Abstract
Background
Most cocaine abusers also abuse alcohol, but little is known about interactions that promote co-abuse. These experiments in rhesus monkeys determined the effects of >8 weeks of ethanol (EtOH) consumption on cocaine self-administration (n=6), effects of dopamine (DA) receptor antagonists on cocaine reinforcement (n=3–4 per drug) and the ability of the D2-like DA receptor agonist quinpirole to elicit yawning (n=3).
Methods
Monkeys self-administered cocaine (0.0–1.0 mg/kg/injection, i.v.) under a 300-second fixed-interval schedule and the above-listed variables were measured before EtOH exposure. Next, monkeys consumed a sweetened, 4% EtOH solution in the home cage under binge-like conditions: one hour, 5 days/week with daily intake equaling 2.0 g/kg EtOH. After approximately 8 weeks, measures were re-determined, then EtOH drinking was discontinued. Finally, acute effects of EtOH on cocaine self-administration were determined by infusing EtOH (0.0–1.0 g/kg. i.v.) prior to cocaine self-administration sessions (n=4).
Results
In 5 of 6 monkeys, EtOH drinking increased self-administration of low cocaine doses but did not alter reinforcing effects of higher doses. Self-administration returned to baseline after EtOH access was terminated (n=3). Effects of DA receptor antagonists on cocaine self-administration were not consistently altered after EtOH consumption, but the ability of quinpirole to induce yawning was enhanced in 2 of 3 monkeys. Acute EtOH infusions only decreased self-administration of lower cocaine doses.
Conclusions
Taken together, the data suggest that long-term EtOH exposure can increase sensitivity to cocaine, possibly by increasing D3 receptor sensitivity. Data do not support a role for acute pharmacological interactions in promoting cocaine/EtOH co-abuse.
Keywords: alcohol, co-abuse, cocaine, nonhuman primates, self-administration
1. INTRODUCTION
Despite decades of research, cocaine abuse persists as a major public health problem and no pharmacotherapy has proven suitable for widespread clinical use (Kampman, 2010; Karila et al., 2011). The lack of success in translating preclinical hypotheses to effective medications indicates that significant barriers remain, but reasons for the disconnect between preclinical and clinical results are poorly understood. One clinical reality rarely incorporated into animal models is co-abuse of other substances. Humans who use or are dependent on multiple substances are typically excluded from clinical studies, and nearly all studies in laboratory animals limit exposure to a single drug of interest. This is understandable because characterizing the effects of two substances in combination is complex. In the case of alcohol and cocaine, however, such studies are critical. Estimates indicate that up to 90% of cocaine abusers also abuse alcohol (Grant and Harford, 1990; Helzer and Pryzbeck, 1988; Kampman et al., 2013; Tziortzis et al., 2011). Importantly, individuals who co-abuse alcohol commonly have more severe cocaine dependence, are more adversely affected by their drug use and are less likely to remain in treatment (Carroll et al., 1993; Heil et al., 2000; Higgins et al., 1994). Alarmingly, alcoholic cocaine users also report greater rates of unwanted sexual encounters and suicidal and homicidal behavior (Heil et al., 2000; Salloum et al., 1996).
The mechanistic basis for the pervasive co-abuse of alcohol and cocaine is incompletely understood, and studies characterizing the interactions between the drugs have produced mixed results. Enhancement of cocaine’s abuse-related effects by EtOH would be expected based on its ability to increase striatal dopamine (DA) concentrations (e.g., Bradberry, 2002; Di Chiara and Imperato, 1988; Yoder et al., 2009). Moreover, similar alterations in brain DA systems—particularly D2 receptors—have been observed in alcoholics and cocaine abusers (e.g., Cosgrove, 2010; Volkow et al., 1996,1999, 2002). When combined, cardiovascular effects of cocaine and EtOH can be enhanced, while effects on cognitive performance may be attenuated (Farre et al., 1993; Foltin and Fischman, 1988; Foltin et al., 1993; Higgins et al., 1992). Experiments in laboratory animals have also indicated that co-administration of cocaine and EtOH can produce more pronounced effects than either drug alone. These studies have primarily focused on motor endpoints such as locomotion and rates of lever pressing under schedules of reinforcement (Aston-Jones et al., 1984; Masur et al., 1989; Misra et al., 1989; Rech et al., 1978) rather than variables that more directly reflect cocaine’s abuse-related effects. Only two studies have examined the effects of EtOH on cocaine self-administration in laboratory animals. In the first (Aspen and Winger, 1997), EtOH increased self-administration of cocaine and another DA uptake blocker, but not a mu opioid receptor agonist, but only in some monkeys. In the second (Winger et al., 2007), demand functions for cocaine/EtOH combinations were intermediate to those of either drug alone. In contrast, studies in humans demonstrate that alcohol can increase cocaine’s reinforcing and pleasurable subjective effects (Farre et al., 1993; Higgins et al., 1996). Thus, although there is some evidence to suggest that EtOH can enhance some effects of cocaine, the data addressing this critical question are limited and involve only acute EtOH exposure. Due to limitations of studies with human drug abusers, including unknown extent of past and current drug use, unknown durations of abstinence, comorbid psychiatric disorders and inability to collect pre-drug “baseline” data, well-controlled, longitudinal experiments in animals are a critical step in understanding the causes and effects of polysubstance abuse.
The present studies were designed to assess two potential mechanisms by which longterm binge-like EtOH consumption may increase cocaine use. One possibility is that chronic exposure to EtOH results in enhancement of the reinforcing effects of cocaine. To address this possibility, dose-effect curves for cocaine self-administration were determined prior to EtOH exposure and again after monkeys had consumed EtOH 5 days per week for 8 weeks. Bottles containing a sweetened 4% EtOH solution were hung on monkeys’ home cages for one hour; monkeys could consume up to 2.0 g/kg per day. This regimen is consistent with the definition of “binge drinking” promulgated by the National Institute Alcohol Abuse and Alcoholism: “a pattern of drinking that brings blood alcohol concentration (BAC) levels to 0.08 g/dl…in about two hours;” http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Because the reinforcing effects of cocaine have been associated with DA D1-like and D2-like receptors, the sensitivity of cocaine self-administration to antagonists of these receptors was determined under both conditions. In addition, the ability of the D2-like DA receptor agonist quinpirole to elicit yawning was examined before and during the period of EtOH drinking. Although it interacts with all D2-like receptor subtypes, yawning elicited by quinpirole has been thoroughly characterized as an effect mediated by the D3 receptor subtype of the D2-like family of DA receptors in rodents and monkeys (e.g., Collins et al., 2005, 2007; Martelle et al., 2007). Finally, after daily EtOH drinking was discontinued, the possibility that acute pharmacological interactions could increase cocaine reinforcement was tested by infusing EtOH intravenously just prior to self-administration sessions.
