Abstract
Purpose
This study primarily sought to determine if the Small Animal Radiation Research Platform (SARRP) can create a rat radiation cystitis (RC) model via targeted bladder irradiation (phase I). The response to treatment of early phase RC in rats via transurethral catheter instillation of liposomal tacrolimus (lipo-tacrolimus) was examined in phase II.
Materials and Methods
In phase I, 16 adult female Sprague-Dawley rats were used and their metabolic urination patterns were analyzed before and after exposure to 20, 30, or 40 Gy radiation. In phase II, irradiated rats were randomly assigned to receive a single instillation of either saline or lipo-tacrolimus.
Results
The 40 Gy radiation dose induced statistically significant reductions in inter-micturition intervals (IMI) compared to the lower doses of radiation. 40 Gy radiation caused a significant reduction in mean IMI by approximately 20 minutes (p < 0.0001). Histological analysis indicated degenerative type epithelial changes and urothelial swelling, with evidence of pseudocarcinomatous epithelial hyperplasia. Therefore, 40 Gy was chosen for the phase II efficacy study. There was no measurable change in total voided urine volume after irradiation or after instillation of lipo-tacrolimus or saline. Lipo-tacrolimus treatment significantly increased post-irradiation IMI values by approximately 30 minutes (p < 0.001) back to baseline levels.
Conclusions
The RC rat model demonstrated a dose-dependent decrease in IMI without inducing short-term skin or gastrointestinal damage. This study demonstrated that lipo-tacrolimus may be a promising new intravesical therapy for the rare and serious condition of RC.
Keywords: radiation cystitis, bladder, liposome, tacrolimus, drug delivery
INTRODUCTION
Radiation is often used in the management of pelvic malignancies, either as primary or adjuvant treatment. The principle aim of radiation therapy (RT) is to deliver the highest possible dose of radiation to a targeted region while simultaneously sparing normal tissues. Nevertheless, in the case of pelvic irradiation, a degree of healthy bladder tissue involvement is inevitable.1
Radiation cystitis (RC) can occur after the completion of pelvic radiotherapy and presents a range of clinical symptoms for which there are no recommended standard management treatments.2 Pelvic irradiation may result in damage to multiple bladder cell types including urothelial, neuronal, detrusor, and vascular smooth muscle cells. RC can reduce bladder capacity and compliance. The extent of the injury can vary depending on several factors, all of which can severely degrade cancer survivors’ quality of life and require long-term follow-up and treatment.3 These cases can be challenging problems to the urologist and a source of substantial morbidity and sometimes mortality for patients.
The discovery and assessment of innovative therapies and treatment-related toxicity in cancer survivorship research has relied heavily on the use of small animals. The current irradiation technique typically used for animals involves simple single beam/single fraction delivery. This greatly differs from that used in human treatment, which incorporates the use of advanced three-dimensional (3D) imaging, planning and computer-controlled delivery. Furthermore, there is a lack of conformity between the uniform doses of radiation used on research animals and the non-uniform doses administered in standard clinical conformal RT.4
A proposed tool to overcome this disparity is an image-guided small animal irradiator, such as the Small Animal Radiation Research Platform (SARRP). SARRP provides a sophisticated method of delivering radiation in the research setting and reconciles many of the concerns associated with standard preclinical radiation devices.5 Current management methods vary by the degree of radiation cystitis. Acute RC is managed through the use of anticholinergics and other agents for symptomatic relief. Chronic RC is treated either systemically with little effect, or with intravesically instilled agents that focus on sterilization, lavage, and arrest of focal bleeding points; however, as doses escalate, intravesical treatments frequently result in increased toxicity.6
Tacrolimus, a calcineurin inhibitor, hinders the production and release of pro-inflammatory cytokines in T-cells. It serves as a potent immunosuppressant that improves barrier function of the skin and mucosa.7 Systemic administration has a high incidence of adverse events, such as nephrotoxicity and hypertension, which increases morbidity due to a direct inhibitory effect on cell mediated immunity.8, 9 However, when used site-specifically to treat dermal inflammatory conditions in an ointment or lotion formulation, minimal adverse events occur.10
This knowledge has prompted studies to investigate intravesical instillation of tacrolimus into the bladder as a potential treatment for radiative, hemorrhagic cystitis. However, the delivery of this pharmaceutical is limited due to its hydrophobic nature. When used with hydrophilic substances such as liposomes, tacrolimus becomes highly soluble.11 Pharmacokinetic study revealed that the use of liposomes as a delivery vehicle increased endocytosis while decreasing systemic exposure and vehicle related toxicity in comparison to non-encapsulated tacrolimus in rats.12, 13
In this current study, we hypothesized that the SARRP would permit the development of a rodent model for acute radiation cystitis. Using this model, we further hypothesized that liposome-encapsulated tacrolimus would be an effective treatment for this condition.
