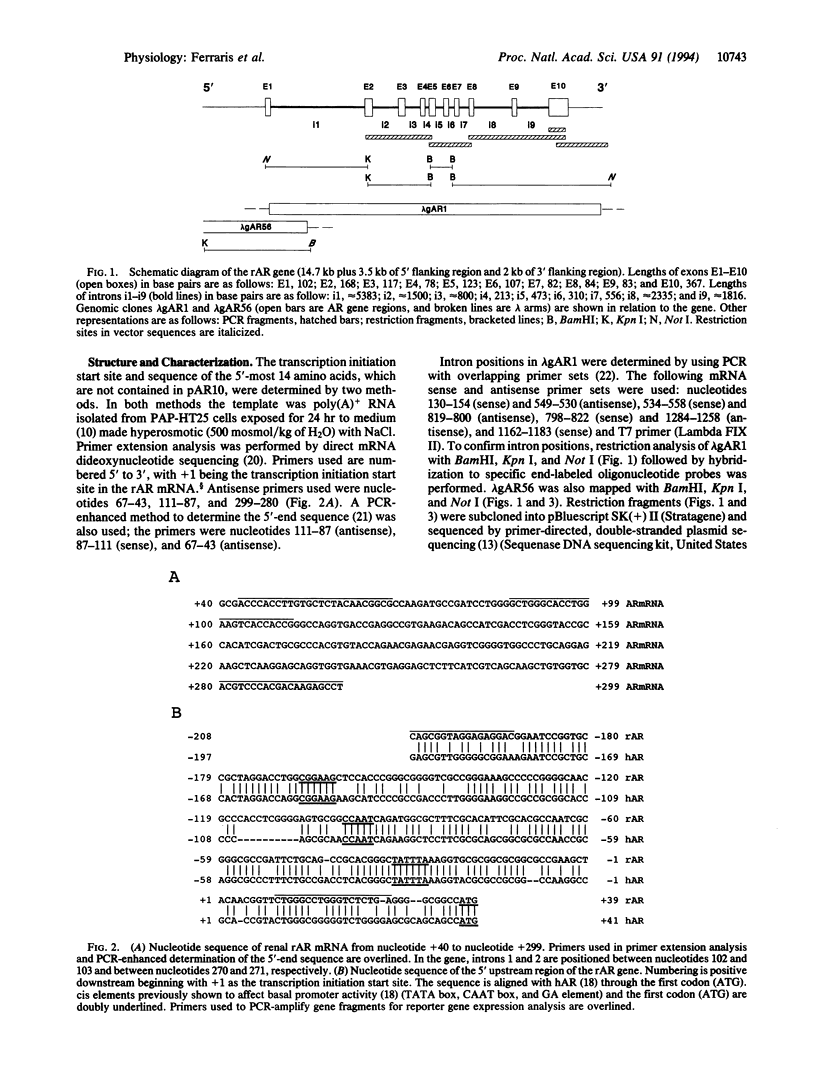
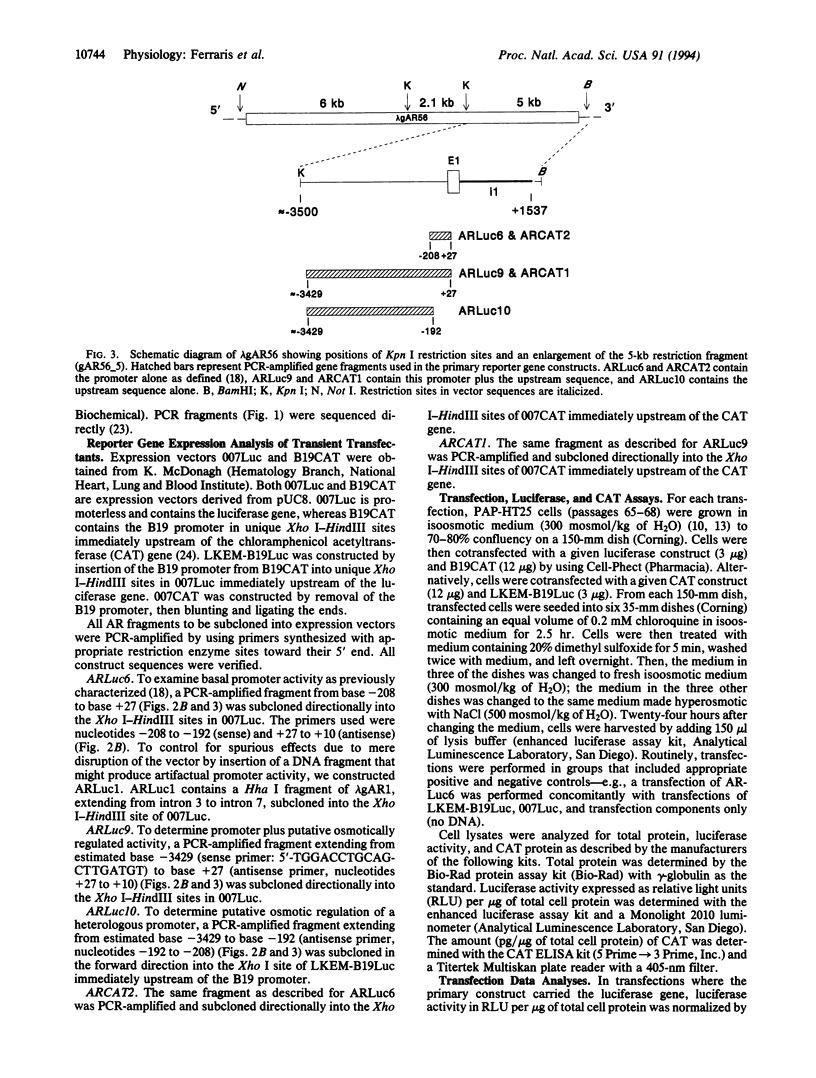
Abstract
Diverse organisms accumulate organic osmolytes to adapt to hyperosmotic stress. The molecular basis of eukaryotic gene osmoregulation remains obscure. Aldose reductase [AR; alditol:NAD(P)+ 1-oxidoreductase, EC 1.1.1.21], which catalyzes the conversion of glucose to sorbitol (an organic osmolyte), is induced in renal medullary cells under hyperosmotic conditions. Elevated extracellular NaCl increases AR mRNA transcription in PAP-HT25 cells, a cell line derived from the rabbit renal papilla. We have cloned and characterized the rabbit AR gene to determine how it is regulated by hyperosmolality. The length of the gene, not including 5' or 3' flanking regions, is approximately 14.7 kilobases (kb) organized into 10 exons and 9 introns. The transcription start site is 36 base pairs upstream of the initiator methionine codon. A 5-kb fragment containing approximately 3.5 kb of 5' flanking region was isolated. The 3.5-kb sequence was examined for basal promoter activity and hyperosmotic response in luciferase reporter gene constructs. A 235-base-pair fragment (base pairs -208 to +27) was able to drive the downstream reporter gene in transfected PAP-HT25 cells under isoosmotic conditions (300 mosmol/kg of H2O). When this fragment plus the remaining upstream sequence (from approximately base pair -3429 to base pair +27) was used, cells in hyperosmotic medium (500 mosmol/kg of H2O) showed about 40-fold induction of luciferase expression compared with cells in isoosmotic medium. The upstream fragment (from approximately base pair -3429 to base pair -192) also conferred osmotic response to a heterologous promoter (B19). This finding evidences putative osmotic response element(s) (OREs) within a specific DNA fragment in a eukaryotic genome. Identification and characterization of OREs within this fragment and their associated trans-acting factors should reveal the molecular mechanisms of gene regulation in osmotic stress.