2. MATERIALS AND METHODS
2.1. Subjects
Eight adult male rhesus monkeys (Macaca mulatta) served as subjects. Each monkey was fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate Products). Monkeys were housed individually in stainless steel cages in which water was available ad libitum. Monkeys were weighed weekly and fed enough food (Purina LabDiet Chow, St. Louis, MO), fresh fruit and vegetables daily to maintain healthy body weights without becoming obese as determined by daily inspection and periodic veterinary examinations. Body weights did not change significantly during these studies. Environmental enrichment was provided as outlined in the Institutional Animal Care and Use Committee’s Non-Human Primate Environmental Enrichment Plan. Animal housing and handling and all experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the Animal Care and Use Committee of Wake Forest University.
Each monkey was prepared with an indwelling venous catheter and subcutaneous vascular access port (VAP; Access Technologies, Skokie, IL) under sterile surgical conditions. An antibiotic (25 mg/kg kefzol, i.m.; Cefazolin sodium, Marsam Pharmaceuticals, Inc., Cherry Hill, NJ) was administered 1 hour prior to surgery. Anesthesia was induced with ketamine (15 mg/kg, i.m.) and maintained with ketamine supplements. A catheter was inserted into a major vein (femoral or internal or external jugular) to the level of the vena cava. The distal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was attached to a VAP, which was placed in a subcutaneous pocket formed by blunt dissection.
Five to seven days per week, each monkey was seated in a primate chair and placed into a ventilated, sound-attenuating chamber (0.76 × 0.76 × 1.5 mm; Med Associates, St. Albans, VT). The back of the animal was cleaned with 3.15% chlorhexidine and 70% isopropyl alcohol (Prevantics Swab, PDI Inc, Orangeburg, NY) and the VAP was connected to an infusion pump (Cole-Parmer Instrument Co., Niles, IL) located outside the chamber via a 22-gauge Huber Point Needle (Access Technologies) and tubing. The pump was operated for approximately 3 seconds to fill the port and catheter with the concentration of cocaine available for the session. A response key was located on one side of the chamber, above which was a horizontal row of three stimulus lights (green, red and white). A food receptacle was located below the key and was connected with a Tygon tube to a pellet dispenser (Med Associates) located on the top of the chamber for delivery of 1-g banana-flavored food pellets (Bio-Serv, Frenchtown, NJ). Experimental events occurring in these chambers were programmed and controlled by Med-PC software.
2.2. Cocaine self-administration
Monkeys were initially trained to respond on the key to receive a food pellet under a 300-second fixed interval schedule (FI 300). Initially, the green light was illuminated and every response produced a pellet and a 3-second illumination of the red light followed by a 10-second timeout (TO) period in which no lights were illuminated and responding had no scheduled consequences. Next, conditions were changed such that, after illumination of the green light, the first response after 1 second elapsed resulted in delivery of a food pellet (FI 1 second). If 30 seconds elapsed after the 1-second interval (a 30-second limited hold, LH), the green light was extinguished and a 10-second TO was initiated. Gradually, the FI and timeout durations were increased and the LH was decreased until all monkeys responded reliably on the terminal parameters: a FI 300-sec schedule with a 10-sec LH and 60-sec TO. This schedule was presented 10 times each day, for a total session length of approximately 60 min. When monkeys responded reliably for 10 pellets each day, the reinforcer was changed to an infusion of 0.1 mg/kg cocaine (the maintenance dose). Dose-response curves were generated by substituting saline or single doses of cocaine (0.001–1.0 mg/kg/injection) for at least five days and until responding stabilized, defined as three consecutive days with ±1 infusions and no upward or downward trend. Cocaine self-administration sessions were conducted between 8 and 10 am 5–7 days per week.
2.3. Effects of dopamine receptor antagonists on cocaine self-administration
When self-administration of the maintenance dose of cocaine was stable, saline or a dose of a DA receptor antagonist was administered i.v. prior to the cocaine self-administration in subsets of a group of four monkeys (R-1606, R-1607, R-1757 and R-1758). Drugs tested (with doses and pretreatment times) were the D1-like receptor agonist SCH 23390 (0.01–0.3 mg/kg, 10 min, n=3), the D2-like receptor antagonist eticlopride (0.001–0.01 mg/kg, 10 min, n=4) and the D3/D4 receptor antagonist buspirone (0.03–1.0 mg/kg, 5 min, n=3). Effects were redetermined during the period of daily EtOH consumption. Although testing of one drug was completed before another drug was tested, drugs were tested in mixed order across monkeys, and doses were tested in mixed order in each monkey. In most cases, monkeys received saline and each dose that had effects (except those with clearly pronounced sedating and/or response rate-decreasing effects) at least twice in mixed order with test sessions occurring no more than twice per week.
2.4. Quinpirole-induced yawning
Yawning elicited by the DA D2-like receptor agonist quinpirole was measured in three monkeys (R-1607, R-1608, R-1758). While the monkey was seated in a primate chair in a quiet room, yawns were recorded with a video camera for 30 minutes (baseline). Next, at 30-min intervals, saline was administered i.v. followed by incremental doses of quinpirole (0.0032–0.3 mg/kg in ascending order) using cumulative dosing procedures (see Bergman and Spealman, 1988). Occurrences of yawning were recorded from the videos by two observers. A yawn was defined as full extension of the jaws, withdrawal of the lips and exposure of the teeth (Code and Tang, 1991). Initial inter-rater variability was <5%. In rare cases of a discrepancy, the video was checked by a third observer and a consensus was reached. The primary dependent variable was total yawns in the 30-min baseline period, or in the 30-min period after each injection. Yawning experiments were conducted between 8 and 11 am. The effects of quinpirole were redetermined during the period of daily EtOH consumption; monkeys received neither cocaine nor EtOH for at least 24 hours prior to each experiment. That is, no cocaine or EtOH drinking session was conducted on the day prior to each yawning experiment. The second yawning experiment was conducted after a total of 11.5 weeks of ethanol drinking in R-1607 and R-1607 and after 54.0 weeks of drinking in R-1758.
