Abstract
Background
Rotator cuff tears are common conditions that often require surgical repair to improve function and relieve pain. Unfortunately, repair failure remains a common problem following rotator cuff repair surgery. Several factors may contribute to repair failure including age, tear size, and time from injury. However, the mechanical mechanisms resulting in repair failure are not well understood making clinical management difficult. Specifically, altered scapular motion (termed scapular dyskinesis) may be one important and modifiable factor contributing to the risk of repair failure. Therefore, the objective of this study was to determine the effect of scapular dyskinesis on supraspinatus tendon healing following repair.
Methods
A rat model of scapular dyskinesis was used. 70 adult male Sprague-Dawley rats (400-450 grams) were randomized into two groups: nerve transection of the accessory and long-thoracic nerves (SD) or sham nerve transection (Sham Control). Following this procedure, all rats underwent unilateral detachment and repair of the supraspinatus tendon. All rats were sacrificed at 2, 4, and 8 weeks after surgery. Shoulder function, passive joint mechanics, and tendon properties (mechanical, histological, organizational, and compositional) were evaluated.
Results
Scapular dyskinesis alters joint function and may lead to compromised supraspinatus tendon properties. Specifically, diminished mechanical properties, altered histology, and decreased tendon organization was observed for some parameters.
Conclusion
This study identifies scapular dyskinesis as one underlying mechanism leading to compromise of supraspinatus healing following repair. Identifying modifiable factors that lead to compromised tendon healing will help improve clinical outcomes following repair.
Level of evidence
Basic Science, in-vivo Animal Study.
Keywords: scapular dyskinesis, tendon, rotator cuff repair, animal model, rat, shoulder
Introduction
Rotator cuff tears are common conditions that often require surgical repair to improve function and relieve pain. Unfortunately, despite successes in relieving pain, the success associated with repair integrity is mixed, with 5-95% of patients having recurrent tears,4, 11, 13, 14, 17, resulting in decreased shoulder strength. Several factors may contribute to repair failure including age, tear size, and time from injury; however, mechanical mechanisms contributing to repair failure are not well-established, making clinical management difficult. Identifying modifiable factors that lead to compromised tendon healing will help improve clinical outcomes following repair.
Tendon healing following repair does not regenerate the normal tendon-bone interface and instead leads to the formation of a fibrovascular scar.12, 25 This scar is mechanically inferior to native tendon and is therefore prone to re-rupture. Several augmentation (conservative and surgical) strategies have been developed in an attempt to improve healing rates and re-establish tendon insertion architecture. Conservative rehabilitation protocols are typically implemented pre-operatively in an attempt to correct deficits and post-operatively in an attempt to improve healing and outcomes.22 Biologics, such as growth factors, cytokines, and stem cells, and tissue grafting approaches have been introduced at the repair site in an attempt to promote healing and strengthen repairs.2, 3, 34, 37, 44
Mechanical loading plays an important role in tendon-to-bone healing following repair.43 However, the optimal loading conditions in the treatment of healing repair tissues are not well-defined. Specifically, previous studies have shown conflicting results, regarding immobilization, passive motion, and increased loading.5, 7, 16, 18, 19, 31 In general, low loading regimens and immobilization seems to promote healing while excessive or abnormal joint loading may have detrimental effects. The goal of pre- and post-operative rehabilitation protocols is to optimize the mechanical loading environment of the tendon and improve healing potential.
At the shoulder, abnormal scapulothoracic joint kinematics (termed scapular dyskinesis) contributes to tendon injury.24, 27 Specifically, the abnormal loading environment in the presence of scapular dyskinesis alters the tendon composition and diminishes its mechanical properties.36 It is likely that scapular dyskinesis is also detrimental to the supraspinatus tendon following surgical repair, compromising healing. Specifically, in the presence of scapular dyskinesis, the mechanical loading environment of the tendon is likely abnormal in both amount (i.e., overload) and type (i.e., compressive, shear) of loading. These mechanical changes may undermine optimal tendon-to-bone healing. In particular, abnormal joint mechanics may be one mechanical mechanism by which failed healing following cuff repair occurs.
Studies in the knee have demonstrated that deficits in strength and function have negative consequences on the long term outcomes following ACL reconstruction.9, 29 However, in the shoulder, the consequences on rotator cuff tendon healing and functional outcomes following rotator cuff repair in the presence of scapular dyskinesis have not been evaluated. Physical therapy and rehabilitation6, 22, 23 aimed to re-educate scapular muscles and correct positional abnormalities is the current treatment for scapular dyskinesis. Successful pre-operative scapular rehabilitation may be necessary to achieve successful outcomes post-operatively.
Therefore, the objective of this study was to determine the effect of scapular dyskinesis on supraspinatus tendon to bone healing following tendon repair utilizing an established animal model.35, 36 We hypothesized that scapular dyskinesis will result in H1) diminished joint function and passive joint mechanics and H2) decreased supraspinatus tendon-to-bone healing following tendon repair due to the compromised mechanical environment present during healing.