MATERIALS AND METHODS
Animals
Forty three-month-old, 250 g ± 50 g adult female Sprague-Dawley rats (Strain #400, Charles River Laboratories, MA) were used. The protocol was approved by the Institutional Animal Care Committee, Beaumont Health System, Research Institute, Royal Oak, Michigan (IACUC approval number: AL-12-11). 16 rats were used for the development of a RC animal model (phase I). These were arranged into four groups of four, where each group received a different amount of radiation. One rat in each group served as a control. Upon developing a suitable RC rat model, we used 24 rats for the treatment efficacy study (phase II). Three were used as controls and 21 received 40 Gy radiation. Of those irradiated, 12 were treated with lipo-tacrolimus and 9 were given saline.
Equipment
Metabolic Cages
The cages allowed free access to food and water and separated urine from feces. On the day of a micturition study, the animals were placed into cages at mid-afternoon. All voiding measurements were taken over a 12-hr overnight period.
Balances
An Ohaus Scout Pro SP401 balance was placed below each metabolic cage with a urine collection container. These were interfaced with a computer that recorded the time and the mass of urine every five seconds.
SARRP
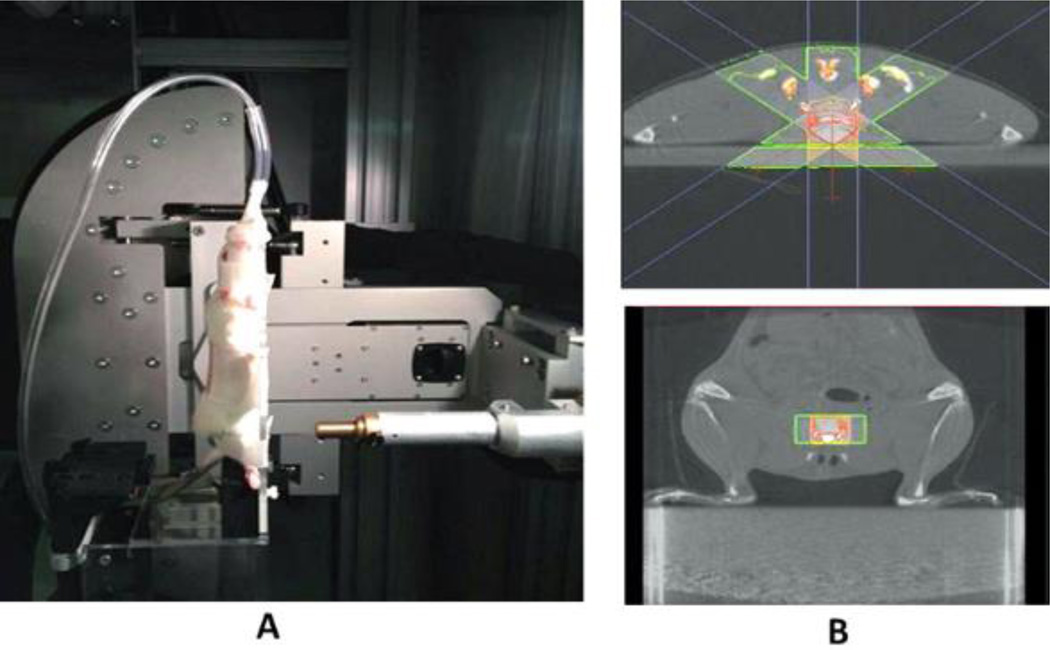
For SARRP irradiation (225 KVp, Xstrahl, Gulmay Medical Inc., Suwanee, GA) and imaging, rats were anesthetized using 1.5 – 2% isoflurane and transferred to a warmed stage for a tail vein injection of Computed Tomography (CT) contrast for bladder visualization. After injection, the animals were positioned vertically on the SARRP platform. Anesthesia was maintained throughout imaging and irradiation. A contrast-enhanced CT was taken using the SARRP. The target for the irradiation was set in the center of the bladder. Treatment planning software determined the precise beam arrangement needed to limit toxicity and focus the total dose on the bladder alone (Figure 1). The animals recovered in a warming tank and returned to general housing.