2.5. Ethanol consumption
Once initial cocaine dose-response curves had been generated, cocaine self-administration sessions were suspended and EtOH was made available for consumption in the home cage using a method similar to that described by Katner et al. (2007). Access to EtOH in a 6% Tang solution was provided via a 500-ml drinking bottle attached to the monkey’s cage for one hour, five days per week in the afternoon. As shown in Table 1 the EtOH concentration and maximum daily EtOH intake increased over 12 days until monkeys reliably consumed a solution of 4% (w/v) EtOH in 6% Tang in a volume sufficient to equal an intake of 1.0 g/kg EtOH. On day 13, for the first three monkeys who underwent this procedure (Cohort 1: R-1607, R-1608, R-1745), the EtOH concentration was increased to 6% for several days as the maximum intake was increased to 2.0 g/kg. Because drinking became erratic and monkeys consumed less than the maximum amount of EtOH during these days, the EtOH concentration was reduced to 5%, then 4% while maximum intake remained at 2.0 g/kg. To avoid this disruption in the second cohort of monkeys (Cohort 2: R-1605, R-1606, R-1757, R-1758, R-1759), the EtOH concentration was not increased above 4%; on Day 13 maximum intake was raised to 1.5 g/kg, then 2.0 g/kg. By day 30, all monkeys were provided enough 4% EtOH to consume a maximum of 2.0 g/kg; this concentration and amount of EtOH was made available 5 days per week for the remainder of the EtOH-drinking period.
Table 1.
Parameters of ethanol access during initial exposure.
| COHORT 1 | COHORT 2 | |||
|---|---|---|---|---|
| Day | [EtOH], w/v | maximum intake (g/kg) |
[EtOH], w/v | maximum intake (g/kg) |
| 1–3 | 1% | 0.5 | 1% | 0.5 |
| 4–6 | 2% | 0.5 | 2% | 0.5 |
| 7–9 | 4% | 0.5 | 4% | 0.5 |
| 10–12 | 4% | 1.0 | 4% | 1.0 |
| 13–15 | 6% | 1.0 | 4% | 1.5 |
| 16–18 | 6% | 1.5 | 4% | 2.0 |
| 19–27 | 6% | 2.0 | 4% | 2.0 |
| 28–29 | 5% | 2.0 | 4% | 2.0 |
| 30+ | 4% | 2.0 | 4% | 2.0 |
To demonstrate that blood EtOH concentration was predictably related to the amount consumed using this procedure, one blood sample (~50 microliters) was collected from the saphenous vein of each of six monkeys after varying amounts of EtOH consumption (i.e., during the progressive increases described above). Blood samples were collected 90 minutes after the 60-min EtOH access period began, sealed in air-tight vials containing 500 microliters of distilled water and 20 microliters of isopropanol (10%; internal standard) and stored at −4°C until assayed using gas chromatography (Agilent 7890A GC system with G1888 Network Headspace Autosampler Santa Clara, CA) supplied with a flame ionization detector and Agilent ChemStation integrator.
After monkeys had consumed EtOH for approximately 8 weeks, cocaine dose-effect curves were re-determined in 6 of the 8 monkeys (all but R-1745 and R-1757). During this time, monkeys continued to drink EtOH in the home cage during the afternoon, at least 4 hours after the end of the morning cocaine self-administration session. EtOH and cocaine access were separated in this manner to eliminate acute pharmacological interactions, to prevent the formation of cocaethylene (e.g., Jatlow et al., 1991) and to ensure that monkeys were not intoxicated or in withdrawal during behavioral sessions. Once the curves were re-determined and effects of antagonists and effects of quinpirole had been re-determined, EtOH access was discontinued and, three weeks later, the reinforcing effects of selected cocaine doses were assessed once more in three monkeys (R-1606, R-1607 and R-1759).
2.6. Acute EtOH treatment
After all above experiments were completed, the effect of acute infusions of EtOH on cocaine self-administration was determined in four monkeys (R-1605, R-1606, R-1607 and R-1758). These experiments were conducted after home cage EtOH access had been discontinued, while daily cocaine self-administration continued. Once cocaine self-administration was stable, the monkey was seated in a primate chair in the room where self-administration occurred. A needle inserted into the VAP was connected via tubing to a syringe in an infusion pump (Cole-Parmer, Vernon Hills, IL) that delivered saline or a 20% EtOH solution at a rate calculated to deliver a specific EtOH dose (0.25, 0.5 or 1.0 g/kg) over 10 minutes. Following the infusion, the monkey was immediately placed into the operant conditioning chamber and a self-administration session began. Saline and each dose of EtOH was tested twice in combination with a low cocaine dose that resulted in approximately 3 or fewer injections being delivered (0.001 mg/kg per injection for R-1607, 0.003 mg/kg per injection for R-1606 and R-1758 and 0.01 mg/kg per injection for R-1605). Saline and 1.0 g/kg EtOH were then tested twice in combination with the maintenance dose of cocaine (0.1 mg/kg per injection).
2.7. Data presentation and analysis
Because the potency of cocaine as a reinforcer and the potency of quinpirole to elicit yawns differed across monkeys, individual data are presented. Regarding the effects of DA receptor antagonists, for each drug, an ED50 value (that is, the dose calculated to produce a 50% decrease in self-administration) was determined in each monkey by interpolation of the linear portion of the dose-effect curve. A paired t-test was used to compare the average ED50 before and during the period of EtOH drinking. To analyze effects of acute EtOH administration on cocaine self-administration, group-averaged data were subjected to a one-way ANOVA for each dose of cocaine, followed by a Dunnett’s test to determine which EtOH treatments resulted in a significant difference form the effects of saline infusion.
2.8. Drugs
Ethanol (95% ethyl alcohol) was obtained from The Warner-Graham Company (Cockeysville, MD) and diluted each morning prior to mixing with Tang. (−)-Cocaine HCl was obtained from the National Institute on Drug Abuse (Bethesda, MD). (−)-Quinpirole hydrochloride, R(+)-SCH23390 hydrochloride, eticlopride hydrochloride and buspirone hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO). Cocaine, quinpirole and eticlopride were dissolved in sterile 0.9% saline. Buspirone and SCH 23390 were dissolved in sterile water. Drugs, were sterile-filtered prior to intravenous administration. Doses are expressed based on the salt form.