Materials and Methods
Study Design
A rat model was used in this Institutional Animal Care and Use Committee approved study. Seventy male Sprague Dawley rats (400-450 g) were randomized into 2 groups: nerve transection to create scapular dyskinesis (SD + Supra Repair group, n=35) or sham nerve transection (Sham + Supra Repair Group, n=35). For the nerve transection surgery, the spinal accessory and long thoracic nerves were visualized and transected as described by Reuther et al.36 For the sham nerve transection surgery, the nerves were visualized but were not transected. Following this procedure, all rats underwent unilateral detachment and repair of the supraspinatus tendon as described by Thomopoulos et al..42 Pre- and post-operative analgesics (buprenorphine, 0.05 mg/kg) were administered up to 2 days following surgery. Animals then returned to unrestricted cage activity and were sacrificed 2, 4, and 8 weeks following surgery and either frozen (for mechanical testing at 4 and 8 weeks, n=10) or fixed in formalin (for histology and immunohistochemistry at each time point, n=5)
Detachment and Repair Surgery
Briefly, the supraspinatus tendon was visualized and a grasping stitch placed, using a 5-0 polypropylene suture (Surgipro II, Covidien, Mansfield, MA, USA). The tendon was then detached at its insertion using a #11 scalpel blade and allowed to freely retract. For repair, a 5mm diameter burr (Multipro 395, Dremel, Mt. Prospect, IL, USA) was then used to remove any remaining fibrocartilage at the insertion site. A 0.5 mm anterior to posterior hole was drilled through the greater tuberosity of the humerus distal to the insertion site and suture was passed through the bone tunnel, and the tendon reapposed to the insertion site. Closure was achieved by suturing the deltoid muscle and the skin was closed using staples.
Quantitative Ambulatory Assessment
Forelimb ground reaction forces (medial/lateral, braking, propulsion, and vertical) were quantified prior to sacrifice using an instrumented walkway.38 Data was collected one day prior to surgery to obtain baseline, uninjured values and then collected at days 5, 7, 14, 28, 42, and 56 following surgery. All data was normalized by body-weight.
Passive Joint Mechanics
Passive range of motion measurements were performed prior to sacrifice.36, 39 Data was collected one day prior to surgery to obtain baseline, uninjured values and then at 2, 4, and 8 weeks following surgery. All data was normalized to baseline values.
Sample Preparation for Mechanical Testing
Following ambulatory and passive joint mechanics measurements, animals were sacrificed and frozen for subsequent mechanical testing. At the time of testing, the animals were thawed and the humerus was dissected out with the supraspinatus tendon intact. Upon dissection, successful denervation was confirmed in all animals in the SD + Supra repair group through observation of serratus anterior and trapezius muscle thinning and atrophy. The tendon was fine dissected under a microscope to remove muscle and any excess, non-loadbearing tissue near the repaired insertion site. Suture from the surgical repair was left embedded within the tendon to avoid damage due to dissection. Cross-sectional area was measured using a custom laser device.10
Tendon Mechanical Testing
Elastic and viscoelastic mechanical properties of the insertion and mid-substance of the supraspinatus tendon were determined using uniaxial tensile testing.15 Briefly, Verhoeff stain lines were placed along the length of the supraspinatus tendon to divide the insertion and mid-substance regions for local optical strain measurements. The humerus was fully embedded in a holding fixture using polymethylmethacrylate (PMMA) and inserted into a custom testing fixture and secured to a base fixture. The proximal tendon was annealed in sand paper and gripped using custom serrated grips. The specimen was immersed in PBS at 37°C during testing. Tensile testing consisted of: preconditioning for 10 cycles from 0.1 N to 0.5 N, stress relaxation to 5% strain at a rate of 5 %/sec for 600 sec, and ramp to failure at 0.3%/sec. Elastic properties were calculated using a linear regression from the linear region of the stress-strain curves. For viscoelastic parameters, percent relaxation was determined using the peak and equilibrium loads.
Tendon Histology
Histologic analysis was performed to examine cellular and organizational changes in the supraspinatus tendon. Tissues were harvested immediately after sacrifice and processed using standard paraffin procedures. Sagittal sections (7 um) were collected and stained with Hematoxylin–Eosin (H&E). Stained supraspinatus tendon sections were imaged at the insertion site and mid-substance using a microscope at 200X and 100X magnification using traditional and polarized light, respectively. Cell density and cell shape were independently graded by three blinded investigators, who were provided with previously prepared standard images, using a scale of 1-3 (1=low, 2= moderate, 3=high) for cellularity and 1-3 (1=spindle shaped, 2= mixed, 3= rounded) for cell shape. Previous studies indicate that cell shape and cell density are important indicators in assessing tendon damage and healing.8, 40 Polarized light images were analyzed using custom software to evaluate the angular deviation of the collagen orientation, a measure of fiber distribution spread.15
Tendon Immunohistochemistry
The distribution of extracellular matrix (ECM) proteins was localized using immunohistochemical techniques.36 Tissue specimens were stained for a protein consistent with cartilage (collagen type II), a protein consistent with scar formation (collagen type III), a protein consistent with tendon (the proteoglycan decorin), and an inflammatory marker (IL1-β) (S-Table 1). The proteins were visualized using DAB, which makes the antibody-protein conjugate turn brown. Given the potential variations in staining intensities, all sections were stained at the same time with the same antibody. Negative controls were used and no background staining was observed. The insertion site and mid-substance of each tendon were evaluated separately. Staining results were independently graded by three blinded investigators, who were provided with previously prepared standard images, using a scale of 0-3 (0=undetectable, 1=low, 2=medium, 3=high), and the mode was used as the final score.