Figure 1.
The SARRP animal stage was modified to accommodate rats positioned vertically. (A). SARRP X-ray unit in treatment position. (B). SARRP CT with bladder targeting using Slicer-3D treatment planning software. The volume highlighted in red will receive the total dose split amongst three beams to spare skin and normal tissue.
Lipo-Tacrolimus
A formulation of tacrolimus encapsulated in multi-lamellar sphingomyelin liposomes was obtained from Lipella Pharmaceuticals (Pittsburgh, PA), prepared as 10% w/w using a previously described method.13 For treatment, 20 mg of lipo-tacrolimus powder was suspended with 10 mL sterile saline. A 0.5 mL volume was used for instillation.
Studies
Phase I
After a one-week acclimation, baseline micturition measurements were acquired twice weekly for two weeks. Each group received a unique dose of bladder-targeted radiation via the SARRP: 0, 20, 30, or 40 Gy. Following irradiation, animals were returned to social, general housing for a minimum of 24 hours before continuing metabolic cage measurements twice per week for a total of six weeks. The animals were placed in these housing units between each measurement. Radiation doses and treatment plans were developed to produce acute effects in the bladder in order to develop a viable bladder model for the evaluation of lipo-tacrolimus.
Histology
Six weeks after irradiation, all rats were euthanized and the bladder was harvested and cryopreserved using Tissue Freezing Medium gel (TFM-5, Triangle Biomedical Sciences) for histological analysis. The cryopreserved bladder was cut into 8 µm thick sections and the air dried frozen sections were fixed in 10% buffered formalin for 20 minutes and stained with Hematoxylin & Eosin (H&E). Stained sections were sealed with Permount mounting media using glass coverslips.
Analysis
IMI distributions were obtained from the voiding studies via a program that iterates through the raw balance data to find the intervals between each micturition event. A micturition event was defined as weight increase in excess of 150 mg over each five-second period. For each group, the IMIs before radiation were compared with the values after radiation using a (non-parametric) Wilcoxon rank-sums test due to a small sample size.
Phase II
Tests for lipo-tacrolimus treatment efficacy were initiated following findings that 40 Gy bladder irradiation causes a significant decrease in IMI. Initially, four baseline voiding studies for each rat were performed over two weeks. 40 Gy of radiation was administered to 21 rats. Four post-radiation voiding studies were completed over a two-week period following the minimum 24-hour general housing rest period. 12 rats received an intravesical instillation of lipo-tacrolimus and 9 received saline. Four measurements were taken post-instillation over a two-week period.
Instillation
Rats were anesthetized using 1.5–2% isoflurane and given an injection of buprenorphine variable to body weight. Intravesical instillation of lipo-tacrolimus was accomplished using a catheterized transurethral approach. The 0.5 mL volume was instilled over a period of 30 minutes. Subjects were then recovered from anesthesia and returned to general housing.
Analysis
The average IMI at each time-point was used in the analysis. A one-way ANOVA test was performed. The data were grouped based on time-points (baseline, post-radiation, and post-instillation) and the treatment groups (saline and lipo-tacrolimus). Multiple logistic regression analysis was performed using SAS JMP software to identify any confounding variables. Post-hoc analysis utilized pair-wise Student’s t-tests. The time-points and treatment groupings resulted in fifteen total pair-wise comparisons. Voiding consistency was analyzed by applying the pair-wise t-tests to the total volume from each measurement.
Statistical Analysis
The chosen α level is 0.05. The Bonferroni correction was applied to establish significance for the pair-wise t-tests.14
RESULTS
Radiation Tolerance
Contrast-enhanced CT-guided irradiation on the SARRP was well tolerated by animals in all groups. There was no animal mortality, weight loss, or significant damage to skin or gastro-intestinal function, although some hair loss was noted at higher doses.