3. RESULTS
3.1. Initial EtOH drinking
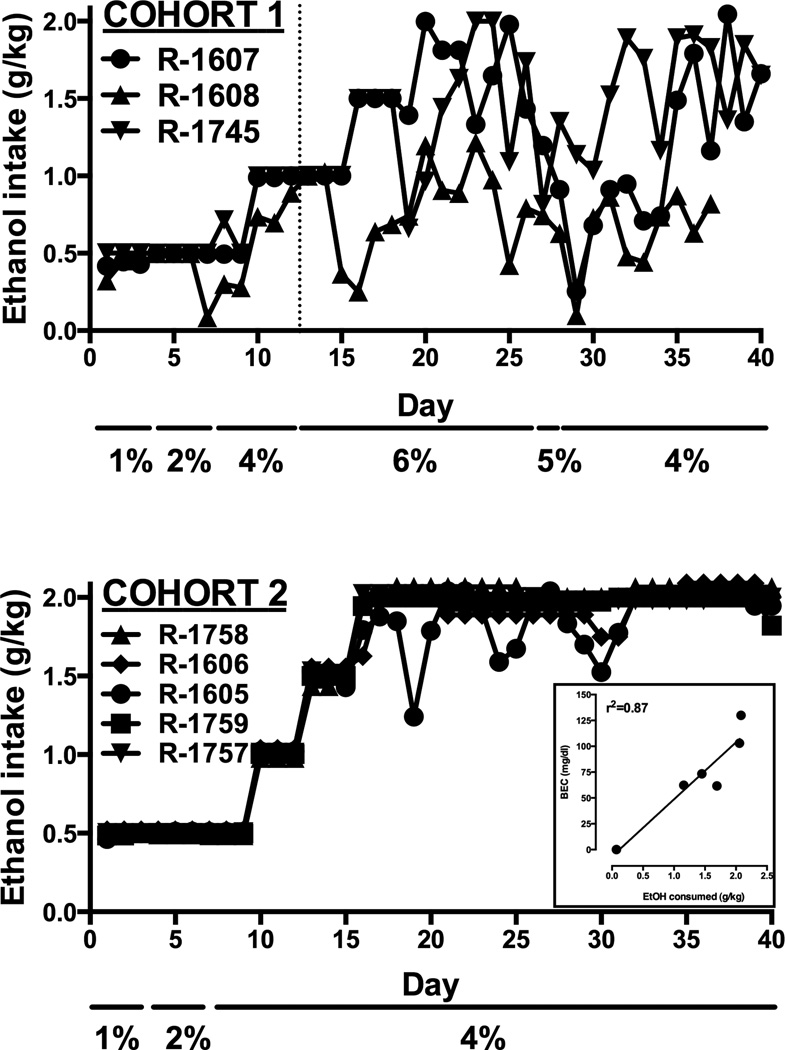
In the first group of three monkeys exposed to this procedure (Cohort 1), as the EtOH concentration and maximum dose was increased over the first 12 days, two monkeys reliably consumed all available EtOH and the third (R-1608) approached maximum intake (Fig. 1, left). On day 13 the EtOH concentration was increased to 6% and the maximum allowed EtOH consumption continued to be increased every three days (see Table 1). Access to this EtOH concentration disrupted EtOH consumption in all monkeys. Therefore, on Day 28 the concentration was decreased to 5% and then to 4% on Day 30. EtOH intake gradually stabilized at near-maximal levels by day 40 in two of the three monkeys. In the second cohort of five monkeys, EtOH concentration was not increased above 4% (see Table 1) and as a result, EtOH consumption was more stable and reached maximal levels in all monkeys (Fig. 1, right). The total amount of EtOH consumed over approximately 8 weeks is shown in Table 2. A significant positive correlation (r2=0.87, p<0.01) was observed between the amount of EtOH consumed and resulting blood ethanol concentration as measured by gas chromatography (Fig. 1, bottom, inset).
Figure 1.
Initial ethanol drinking in two cohorts of rhesus monkeys. Ordinates: Ethanol intake (g/kg), abscisse: Day of ethanol exposure. Additional numbers along abscissae indicate the concentration on ethanol available each day. Inset: Relationship between amount of EtOH consumed and blood ethanol concentration.
Table 2.
EtOH consumption before re-determination of the cocaine self-administration dose-effect curvea or an equivalent time periodb.
| Sessions | Total intake (g/kg) | |
|---|---|---|
| Cohort 1 | ||
| R-1607a | 37 | 39.0 |
| R-1608a | 37 | 23.9 |
| R-1745b | 39 | 46.9 |
| Cohort 2 | ||
| R-1605a | 40 | 58.7 |
| R-1606a | 39 | 59.2 |
| R-1758a | 38 | 58.7 |
| R-1759a | 40 | 61.0 |
| R-1757b | 38 | 58.0 |
| Mean ± SD | 38.9 ± 1.1 | 55.6 ± 7.7 |
3.2. Effects of EtOH drinking on cocaine self-administration
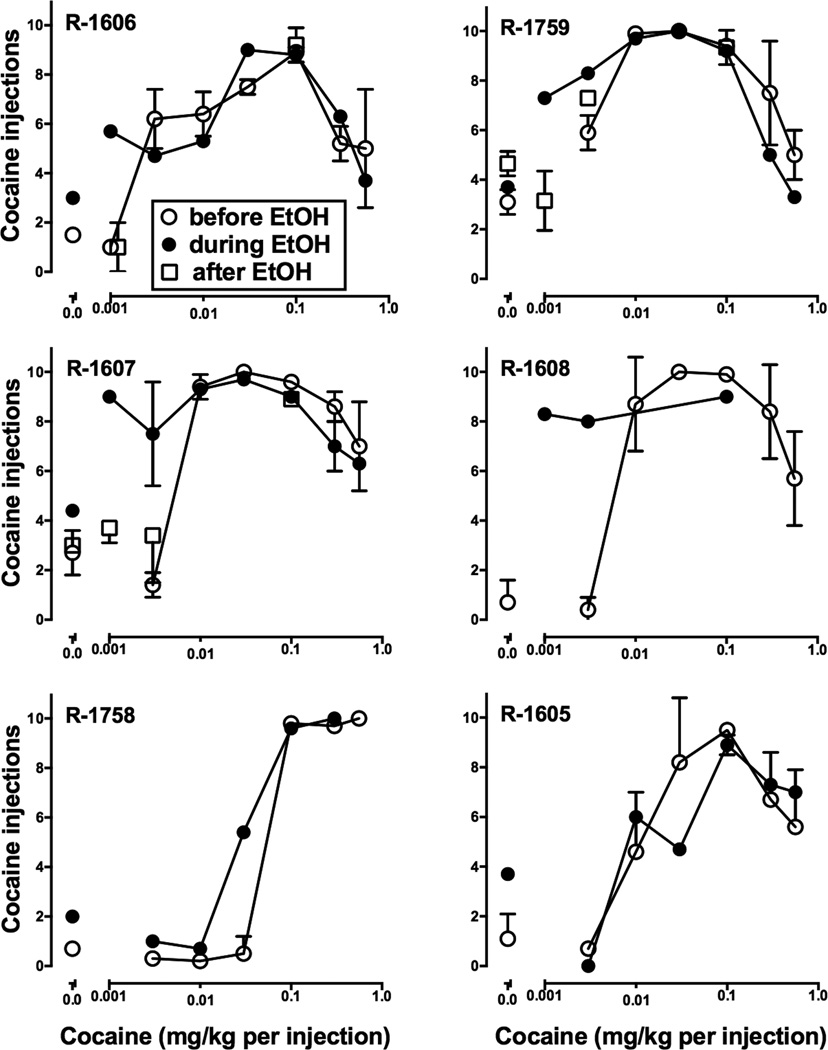
Prior to EtOH exposure, cocaine dose-effect curves were typical of self-administration under a FI 300-sec schedule. Increasing the available dose of cocaine resulted in an increase in infusions delivered that approached or reached the maximum (10 infusions) when 0.01–0.1 mg/kg cocaine was self-administered (Fig. 2). Availability of higher cocaine doses resulted in a decreases in the number of infusions delivered in five of six monkeys. Once monkeys had consumed EtOH 5 days per week for approximately 8 weeks in the absence of cocaine (see Table 2), the dose-effect curve for cocaine self-administration was re-determined. Five of the six monkeys (all except R-1605) self-administered more cocaine at one or more doses than they had before EtOH drinking began (Fig. 2). Typically, lower doses of cocaine that lacked reinforcing effects at baseline were robustly self-administered after 8 weeks of drinking EtOH. Higher doses representing the peak and descending limbs of the curves were not affected, and there was no noticeable effect of cocaine self-administration on home-cage EtOH drinking later in the day (not shown). Once experiments with DA receptor antagonists and quinpirole were completed, daily EtOH drinking was discontinued and, after three weeks, the reinforcing effects of selected doses were again determined in three monkeys (Fig. 2). Discontinuing daily EtOH drinking did not alter self-administration of the maintenance dose of cocaine. However, lower cocaine doses, which had been self-administered during the period of EtOH drinking, no longer had reinforcing effects.