Statistics
Statistical analysis was performed according to our specific study hypotheses rather than by the global study design. For ambulatory assessment, measurements were occasionally absent for a specific animal on a specific day. Therefore, multiple imputations were conducted using the Markov chain Monte Carlo method for missing data points (~10%). For both ambulatory assessment and passive joint mechanics, significance was assessed using a 2-way ANOVA with repeated measures on time with follow-up t-tests between groups at each time point. Tissue mechanics between groups were assessed using t-tests (n=10). Based on previous data on rat tendon mechanical properties by Gimbel et al, Peltz et al, and Perry et al.,15, 30, 33 a priori power analysis was performed to determine sample size to achieve a power of 0.8. Histology and immunohistochemistry scores were evaluated using Mann-Whitney tests (n=3-5). Significance was set at p<0.05; trends at p<0.1.
Results
Ambulatory Data
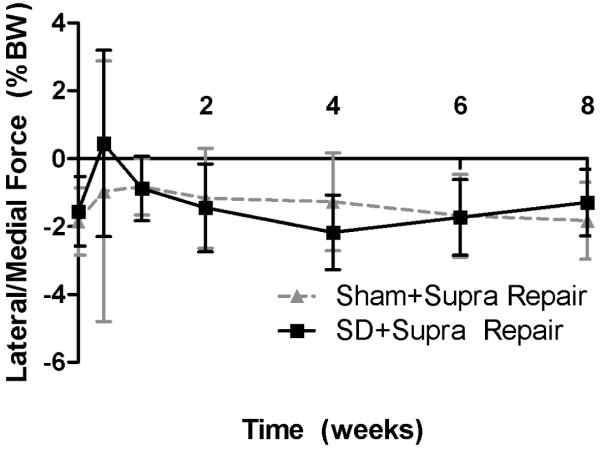
Shoulder joint function was significantly altered in the SD + Supra Repair group compared to the Sham + Supra Repair group (Figure 1). Gross observation demonstrated alteration in scapular movements, consistent with scapular “winging” as observed in the human. Specifically, propulsion force was significantly increased in the SD + Supra Repair group compared to the Sham + Supra Repair group at every time point, except 5 days post-injury (Figure 1A). Braking force was significantly decreased in the SD + Supra Repair group compared to the Sham + Supra Repair group at 6 and 8 weeks post-injury (Figure 1B). No differences in vertical or medial/lateral forces were observed between groups (Figure 1C, 1D). No significant differences in spatio-temporal parameters (step width, stride length, or speed) were observed between groups (data not shown), demonstrating lack of a difference in compensation between groups by the uninjured contralateral limb, as observed by Perry et al.32
Figure 1.
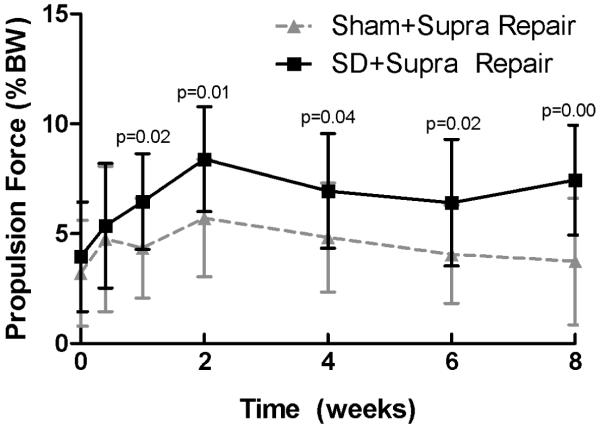
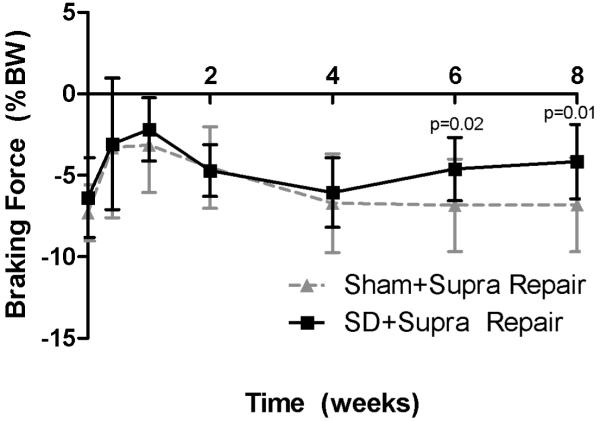
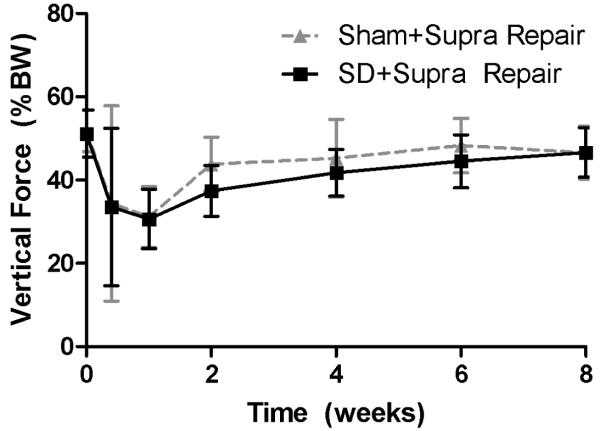
(A) The SD + Supra Repair group had increased propulsion force compared to the Sham + Supra Repair group at most time-points (except 5 days following repair). (B) The SD + Supra Repair group had decreased breaking force compared to the Sham + Supra Repair group at later time-points (6 and 8 weeks). (C) No difference in vertical force was observed. (D) No difference in medial/lateral force was observed. Data is shown as mean ± standard deviation.
