carotid atherosclerosis. Stroke. 2009;40(7):2307–12. doi: 10.1161/STROKEAHA.109.554121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, et al. Fine mapping study reveals novel candidate genes for carotid intima-media thickness in Dominican Republican families. Circ Cardiovasc Genet. 2012;5(2):234–41. doi: 10.1161/CIRCGENETICS.111.961763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardener H, et al. Carotid plaque and candidate genes related to inflammation and endothelial function in Hispanics from northern Manhattan. Stroke. 2011;42(4):889–96. doi: 10.1161/STROKEAHA.110.591065. [DOI] [PMC free article] [PubMed] [Google Scholar]