Figure 2.
Cocaine self-administration before (n=6), during (n=6) and after (n=3; R-1606, R-1759, R-1607) the period of daily ethanol drinking in individual monkeys. Note that, for R-1606, the 0.001 mg/kg “after EtOH” data point was slightly offset to better distinguish it from the “before EtOH” point at that dose. Ordinates: Number of cocaine injections, abscissae: cocaine dose (mg/kg per injection) available for self-administration.
3.3. Effects of EtOH drinking on the effects of dopamine receptor antagonists
Administration of SCH 23390, eticlopride and buspirone resulted in dose-dependent decreases in responding maintained by 0.1 mg/kg cocaine in all monkeys both before and during the EtOH-drinking period. Analysis of ED50 values indicated that the effects of these drugs did not differ significantly once monkeys had accumulated a history of drinking EtOH (Table 3).
Table 3.
ED50 values of DA receptor antagonists for decreasing self-administration of 0.1 mg/kg cocaine.
| Before EtOH | During EtOH | |
|---|---|---|
| SCH 23390 | ||
| R-1607 | 0.007 | 0.006 |
| R-1758 | 0.004 | 0.004 |
| R-1606 | 0.005 | 0.002 |
| Mean | 0.005 | 0.004 |
| SD | 0.002 | 0.002 |
| Eticlopride R-1607 | 0.116 | 0.081 |
| R-1758 | 0.067 | 0.139 |
| R-1606 | 0.037 | 0.168 |
| R-1757 | 0.238 | 0.019 |
| Mean | 0.114 | 0.102 |
| SD | 0.089 | 0.066 |
| Buspirone | ||
| R-1607 | 0.748 | 0.804 |
| R-1758 | 0.581 | 0.085 |
| R-1757 | 0.127 | 0.110 |
| Mean | 0.485 | 0.333 |
| SD | 0.322 | 0.409 |
3.4. Effects of EtOH drinking on quinpirole-induced yawning
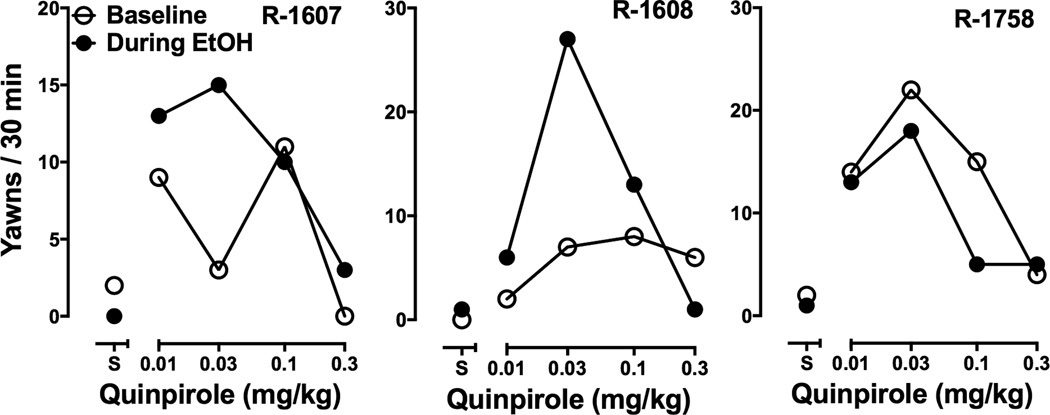
Saline administration resulted in fewer that three yawns in all three monkeys regardless of EtOH drinking status (Fig. 3). The D2-like DA receptor agonist quinpirole elicited yawning as reported previously (Martelle et al., 2007). In R-1607 and R-1608, the effects of quinpirole were greater during the period that EtOH was being consumed. The effects of quinpirole did not differ in R-1758; although fewer yawns were observed during the period of EtOH drinking, the rate of yawning after saline administration was slightly higher.
Figure 3.
Quinpirole-induced yawning in individual monkeys. Ordinates: number of yawns during the 30-min observation period, abscissae: dose (mg/kg) of quinpirole or saline, S, administered.
3.5 Effects of acute EtOH infusions on cocaine self-administration
When monkeys self-administered a relatively low dose of cocaine which resulted in delivery of approximately three injections, i.v. administration of saline, 0.25 or 0.5 g/kg EtOH before the session did not alter cocaine self-administration, and 1.0 g/kg EtOH decreased responding (Table 4). A repeated-measures one-way ANOVA determined a main effect of EtOH dose (F4,12=5.35, p = 0.01), and a post-hoc Dunnett’s test revealed a significant effect of the highest EtOH dose (p<0.05). When monkeys self-administered the maintenance dose of cocaine, i.v. administration of saline or 1.0 g/kg EtOH was without effect.