Passive Joint Mechanics
No differences in passive joint mechanics were observed between groups at any time point (S-Table 2).
Tendon Mechanics
Mechanical properties were altered in the SD + Supra Repair group compared to the Sham + Supra Repair group for some parameters. Specifically, insertion cross-sectional area was significantly decreased in the SD + Supra Repair group compared to the Sham + Supra Repair group at 4 weeks, with a similar trend at the tendon mid-substance (Figure 2A, 2B). No differences in insertion elastic modulus were observed between groups (Figure 3A). Mid-substance elastic modulus was significantly decreased in the SD + Supra Repair group compared to the Sham + Supra Repair group at 4 weeks (Figure 3B). No differences in maximal load were observed (data not shown). For viscoelastic properties, a trend toward increased percent relaxation in the SD + Supra Repair group compared to the Sham + Supra Repair group was observed at 8 weeks post-injury (Figure 4).
Figure 2.
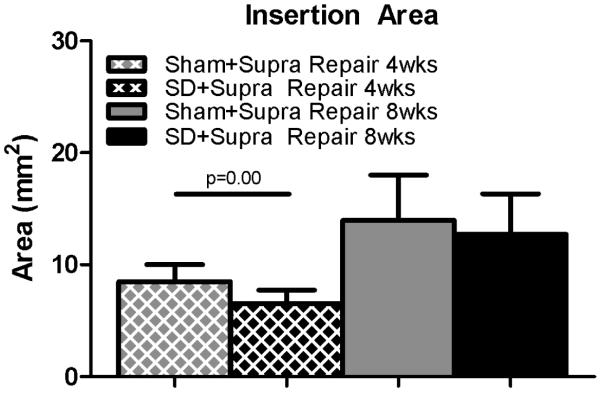
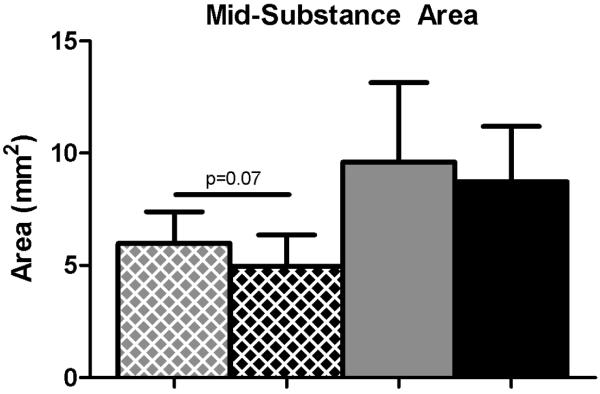
(A) The SD + Supra Repair group had decreased cross-sectional area at the insertion compared to the Sham + Supra Repair group at 4 weeks following repair. (B) A trend toward decreased cross-sectional area was observed in the SD + Supra Repair group compared to the Sham + Supra Repair group at the mid-substance at 4 weeks following repair. Data is shown as mean ± standard deviation.
Figure 3.
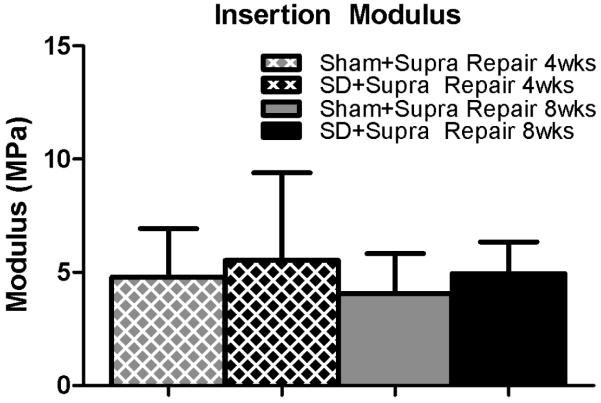
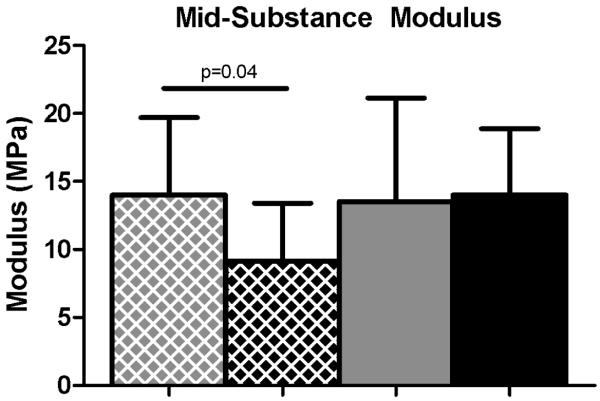
(A) No differences were observed in insertion elastic modulus. (B) The SD + Supra Repair group had decreased elastic modulus compared to the Sham + Supra Repair group at 4 weeks following repair. Data is shown as mean ± standard deviation.
Figure 4.