Phase I
RC Rat Model
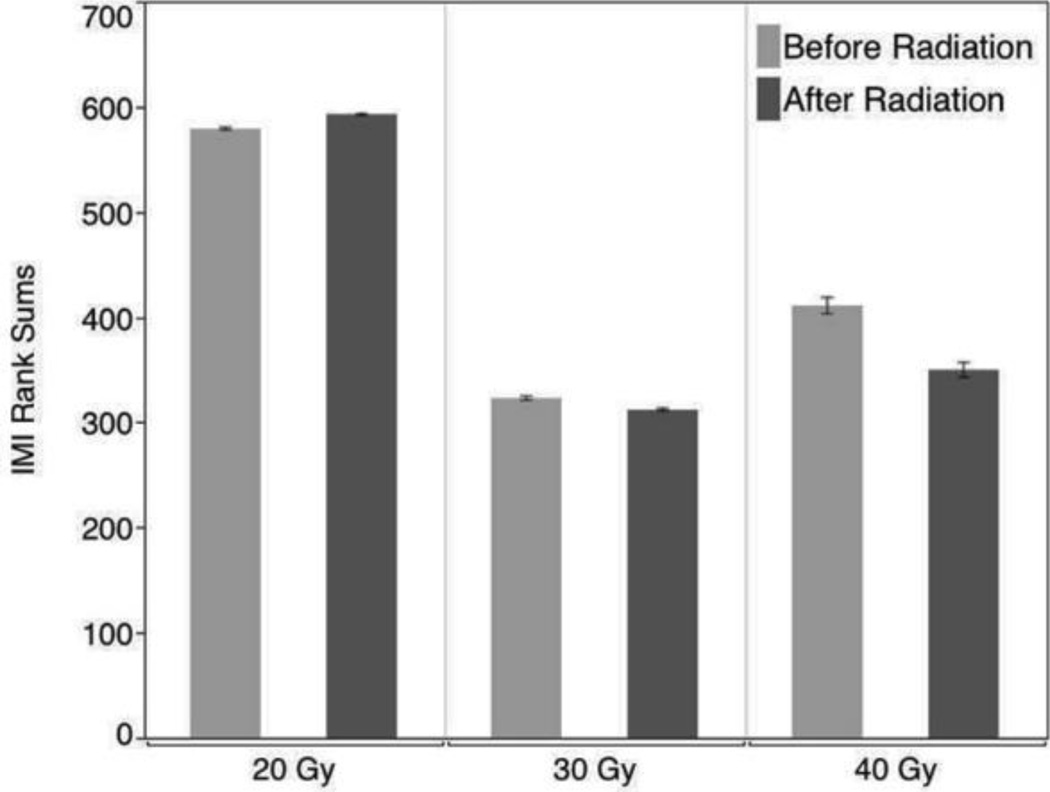
Baseline and post-radiation IMIs were not significantly different for the 20 Gy group (p = 0.69). Baseline IMI signals for the remaining two dosage groups (30 Gy and 40 Gy) were not statistically distinguishable. The score means of IMI rank-sums for each three-rat dose group were 127 and 131, respectively. This difference is relatively small, and thus the null hypothesis (that the baseline radiation IMI signals for both groups are the same) cannot be rejected. The p-value of the corresponding chi-square test is 0.67. Application of 30 Gy and 40 Gy radiation doses caused a dose-dependent change in IMI probability density. With both increased doses, radiation appeared to affect the IMI signal by shifting the probability density functions toward shorter IMI values. However, only the effect of the 40 Gy dose was strong enough to arrive at a statistically significant effect (p = 0.0024). Figure 2 shows the relative differences between the before and after groups at each radiation level.
Figure 2.
Relative IMI rank-sum scores based on radiation treatment strength. The bladder was irradiated with 20, 30 and 40 Gy doses. 20 Gy did not show significant change in the IMI probability density. When compared with 30 Gy, the 40 Gy radiation treatment produced more of a significant decrease in the IMI distribution with a statistically significant effect (p = 0.0024).