Table 4.
Cocaine injections delivered (± SEM, n=4) at baseline and after i.v. infusions of saline or EtOH.
| Low dose of cocaine (0.001 – 0.01 mg/kg per injection) | |
| Infusion | Injections |
| no infusion | 2.8 ± 0.4 |
| saline | 2.9 ± 0.5 |
| 0.25 g/kg EtOH | 2.9 ± 0.3 |
| 0.5 g/kg EtOH | 2.0 ± 0.2 |
| 1.0 g/kg EtOH | 1.1 ± 0.5* |
| Maintenance dose of cocaine (0.1 mg/kg per injection) | |
| Infusion | Injections |
| no infusion | 9.1 ± 0.4 |
| saline | 8.6 ± 0.1 |
| 1.0 g/kg EtOH | 9.0 ± 0.2 |
, p < 0.05 compared to corresponding saline point.
4. DISCUSSION
The primary finding of these studies is that chronic (~8-week) binge-like consumption of EtOH by rhesus monkeys resulted in increased reinforcing effects cocaine. Responding during availability of low cocaine doses, which was not different from responding for saline injections prior to EtOH consumption, was robustly increased in most animals. That the change in cocaine reinforcement over time was due to EtOH exposure, rather than a simple drift in cocaine’s effects over time, was confirmed in three monkeys by re-examining the relevant cocaine does after EtOH access was discontinued. When the reinforcing effects of those doses were retested, responding had returned to saline-like baseline levels. Moreover, previous studies have demonstrated that continued access to self-administered cocaine is associated with tolerance to the effects of higher cocaine dose with no change in potency and no increase in reinforcing effects of lower does (Czoty et al., 2006).
A noteworthy feature of the design of these experiments is that exposure to EtOH and cocaine were separated in time. This was done to avoid two potential confounds. First, EtOH was made available after cocaine each day in order to avoid the monkeys being intoxicated or in acute withdrawal during cocaine self-administration sessions. Second, co-administration of EtOH and cocaine results in generation of cocaethylene. This metabolite could confound results because it has pharmacodynamic, neurochemical and behavioral effects similar to cocaine, with a longer half-life (e.g., Hearn et al., 1991; Iyer et al., 1995; Jatlow et al., 1991; Katz et al., 1992). An additional concern is that cocaethylene could potentiate the cardiovascular toxicity associated with cocaine and EtOH use. Because EtOH consumption occurred in the afternoon, several hours after cocaine self-administration concluded, the increase in cocaine self-administration observed in these studies is more likely to be the result of neuroadaptations occurring during long-term EtOH exposure. Moreover, data from previous experiments (Aspen and Winger, 1997; Winger et al., 2007) indicate that acute interactions between cocaine and EtOH do not increase in cocaine’s reinforcing effects. Consistent with these findings, in the present study, when EtOH was administered intravenously just prior to cocaine self-administration, it did not increase self-administration of low cocaine doses. Although this seems to be the most parsimonious explanation for the data, an alternate possibility that could be addressed in future studies is that several weeks of EtOH drinking resulted in differential tolerance developing to EtOH’s effects on cocaine reinforcement compared to its sedating effects. A secondary observation of note was that monkeys continued to consume all available EtOH during the afternoon, several hours after cocaine had been self-administered.
Although EtOH and cocaine interact with a number of neurotransmitter systems, brain DA function represents a likely area of convergence. Across species, EtOH shares with cocaine the ability to acutely increase brain DA concentrations (e.g., Bradberry, 2002; Di Chiara and Imperato, 1988; Yoder et al., 2009), which has long been identified as the primary mechanism of cocaine’s abuse-related effects (e.g., Koob and Volkow, 2010; Pierce and Kumaresan, 2006). Moreover, brain-imaging studies in both cocaine and alcohol abusers have indicated that chronic consumption results in decreased brain DA D2-like receptors (for review see Cosgrove 2010) and a hypofunctional state of DA neurotransmission (e.g., Henry et al., 2009; Karkhanis et al., 2015; Parsons et al., 1991; Weiss et al., 1996). The present studies examined whether 8 weeks of EtOH consumption modified the function of D1- or D2-like DA receptors by examining whether EtOH consumption altered the well-documented ability of antagonists to reduce cocaine self-administration (e.g., Bergman et al., 1990; Nader et al., 1999; Woolverton and Virus, 1989; Xi et al., 2005). On average, there were no consistent differences in the ED50 values of the D1-like receptor antagonist SCH 23390, the D2-like receptor antagonist eticlopride or the D3/D4-like antagonist receptor buspirone. Although these results suggest a lack of effect of 8 weeks of binge-like EtOH consumption on DA receptor function, it is possible that the decrease in responding reflects a general disruption of behavior rather than a specific reduction of the reinforcing effects of cocaine.
To examine a potentially more subtle change in D3 receptor sensitivity, quinpirole-elicited yawning was assessed before and during the period of EtOH drinking. Studies in rats have demonstrated that the ascending limb of the quinpirole-induced yawning curve is mediated by D3 receptor stimulation, and that stimulation of D2 receptors by higher quinpirole doses attenuates this effect (Collins et al., 2005, 2007). In the present study, the ability of quinpirole to increase yawning was enhanced during following 8 weeks of EtOH consumption in two of the three monkeys. Interestingly, the one subject that was not affected (R-1758) also displayed a different pattern of effects of EtOH on cocaine self-administration: the reinforcing effects of an intermediate dose, rather than a low dose, was affected in that monkey (see Fig. 2). This subject was also the only one of the six monkeys whose baseline cocaine self-administration dose-effect curve lacked a descending limb, perhaps indicating that this monkey was less sensitive to both EtOH and cocaine. In addition, R-1758 had consumed EtOH for much longer than the other two subjects at the time of the re-determination of quinpirole-induced yawning (54 weeks vs. 11 weeks). Thus it is possible that an EtOH-induced augmentation of quinpirole’s effects may have occurred earlier in R-1758’s drinking history and dissipated. Future studies are needed to more definitively characterize the effects of EtOH consumption on D3 receptor function.