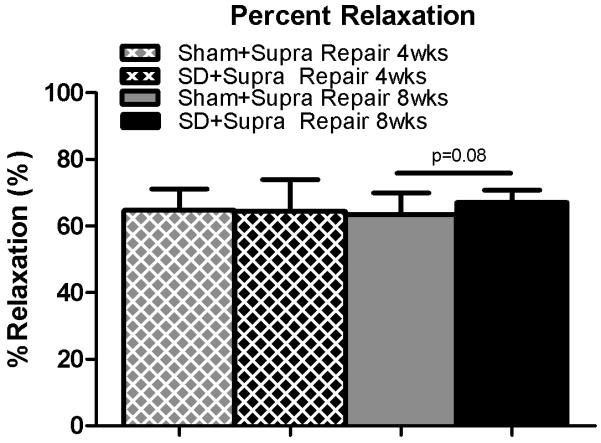
A trend toward increased percent relaxation was observed in the SD + Supra Repair group compared to the Sham + Supra Repair group at 8 weeks following repair. Data is shown as mean ± standard deviation.
Tendon Histology
No differences in cellularity were observed between groups (Table 1). A trend toward a more rounded cell shape was observed at the supraspinatus insertion in the SD + Supra Repair group compared to the Sham + Supra Repair group at 4 weeks (S-Figure 1). No differences in collagen fiber organization were observed at any time point, except for a trend toward greater disorganization in the mid-substance at 2 weeks (S-Figure 2).
Table I.
Tendon Histology
Results for passive joint mechanics demonstrated no differences between groups. Data is shown as normalized by baseline values (change from baseline) and as mean ± standard deviation.
| Histology | Group | Region | 2 weeks | p-value | 4 weeks | p-value | 8 weeks | p-value |
|---|---|---|---|---|---|---|---|---|
| Cell Shape | SD+Supra Repair | INS | 1 (1-2) | 0.11 | 2 (2-2) | 0.08+ | 2 (2-2) | 0.37 |
| Sham+Supra Repair | 2 (2-2) | 1 (1-1) | 2 (1.75-2) | |||||
| SD+Supra Repair | MID | 2 (1.75-2) | 0.5 | 2 (2-2) | 0.50 | 3 (2-3) | 0.27 | |
| Sham+Supra Repair | 2 (1-2) | 2 (2-2) | 2 (2-3) | |||||
| Cell Density |
SD+Supra Repair | INS | 2 (2-3) | 0.42 | 3 (2-3) | 0.50 | 2 (1-2) | 0.45 |
| Sham+Supra Repair | 2 (2-3) | 3 (2-3) | 2 (1-3) | |||||
| SD+Supra Repair | MID | 3 (3-3) | 0.50 | 3 (3-3) | 0.16 | 2 (2-2) | 0.35 | |
| Sham+Supra Repair | 3 (3-3) | 3 (2-3) | 2 (2-3) |
Tendon Immunohistochemistry
No differences were observed at any time point or in any region for the proteins studied (S-Table 3).
Discussion
While the prevalence of rotator cuff repair failures is well-documented, the mechanical mechanisms by which failure occurs are not well-established, making clinical management difficult. Previous studies have demonstrated a strong association between abnormal scapulothoracic joint kinematics (termed scapular dyskinesis) and shoulder injury.27 Using an established animal model, we prescribed scapular dyskinesis and rigorously evaluated the effect on supraspinatus tendon healing following cuff repair.
Consistent with our first hypothesis, scapular dyskinesis significantly altered joint function. Specifically, propulsion force was significantly increased in the SD + Supra Repair group compared to the Sham + Supra Repair while braking force was significantly decreased at later time points. These changes indicate an alteration in the loading environment at the shoulder due to scapular dyskinesis and may place the healing supraspinatus tendon at increased risk of re-rupture. These findings are consistent with but less dramatic than previous findings by Reuther et al. that have also identified significant changes in joint function in propulsion force and in additional parameters, such as vertical ground reaction force, due to scapular dyskinesis alone.36 A higher propulsion force may place increased stresses on the healing supraspinatus, compromising the mechanical integrity of the repair. Decreased braking force, which was observed only at later time points, may be indicative of a functional adaptation to prevent further damage. During forward locomotion, the rat undergoes large amounts of forward flexion, requiring substantial rotation of both the humerus and scapula at the shoulder joint. In this scapular dyskinesis model, the scapular rotators, the trapezius and serratus anterior, are not functioning and the scapula is unable to rotate, which likely led to the observed alterations in braking and propulsion forces. However, with no change in the medial or lateral forces, the results indicate that the serratus anterior and trapezius did not participate in these actions, or were compensated for by surrounding structures. Interestingly, no changes in vertical forces were observed, contrary to previous findings from Reuther et al. using this model.36 Vertical force corresponds to weight-bearing and pain and it is possible that the pain due to the repair surgery was greater than that due to the effect of scapular dyskinesis on function, masking any underlying alterations due to scapular dyskinesis. Loading environment is important in healing tissues and can differ in amount/magnitude (i.e., immobilization, exercise, over use) and type (i.e., tensile, compressive, shear). In this study, scapular dyskinesis likely alters the type of loading the tendon experiences much more than amount/magnitude, thus altering the mechanical integrity of the healing tendon.