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnasco S. M., Uchida S., Balaban R. S., Kador P. F., Burg M. B. Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1718–1720. doi: 10.1073/pnas.84.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnasco S., Balaban R., Fales H. M., Yang Y. M., Burg M. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem. 1986 May 5;261(13):5872–5877. [PubMed] [Google Scholar]
- Bedford J. J., Bagnasco S. M., Kador P. F., Harris H. W., Jr, Burg M. B. Characterization and purification of a mammalian osmoregulatory protein, aldose reductase, induced in renal medullary cells by high extracellular NaCl. J Biol Chem. 1987 Oct 15;262(29):14255–14259. [PubMed] [Google Scholar]
- Bruzdzinski C. J., Gelehrter T. D. Determination of exon-intron structure: a novel application of the polymerase chain reaction technique. DNA. 1989 Nov;8(9):691–696. doi: 10.1089/dna.1.1989.8.691. [DOI] [PubMed] [Google Scholar]
- Cowley B. D., Jr, Ferraris J. D., Carper D., Burg M. B. In vivo osmoregulation of aldose reductase mRNA, protein, and sorbitol in renal medulla. Am J Physiol. 1990 Jan;258(1 Pt 2):F154–F161. doi: 10.1152/ajprenal.1990.258.1.F154. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Burg M. B. Renal medullary organic osmolytes. Physiol Rev. 1991 Oct;71(4):1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Martin B., Murphy H. R., Uchida S., Murer H., Cowley B. D., Jr, Handler J. S., Burg M. B. Molecular cloning of cDNA coding for kidney aldose reductase. Regulation of specific mRNA accumulation by NaCl-mediated osmotic stress. J Biol Chem. 1989 Oct 5;264(28):16815–16821. [PubMed] [Google Scholar]
- Graham A., Brown L., Hedge P. J., Gammack A. J., Markham A. F. Structure of the human aldose reductase gene. J Biol Chem. 1991 Apr 15;266(11):6872–6877. [PubMed] [Google Scholar]
- Graham C., Szpirer C., Levan G., Carper D. Characterization of the aldose reductase-encoding gene family in rat. Gene. 1991 Nov 15;107(2):259–267. doi: 10.1016/0378-1119(91)90326-7. [DOI] [PubMed] [Google Scholar]