There results suggest that 11 weeks of EtOH drinking may increase cocaine reinforcement via alterations in D3 (and possibly D2) receptors. There is great interest in drugs that target D3 receptors as potential pharmacotherapies for abuse of several drugs, including cocaine and alcohol (Heidbreder and Newman, 2010). Data from rodent models suggests that D3 receptor antagonists can decrease EtOH consumption (Thanos et al., 2005; Heidbreder et al., 2007; Rice et al., 2012). Moreover, D3 receptors have been shown to be altered by chronic EtOH drinking in rodents and humans (Erritzoe et al., 2014; Vengeline et al., 2006). Although an increase in D3 function may seem counterintuitive considering that chronic EtOH has been shown to decrease D2-like receptor availability and dopamine levels (see above). However, prior brain imaging studies showing decreases in D2-like receptors in cocaine and alcohol abusers have used radiotracers that do not discriminate between subtypes of D2-like receptors. It is possible that the overall decrease in D2-like receptors is the net effect of a large decrease in the more plentiful D2 subtype and an increase in the less numerous, more spatially restricted D3 subtype. This hypothesis is supported by recent brain imaging studies that used more selective radiotracers to document elevations in D3 receptor availability in chronic cocaine users and alcoholics (Erritzoe et al., 2014; Payer et al., 2014). Future studies should more closely investigate the effects of EtOH and combined cocaine/EtOH exposure on D3 and D2 receptor number and function, in addition to assessing potential involvement of other neurotransmitter systems.
Results of the present studies suggest that chronic but not acute EtOH exposure can increase sensitivity to the reinforcing effects of low doses of cocaine. It is important to note that there are other behavioral mechanisms that may also promote co-abuse that were not examined. For example, it is clear that some cocaine users drink alcohol during cocaine binges to mitigate negative effects of cocaine such as insomnia, anxiety and agitation (e.g., Chitwood, 1985). Such effects have been observed in laboratory animals as well. For example, relatively low doses of cocaine were found to attenuate the anxiolytic effects of EtOH in rats, even though these cocaine doses increased ataxic effects of EtOH (Aston-Jones et al., 1984). Thus, the interactions identified in the present study may represent only one possible mechanism by which chronic EtOH and cocaine interact. Future studies should directly examine whether anxiolytic effects of EtOH play a role in enhancing cocaine self-administration or ameliorating putative withdrawal or other negative effects.
The results of these studies have important implications for both preclinical and clinical research and treatment of cocaine and alcohol co-abuse. In the process of developing putative pharmacotherapies for cocaine abuse, for example, the vast majority of laboratory animal experiments expose animals to only the abused drug of interest. However, if chronic EtOH consumption alters the behavioral pharmacology of cocaine, hypotheses generated in subjects exposed only to cocaine may not be translatable to most cocaine users who also use alcohol. Thus it is possible that, in an effort to control for polysubstance abuse, researchers have created a pool of subjects that are not representative of the clinical population they are intended to model. Despite the added cost and complexity of experimental design, preclinical researchers should consider incorporating exposure to multiple abused substances into models that assess the effectiveness of putative medications. A similar suggestion could be made for the design of clinical trials. If chronic alcohol use alters the effects of cocaine, then clinical trials of putative pharmacotherapies should not exclude polysubstance abusers. A clinical trial examining the efficacy of modafinil for cocaine dependence (Anderson et al., 2009) suggests that this is a legitimate concern. When given to cocaine-dependent patients for 12 weeks, placebo and modafinil did not differ in their effects on cocaine use. However, in follow-up analyses, modafinil was significantly better than placebo in subjects who had never been alcohol-dependent. Finally, treatment providers currently differ on whether patients may use licit drugs (i.e., alcohol, nicotine and caffeine) during treatment for cocaine dependence. The present results indicating that alcohol use can increase the reinforcing effects of cocaine suggest that alcohol use may detract from efforts to maintain abstinence. Future studies should examine conditions that more closely resemble clinical co-abuse, including concurrent availability of EtOH and cocaine and access to EtOH that is not limited in amount or duration as it was in the present studies.
The vast majority of cocaine abusers also use or abuse alcohol; understanding the behavioral and neuropharmacological interactions of long-term exposure to these drugs is essential in developing effective treatments. In the present studies, 8 weeks of ethanol consumption by rhesus monkeys increased cocaine self-administration; similar behavioral effects were not observed when EtOH was administered acutely. One possible conclusion is that chronic EtOH drinking produced changes in brain substrates that mediate the reinforcing effects of cocaine, possibly D3 and/or D2 receptors.
Highlights.
Chronic EtOH consumption increased self-administration of low cocaine doses.
Self-administration returned to baseline after EtOH drinking was discontinued.
EtOH drinking enhanced quinpirole-induced yawning in 2 of 3 monkeys.
Cocaine self-administration was not increased after acute EtOH exposure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson AL, Reid MA, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciarulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspen JM, Winger G. Ethanol effects on self-administration of alfentanil, cocaine and nomifensine in rhesus monkeys. Psychopharmacology. 1997;130:222–227. doi: 10.1007/s002130050232. [DOI] [PubMed] [Google Scholar]
- Aston-Jones S, Aston-Jones G, Koob GF. Cocaine antagonizes the anxiolytic effects of ethanol. Psychopharmacology. 1984;84:28–31. doi: 10.1007/BF00432019. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D1 and D2 antagonists. Behav. Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Bergman J, Spealman RD. Behavioral effects of histamine H1 antagonists: comparison with other drugs and modification by haloperidol. J. Pharmacol. Exp. Ther. 1988;245:471–478. [PubMed] [Google Scholar]