Contrary to our first hypothesis, scapular dyskinesis had no effect on passive joint mechanics. Sarver et al previously demonstrated that increased stiffness and decreased range of motion is observed following cuff repair.39 This may be attributed to scar formation in the healing tissue and joint capsule or muscular adaptations in response to the repair surgery. While tendon stiffening, capsular tightening, and muscular dysfunction are likely to contribute to changes in glenohumeral joint stiffness and range of motion, the excessive scar formation present following repair surgery may mask these underlying issues and therefore differences in passive joint mechanics between groups were not identified. It is likely that the surgical repair has a greater effect on passive joint mechanics than the effect of scapular dyskinesis.
Consistent with our second hypothesis, scapular dyskinesis was detrimental to supraspinatus tendon mechanical properties for some parameters. Specifically, mid-substance modulus was significantly diminished at 4 weeks in the SD + Supra Repair group compared to the Sham + Supra Repair group. However, this change did not persist at 8 weeks. Additionally, a trend toward a more rounded cell shape was observed in the supraspinatus insertion at 4 weeks, which may be indicative of compressive loading. In the mid-substance region, the tendon was also more disorganized in the SD + Supra Repair group compared to control at 2 weeks and is consistent with the mechanical changes observed at this time point. The location specific tendon changes (mid-substance region) are similar to findings observed previously by Reuther et al. in the supraspinatus tendon in the presence of scapular dyskinesis36 and may be due to its anatomic location under the acromial arch during forward flexion, resulting in impingement and an altered loading environment. Surprisingly, decreased cross-sectional area was observed in the SD + Supra Repair group compared to the Sham + Supra Repair group at 4 weeks. This finding may be related to the reduced subacromial space present in the SD + Supra Repair group due to the diminished upward rotation of the scapula, allowing less space for tissue proliferation and matrix production. This finding did not persist at 8 weeks and an improvement in tissue elasticity is apparent (no difference in elastic modulus). This may be related to the compensatory decrease in braking force observed at later time points (6 and 8 weeks), reducing stress on the repair site and preserving mechanical properties. However, a trend toward increased percent relaxation (a viscoelastic parameter) was observed in the SD + Supra Repair group compared to the Sham + Supra Repair group at 8 weeks post-injury. Previous studies have found increased percent relaxation in injured tendon, indicative of inferior tissue properties and poor healing.1, 8
Contrary to our second hypothesis, no differences were observed in insertion modulus or maximal load. While increased insertion modulus may be an indicator of tendon healing, Reuther et al. has previously demonstrated that scapular dyskinesis has no effect on native insertion site properties.36 Additionally, while maximal load is an important indicator of repair strength, the typical loads at which the supraspinatus operates is not near this threshold. Material properties, such as elastic modulus and percent relaxation, which highlight the quality of the tissue, may be a better indicator of repair integrity and functionality then maximal load at failure. Fatigue properties, which may be more consistent with modes of failure seen in the clinical condition, could be evaluated in future studies. In general, these findings indicate that there may be mechanical consequences associated with poor scapulothoracic joint kinematics for the healing supraspinatus tendon following repair.
Interestingly, no differences were observed in protein expression, as measured by immunohistochemical techniques. Tendon healing is characterized by a reactive scar formation and involves excessive inflammation, cell proliferation, and increased matrix synthesis characterized by increased deposition of type III collagen.20 This heightened response to tendon injury and repair may mask any effect of scapular dyskinesis in this study. As observed with passive joint mechanics, surgical repair may have a greater effect on tendon composition than the effect of scapular dyskinesis.
This study has several limitations. First, the use of a quadruped animal does not exactly replicate the human shoulder. However, the presence of the acromial arch and its position over the rotator cuff is similar to the human shoulder and is essential in our model to evaluate the effect of scapular dyskinesis on supraspinatus healing as it passes underneath it. Secondly, acute transection of the supraspinatus tendon and immediate repair does not exactly replicate the human condition. Specifically, rotator cuff tears are typically chronic in nature. However, using an acute transection and repair allows us to examine the mechanical and biologic healing response in a more controlled manner to address our study hypotheses. Thirdly, acute transection of both the spinal accessory and long thoracic nerve to induce scapular dyskinesis does not exactly mimic the most common clinical scenario of scapular dyskinesis. Specifically, the etiology of scapular dyskinesis could be attributed to nerve injury in only approximately 6% of cases41 and several other factors may contribute to scapular dysfunction clinically. However, nerve injuries, due to a traumatic blow, a severe stretch or traction, or laceration, are common in sports26 and can be found secondary to various surgical interventions.21, 28 This model of scapular dyskinesis, created by utilizing a nerve injury mechanism, is appropriate because it does mimic the clinical condition of scapular “winging” and does provide a consistent, repeatable method of inducing scapular dyskinesis, allowing us to address our primary hypothesis. Specifically, this model was designed to investigate the effect of scapular dyskinesis on tendon healing and was not developed to address the etiology of scapular dyskinesis. Importantly, underlying mechanisms and cause and effect relationships can only be addressed in an animal model where time from injury can be controlled and function and properties can be evaluated over time. Future studies could model a more mild case of scapular dyskinesis through surgical transection of one nerve (as opposed to two) or through temporary paralysis of the scapular muscles through Botulinum toxin (Botox) injections. Lastly, while immunohistochemistry is a standard technique to localize proteins in tendon, additional quantitative measures, such as the enzyme-linked immunosorbant assays (ELISA), could be measured to further elucidate biochemical outcomes.