Histology
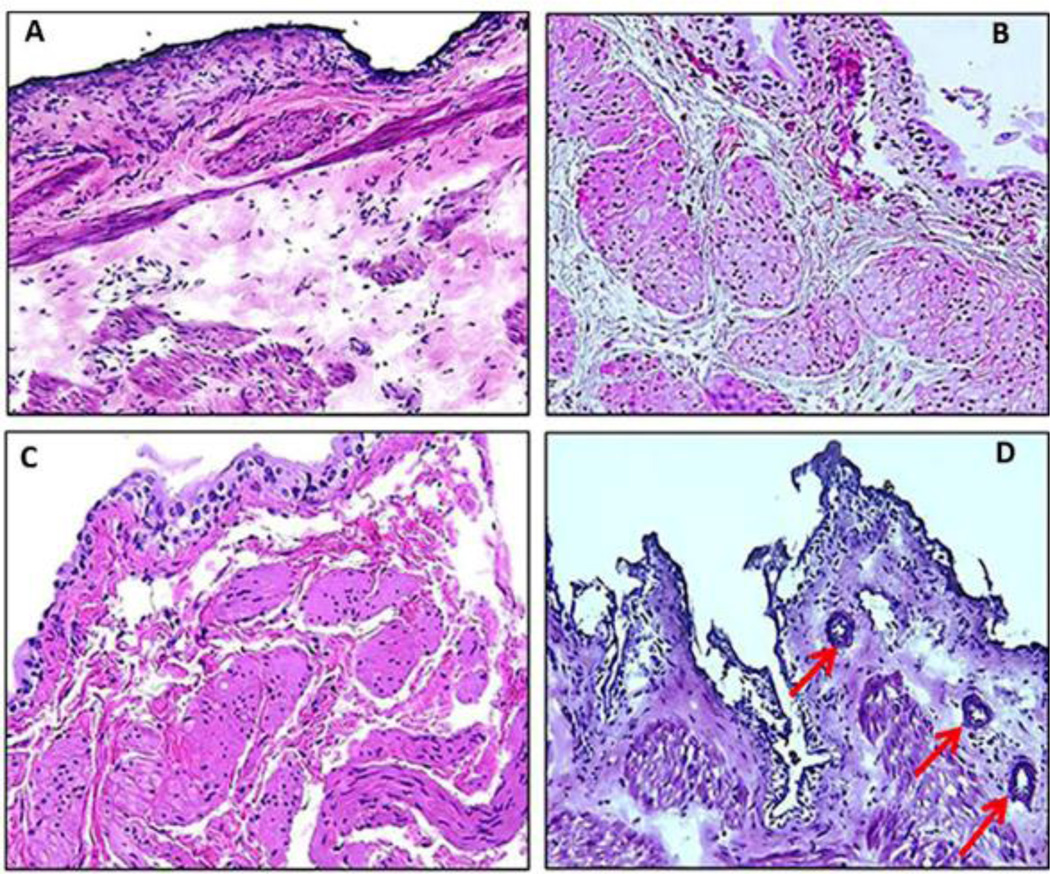
H&E staining of the harvested bladder tissue from different treatment groups was performed to assess histological changes induced by radiation (Figure 3). Bladders from the 20 Gy group did not show any inflammatory changes (Figure 3B). The 30 Gy group showed edematous changes in the lamina propria with accompanying infiltration of immune cells, with ectactic blood vessels in the lamina propria and hyperplastic urothelium (Figure 3C). Degenerative type epithelial changes were seen in bladder harvested from the 40 Gy group, which includes cytoplasmic ballooning due to severe mucosal edema and small nests of urothelial cells in the lamina propria surrounding the blood vessels. The histological findings in Figure 3D are indicative of pseudocarcinomatous urothelial hyperplasia of the bladder, a condition associated with RT. Histology of control bladder (not exposed to radiation) is shown for comparison (Figure 3A).
Figure 3.