- Bradberry CW. Dose-dependent effect of alcohol on extracellular dopamine in mesolimbic striatum of awake rhesus monkeys: comparison with cocaine across individuals. Psychopharmacology. 2002;165:67–76. doi: 10.1007/s00213-002-1233-9. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Bryant KJ. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J. Stud. Alcohol. 1993;54:199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- Chitwood DD. Patterns and consequences of cocaine use. NIDA Res. Monogr. 1985;61:111–129. [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J. Pharmacol. Exp. Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP. Imaging receptor changes in human drug abusers. Curr. Top. Behav. Neurosci. 2010;3:199–217. doi: 10.1007/7854_2009_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Influence of abstinence and conditions of cocaine access on the reinforcing strength of cocaine in nonhuman primates. Drug Alcohol Depend. 2006;85:213–220. doi: 10.1016/j.drugalcdep.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe D, Tziortzi A, Bargiela D, Colasanti A, Searle GE, Gunn RN, Beaver JD, Waldman A, Nutt DJ, Bani M, Merlo-Pich E, Rabiner EA, Lingford-Hughes A. In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology. 2014;39:1703–1712. doi: 10.1038/npp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, Cami J. Alcohol and cocaine interactions in humans. J. Pharmacol. Exp. Ther. 1993;266:1364–1373. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Ethanol and cocaine interactions in humans: cardiovascular consequences. Pharmacol. Biochem. Behav. 1988;31:877–883. doi: 10.1016/0091-3057(88)90399-1. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend. 1993;32:93–106. doi: 10.1016/0376-8716(93)80001-u. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Concurrent and simultaneous use of alcohol with cocaine: results of a national survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Hearn WL, Flynn DD, Hime GW, Cofino JC, Mantero-Atienza E, Wetli CV, Mash DC. Cocaethylene: a unique cocaine metabolite displays high affinity for the dopamine transporter. J. Neurochem. 1991;56:698–701. doi: 10.1111/j.1471-4159.1991.tb08205.x. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Andreoli M, Marcon C, Hutcheson DM, Gardner EL, Ashby CR., Jr Evidence for the role of dopamine D3 receptors in oral operant alcohol self-administration and reinstatement of alcohol-seeking behavior in mice. Addict. Biol. 2007;12:35–50. doi: 10.1111/j.1369-1600.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann. N.Y. Acad. Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Badger GJ, Higgins ST. Alcohol dependence among cocaine-dependent outpatients: demographics, drug use, treatment outcome and other characteristics. J. Stud. Alcohol. 2000;62:14–22. doi: 10.15288/jsa.2001.62.14. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Pryzbeck TR. The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J. Stud. Alcohol. 1988;49:219–224. doi: 10.15288/jsa.1988.49.219. [DOI] [PubMed] [Google Scholar]
- Henry PK, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology. 2009;205:237–247. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Badger GJ. Alcohol dependence and simultaneous cocaine and alcohol use in cocaine-dependent patients. J. Addict. Dis. 1994;13:177–189. doi: 10.1300/j069v13n04_06. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Roll JM, Bickel WK. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Rush CR, Hughes JR, Bickel WK, Lynn M, Capeless MA. Effects of cocaine and alcohol, alone and in combination, on human learning and performance. J. Exp. Anal. Behav. 1992;58:87–105. doi: 10.1901/jeab.1992.58-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer RN, Nobiletti JB, Jatlow PI, Bradberry CW. Cocaine and cocaethylene: effects on extracellular dopamine in the primate. Psychopharmacology. 1995;120:150–155. doi: 10.1007/BF02246187. [DOI] [PubMed] [Google Scholar]
- Jatlow P, Elsworth JD, Bradberry CW, Winger G, Taylor JR, Russell R, Roth RH. Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci. 1991;48:1787–1794. doi: 10.1016/0024-3205(91)90217-y. [DOI] [PubMed] [Google Scholar]
- Kampman KM. What’s new in the treatment of cocaine addiction? Curr. Psychiatry Rep. 2010;12:441–447. doi: 10.1007/s11920-010-0143-5. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133:94–99. doi: 10.1016/j.drugalcdep.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Reynaud M, Aubin HJ, Rolland B, Guardia D, Cottencin O, Benyamina A. Pharmacological treatments for cocaine dependence: is there something new? Curr. Pharmacol. Des. 2011;17:1359–1368. doi: 10.2174/138161211796150873. [DOI] [PubMed] [Google Scholar]
- Katz JL, Terry P, Witkin JM. Comparative behavioral pharmacology and toxicology of cocaine and its ethanol-derived metabolite, cocaine ethyl-ester (cocaethylene) Life Sci. 1992;50:1351–1361. doi: 10.1016/0024-3205(92)90286-x. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulous JK, Jones SR. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 2015;150:24–30. doi: 10.1016/j.drugalcdep.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2000;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J. Pharmacol. Exp. Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Masur J, Souza-Formigoni ML, Pires ML. Increased stimulatory effect by the combined administration of cocaine and alcohol in mice. Alcohol. 1989;6:181–182. doi: 10.1016/0741-8329(89)90015-3. [DOI] [PubMed] [Google Scholar]
- Misra AL, Pontani RB, Vadlamani NL. Interactions of cocaine with barbital, pentobarbital and ethanol. Arch. Int. Pharmacodyn. Ther. 1989;299:44–54. [PubMed] [Google Scholar]
- Nader MA, Green KL, Luedtke RR, Mach RH. The effects of benzamide analogues on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1999;147:143–152. doi: 10.1007/s002130051154. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence form chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, Boileau I. Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [11C]-+PHNO. Neuropsychopharmacology. 2014;39:311–318. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci, Biobehav, Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Rech RH, Vomachka MK, Rickert DE. Interactions between depressants (alcoholtype) and stimulants (amphetamine-type) Pharmacol. Biochem. Behav. 1978;8:143–151. doi: 10.1016/0091-3057(78)90331-3. [DOI] [PubMed] [Google Scholar]
- Rice OV, Patrick J, Schonhar CD, Nimg H, Ashby CR., Jr The effects of the preferential dopamine D(3) receptor antagonist S33138 on ethanol binge drinking in C57BL/6J mice. Synapse. 2012;66:975–978. doi: 10.1002/syn.21575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum IM, Daley DC, Cornelius JR, Kirisci L, Thase ME. Disproportionate lethality in psychiatric patients with concurrent alcohol and cocaine abuse. Am. J. Psychiatry. 1996;153:953–955. doi: 10.1176/ajp.153.7.953. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Katana JM, Ashby CR, Jr, Michaelides M, Gardner EL, Heidbreder CA, Volkow ND. The selective dopamine D3 receptor antagonist SB-277011-A attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacol. Biochem. Behav. 2005;81:190–197. doi: 10.1016/j.pbb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Tziortzis D, Mahoney JJ, III, Kalechstein AD, Newton TF, De La Garza R., III The relationship between impulsivity and craving in cocaine-an d methamphetamine-dependent volunteers. Pharmacol. Biochem. Behav. 2011;98:196–202. doi: 10.1016/j.pbb.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Leonardi-Essmann F, Perreau-Lenz S, Gebicke-Haerter P, Drescher K, Gross G, Spanagel R. The dopamine D3 receptor plays an essential role in alcohol-seeking and relapse. FASEB J. 2006;20:2223–2233. doi: 10.1096/fj.06-6110com. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J. Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hintzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol. Clin. Exp. Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hintzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated definiencies in accumbal dopamine and 5-hydroxytryptamine release independent rats. J. Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR. Modification of ethanol's reinforcing effectiveness in rhesus monkeys by cocaine, flunitrazepam, or gamma-hydroxybutyrate. Psychopharmacology. 2007;193:587–598. doi: 10.1007/s00213-007-0809-9. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol. Biochem. Behav. 1989;32:691–697. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed ratio cocaine self-administration in rats. Eur. J. Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ, Kareken DA. When what you see isn’t what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol. Clin. Exp. Res. 2009;33:139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]