Conclusion
In summary, results suggest the functional consequences associated with scapular dyskinesis may compromise supraspinatus tendon healing following repair by diminishing some tendon mechanical properties. Identification of abnormal joint mechanics as a potential mechanical mechanism of failed rotator cuff healing will help guide clinicians in prescribing treatment strategies for patients with cuff tears. This study supports the need to evaluate joint kinematics and to consider correcting these abnormalities prior to surgical repair. This may allow for optimization of the mechanical loading environment and result in improved tendon healing rates.
Supplementary Material
S-Figure 1 A representative image for the supraspinatus insertion is displayed. A trend toward a more rounded cell shape was observed at the supraspinatus insertion in the SD + Supra Repair group (B) compared to the Sham + Supra Repair group (A) at 4 weeks.
S-Figure 2 (A) No differences were observed in insertion organization. (B) A trend toward increased angular deviation was observed in the SD + Supra Repair group compared to the Sham + Supra Repair group at 2 weeks following repair. Data is shown as mean ± standard deviation.
Table II.
A trend toward a more rounded cell shape was observed in the SD + Supra Repair group compared to the Sham + Supra Repair group at 4 weeks following repair. Data is shown as median and interquartile range (+p<0.1).
Acknowledgements
The authors acknowledge Adam Pardes and Bob Zanes for their assistance. The study was funded by NIH/NIAMS (R01AR056658) and the Penn Center for Musculoskeletal Disorders (P30AR050950).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Abramowitch SD, Woo SL, Clineff TD, Debski RE. An evaluation of the quasi-linear viscoelastic properties of the healing medial collateral ligament in a goat model. Ann Biomed Eng. 2004;32:329–335. doi: 10.1023/b:abme.0000017539.85245.6a. 10.1023/B:ABME.0000017539.85245.6a. [DOI] [PubMed] [Google Scholar]
- 2.Adams JE, Zobitz ME, Reach JS, Jr., An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700–709. doi: 10.1016/j.arthro.2006.03.016. 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Beason DP, Connizzo BK, Dourte LM, Mauck RL, Soslowsky LJ, Steinberg DR, et al. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J Shoulder Elbow Surg. 2012;21:245–250. doi: 10.1016/j.jse.2011.10.021. 10.1016/j.jse.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Bigliani LU, Cordasco FA, McIlveen SJ, Musso ES. Operative treatment of failed repairs of the rotator cuff. The Journal of bone and joint surgery American volume. 1992;74:1505–1515. [PubMed] [Google Scholar]
- 5.Brophy RH, Kovacevic D, Imhauser CW, Stasiak M, Bedi A, Fox AJ, et al. Effect of short-duration low-magnitude cyclic loading versus immobilization on tendon-bone healing after ACL reconstruction in a rat model. The Journal of bone and joint surgery American volume. 2011;93:381–393. doi: 10.2106/JBJS.I.00933. 10.2106/JBJS.I.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology Part III: The SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19:641–661. doi: 10.1016/s0749-8063(03)00389-x. 10.1016/S0749-8063(03)00389-X. [DOI] [PubMed] [Google Scholar]
- 7.Dagher E, Hays PL, Kawamura S, Godin J, Deng XH, Rodeo SA. Immobilization modulates macrophage accumulation in tendon-bone healing. Clin Orthop Relat Res. 2009;467:281–287. doi: 10.1007/s11999-008-0512-0. 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dourte LM, Perry SM, Getz CL, Soslowsky LJ. Tendon properties remain altered in a chronic rat rotator cuff model. Clin Orthop Relat Res. 2010;468:1485–1492. doi: 10.1007/s11999-009-1206-y. 10.1007/s11999-009-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eitzen I, Holm I, Risberg MA. Preoperative quadriceps strength is a significant predictor of knee function two years after anterior cruciate ligament reconstruction. Br J Sports Med. 2009;43:371–376. doi: 10.1136/bjsm.2008.057059. 10.1136/bjsm.2008.057059. [DOI] [PubMed] [Google Scholar]
- 10.Favata M. Scarless healing in the fetus : implications and strategies for postnatal tendon repair [Thesis (Ph D in Bioengineering)]: University of Pennsylvania. 2006;xi:218. [Google Scholar]
- 11.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. The Journal of bone and joint surgery American volume. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. No doi. [DOI] [PubMed] [Google Scholar]
- 12.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 13.Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994:43–53. [PubMed] [Google Scholar]
- 14.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. The Journal of bone and joint surgery American volume. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Gimbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400–404. doi: 10.1115/1.2721075. 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 17.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. The Journal of bone and joint surgery American volume. 1991;73:982–989. [PubMed] [Google Scholar]
- 18.Hettrich CM, Gasinu S, Beamer BS, Fox A, Ying O, Deng XH, et al. The effect of immobilization on the native and repaired tendon-to-bone interface. J Bone Joint Surg Am. 2013;95:925–930. doi: 10.2106/JBJS.K.01329. 10.2106/JBJS.K.01329. [DOI] [PubMed] [Google Scholar]
- 19.Hettrich CM, Gasinu S, Beamer BS, Stasiak M, Fox A, Birmingham P, et al. The Effect of Mechanical Load on Tendon-to-Bone Healing in a Rat Model. Am J Sports Med. 2014;42(5):1233–41. doi: 10.1177/0363546514526138. 10.1177/0363546514526138. [DOI] [PubMed] [Google Scholar]