H&E staining of the harvested bladder tissue from different treatment groups (Panel A-D). Histology of bladder from control rat group not exposed to radiation is shown for comparison (Panel A). Rat bladder from group irradiated with 20 Gy (Panel B) showed no inflammatory changes whereas group irradiated with 30 Gy (Panel C) showed edematous changes accompanying infiltration of inflammatory cells, dilatation of blood vessels and hyperplastic urothelium. Panel D shows degenerative type epithelial changes in the bladder harvested from rat group irradiated with 40 Gy, which include cytoplasmic ballooning and pseudocarcinomatous epithelial hyperplasia (arrow). Images were taken at 20× magnification.
Phase II
Voiding Consistency
Analyses were performed to determine if the total amount of urine voided during the measurements remained constant throughout the study. Multiple logistic regression analysis separated the confounding factors (treatment and time-point). The p-values for these were 0.25 and 0.22, respectively, which suggested that the volume did not change throughout the study due to either of these factors. A multiple-pairs t-test analysis between each of the time points in each treatment group (Table 1) confirmed that the mean total volume did not significantly differ throughout the study.
Table 1.
The effect of 40 Gy radiation and treatment on the IMIs and total volume for the saline and lipo-tacrolimus treatment groups before radiation, after radiation and after treatment (instillation). The baseline IMIs are statistically similar for both treatment groups, as are the post-radiation IMIs. There is a significant difference between baseline and post-radiation within each group. Lipo-tacrolimus treatment showed a significant improvement in IMI vs. post-radiation and was similar to baseline. The saline instillation caused a less significant increase in the IMI. The total volume remained constant (p > 0.05 for all pair-wise tests) throughout the study.
| Treatment | Time-point | Mean IMI (minutes) | Mean Total Volume (mL) |
|---|---|---|---|
| Baseline | 67.3 ± 8.4 | 5.5 ± 1.6 | |
| Saline | Post-Radiation | 46.2 ± 5.1 | 6.7 ± 1.2 |
| Post-Instillation | 56.6 ± 5.9 | 7.2 ± 1.1 | |
| Baseline | 64.5 ± 5.9 | 6.7 ± 1.7 | |
| Lipo-Tacrolimus | Post-Radiation | 47.7 ± 4.2 | 7.6 ± 1.9 |
| Post-Instillation | 76.8 ± 10.1 | 7.1 ± 1.0 |
Histology
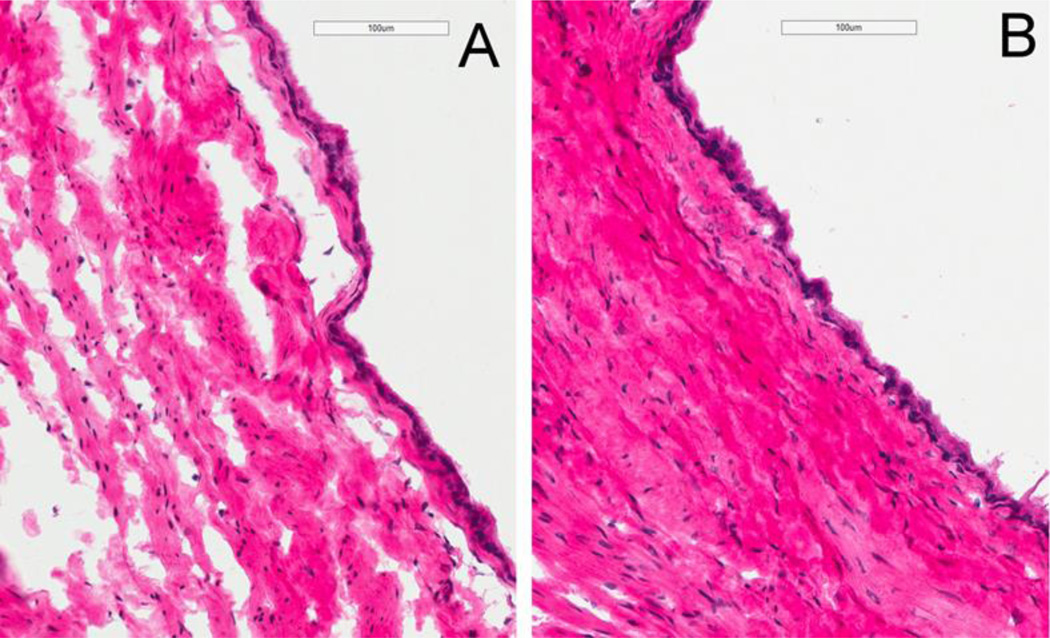
H&E staining of the harvested bladder was performed six weeks after irradiation and two weeks after treatment (Figure 4). Saline treated rats (panel A) showed edematous changes accompanying infiltration of inflammatory cells and hyperplastic urothelium changes whereas lipo-tacrolimus treated rats (Panel B) showed minimal edematous changes.