- Hofmann M. A., Brian D. A. A PCR-enhanced method for determining the 5' end sequence of mRNAs. PCR Methods Appl. 1991 Aug;1(1):43–45. doi: 10.1101/gr.1.1.43. [DOI] [PubMed] [Google Scholar]
- Liu J. M., Fujii H., Green S. W., Komatsu N., Young N. S., Shimada T. Indiscriminate activity from the B19 parvovirus p6 promoter in nonpermissive cells. Virology. 1991 May;182(1):361–364. doi: 10.1016/0042-6822(91)90682-2. [DOI] [PubMed] [Google Scholar]
- Mellies J., Brems R., Villarejo M. The Escherichia coli proU promoter element and its contribution to osmotically signaled transcription activation. J Bacteriol. 1994 Jun;176(12):3638–3645. doi: 10.1128/jb.176.12.3638-3645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T., Garcia-Perez A., Burg M. B. Factors affecting the ratio of different organic osmolytes in renal medullary cells. Am J Physiol. 1990 Nov;259(5 Pt 2):F847–F858. doi: 10.1152/ajprenal.1990.259.5.F847. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Garcia-Perez A., Burg M. B. Osmotic regulation of aldose reductase protein synthesis in renal medullary cells. J Biol Chem. 1989 Oct 5;264(28):16810–16814. [PubMed] [Google Scholar]
- Smardo F. L., Jr, Burg M. B., Garcia-Perez A. Kidney aldose reductase gene transcription is osmotically regulated. Am J Physiol. 1992 Mar;262(3 Pt 1):C776–C782. doi: 10.1152/ajpcell.1992.262.3.C776. [DOI] [PubMed] [Google Scholar]
- Uchida S., Garcia-Perez A., Murphy H., Burg M. Signal for induction of aldose reductase in renal medullary cells by high external NaCl. Am J Physiol. 1989 Mar;256(3 Pt 1):C614–C620. doi: 10.1152/ajpcell.1989.256.3.C614. [DOI] [PubMed] [Google Scholar]
- Uchida S., Green N., Coon H., Triche T., Mims S., Burg M. High NaCl induces stable changes in phenotype and karyotype of renal cells in culture. Am J Physiol. 1987 Aug;253(2 Pt 1):C230–C242. doi: 10.1152/ajpcell.1987.253.2.C230. [DOI] [PubMed] [Google Scholar]
- Wang K., Bohren K. M., Gabbay K. H. Characterization of the human aldose reductase gene promoter. J Biol Chem. 1993 Jul 25;268(21):16052–16058. [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]