- 20.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Kelley MJ, Kane TE, Leggin BG. Spinal accessory nerve palsy: associated signs and symptoms. The Journal of orthopaedic and sports physical therapy. 2008;38:78–86. doi: 10.2519/jospt.2008.2454. 10.2519/jospt.2008.2454. [DOI] [PubMed] [Google Scholar]
- 22.Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26:325–337. doi: 10.1177/03635465980260022801. [DOI] [PubMed] [Google Scholar]
- 23.Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11:142–151. doi: 10.5435/00124635-200303000-00008. No doi. [DOI] [PubMed] [Google Scholar]
- 24.Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20:364–372. doi: 10.5435/JAAOS-20-06-364. 10.5435/JAAOS-20-06-364. [DOI] [PubMed] [Google Scholar]
- 25.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21:228–237. doi: 10.1016/j.jse.2011.11.002. 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhlman GS, McKeag DB. The "burner": a common nerve injury in contact sports. American family physician. 1999;60:2035–2040. 2042. [PubMed] [Google Scholar]
- 27.Ludewig PM, Reynolds JF. The association of scapular kinematics and glenohumeral joint pathologies. The Journal of orthopaedic and sports physical therapy. 2009;39:90–104. doi: 10.2519/jospt.2009.2808. 10.2519/jospt.2009.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Impact of neck dissection on scapular muscle function: a case-controlled electromyographic study. Archives of physical medicine and rehabilitation. 2013;94:113–119. doi: 10.1016/j.apmr.2012.07.017. 10.1016/j.apmr.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 29.McHugh MP, Tyler TF, Gleim GW, Nicholas SJ. Preoperative indicators of motion loss and weakness following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1998;27:407–411. doi: 10.2519/jospt.1998.27.6.407. [DOI] [PubMed] [Google Scholar]
- 30.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27:416–420. doi: 10.1002/jor.20770. 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peltz CD, Sarver JJ, Dourte LM, Wurgler-Hauri CC, Williams GR, Soslowsky LJ. Exercise following a short immobilization period is detrimental to tendon properties and joint mechanics in a rat rotator cuff injury model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28:841–845. doi: 10.1002/jor.21059. 10.1002/jor.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry SM, Getz CL, Soslowsky LJ. Alterations in function after rotator cuff tears in an animal model. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al] 2009;18:296–304. doi: 10.1016/j.jse.2008.10.008. 10.1016/j.jse.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry SM, Getz CL, Soslowsky LJ. After rotator cuff tears, the remaining (intact) tendons are mechanically altered. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al] 2009;18:52–57. doi: 10.1016/j.jse.2008.07.003. 10.1016/j.jse.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry SM, Gupta RR, Van Kleunen J, Ramsey ML, Soslowsky LJ, Glaser DL. Use of small intestine submucosa in a rat model of acute and chronic rotator cuff tear. J Shoulder Elbow Surg. 2007;16:S179–183. doi: 10.1016/j.jse.2007.03.009. 10.1016/j.jse.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Reuther KE, Thomas SJ, Tucker JJ, Vafa RP, Gordon JA, Liu SS, et al. Overuse Activity in the Presence of Scapular Dyskinesis Leads to Shoulder Tendon Damage in a Rat Model. Annals of biomedical engineering. 2014 Sep 30; doi: 10.1007/s10439-014-1137-y. [Epub ahead of print] 10.1007/s10439-014-1137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuther KE, Thomas SJ, Tucker JJ, Yannascoli SM, Caro AC, Vafa RP, et al. Scapular dyskinesis is detrimental to shoulder tendon properties and joint mechanics in a rat model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2014;32:1436–1443. doi: 10.1002/jor.22693. 10.1002/jor.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16:S191–197. doi: 10.1016/j.jse.2007.03.012. 10.1016/j.jse.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Sarver JJ, Dishowitz MI, Kim SY, Soslowsky LJ. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 2010;43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarver JJ, Peltz CD, Dourte L, Reddy S, Williams GR, Soslowsky LJ. After rotator cuff repair, stiffness--but not the loss in range of motion--increased transiently for immobilized shoulders in a rat model. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al] 2008;17:108S–113S. doi: 10.1016/j.jse.2007.08.004. 10.1016/j.jse.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, et al. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al] 2000;9:79–84. Neer Award 1999. [PubMed] [Google Scholar]
- 41.Srikumaran U, Wells JH, Freehill MT, Tan EW, Higgins LD, Warner JJ. Scapular Winging: A Great Masquerader of Shoulder Disorders: AAOS Exhibit Selection. The Journal of bone and joint surgery American volume. 2014;96:e122. doi: 10.2106/JBJS.M.01031. 10.2106/JBJS.M.01031. [DOI] [PubMed] [Google Scholar]
- 42.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454–463. doi: 10.1016/S0736-0266(01)00144-9. 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 43.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. doi: 10.1115/1.1536660. doi:10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 44.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S-Figure 1 A representative image for the supraspinatus insertion is displayed. A trend toward a more rounded cell shape was observed at the supraspinatus insertion in the SD + Supra Repair group (B) compared to the Sham + Supra Repair group (A) at 4 weeks.
S-Figure 2 (A) No differences were observed in insertion organization. (B) A trend toward increased angular deviation was observed in the SD + Supra Repair group compared to the Sham + Supra Repair group at 2 weeks following repair. Data is shown as mean ± standard deviation.