Figure 4.
H&E staining of the harvested rat bladder from 6 weeks after 40 Gy irradiation and 2 weeks after saline instillation (Panel A) showed edematous changes accompanying infiltration of inflammatory cells and hyperplastic urothelium changes whereas the group treated with an instillation of lipo-tacrolimus (Panel B) showed minimal edematous changes. Images were taken at 20× magnification.
Treatment efficacy
The one-way ANOVA test on the IMIs (Table 1) indicated that there was a significant difference between the time-point groups (p < 0.05). Multiple logistic regression analysis was also applied to the IMIs. The effects of the treatment (p = 0.03) and time-points (p < 0.0001) on IMI distribution were significant.
There was no significant difference between the baselines or post-radiation data between each of the treatment groups (e.g. saline v. lipo-tacrolimus), but there was a significant decrease in IMI between baseline and post-radiation IMIs within each treatment group (e.g. saline/baseline v. saline/post-radiation). The IMIs after radiation for each treatment group did not significantly differ. These comparisons support that the RT effects did not differ between the treatment groups.
The post-instillation group for saline did not show a significant difference from the post-radiation group or from the baseline group. The lipo-tacrolimus group showed a significant increase in IMI from post-radiation to post-instillation (p < 0.001). Furthermore, there was no significant difference from baseline after lipo-tacrolimus treatment (p = 0.019). These results indicate that the lipo-tacrolimus had a significant effect in increasing the IMI distribution back to baseline conditions, whereas saline was less clear with regard to its effects, if any.
A comparison between saline and lipo-tacrolimus post-instillation suggests that there is a significant difference between the effects of each treatment (p = 0.0001, with lipo-tacrolimus showing a greater treatment effect by increasing the IMI back to baseline values).
DISCUSSION
Radiation treatment for pelvic malignancies is typically associated with radiation injury to the urinary bladder that can lead to radiation cystitis. The precise mechanism of delivery to particular anatomic locations makes radiation treatment distinct from the systemic approach of chemotherapy. This precision substantially diminishes damage to healthy cells within the body.15 The practice still poses both short- and long-term risks. The extent of this damage varies, and it may result in the development of secondary cancers that are considered to be a tremendous concern in pediatric radiotherapy patients.16 The late sequelae of RT may take months or years to develop and includes bothersome symptoms such as hematuria. Although no definitive treatment is currently available, various interventions are currently used for both radiation and hemorrhagic cystitis.17
Guided by clinical considerations, this rodent model of RC was developed as a conduit between research and human treatment - one that provides the opportunity for testing of innovative treatments. Assessment of the model, as well as treatment, was accomplished through the observation of micturition pattern variances. As an indicator of bladder function, these changes in micturition intervals of irradiated animals were studied non-invasively by measuring changes in overnight voiding patterns. This method makes repeat measurements on the same animal possible, resulting in the reduction of animals necessary for therapeutic studies.
A dose dependent decrease in IMI was observed in the rat group irradiated with a 40 Gy dose. The change in IMI was moderate and insignificant at the 30 Gy dose, but drastic and significant at 40 Gy relative to baseline values. Degenerative type changes observed in rat urothelium in the group irradiated with 40 Gy were consistent with the decreased IMI in this group. The rat group irradiated with 20 Gy showed no significant change in IMI, which was consistent with mild inflammatory changes in histology.
The radiation dosing regimen used in the present study is higher than previous studies that have measured radiation-associated bladder toxicities.18 The CT-guided SARRP limits radiation exposure from surrounding tissue and allows us to test RC therapeutics. Future studies will evaluate low-dose, fractionated radiation delivery schemes and assess damage at longer time points.
Radiation-induced vascular damage in the bladder is considered a major mechanism for the pathogenesis of RC and histological changes are considered to be time and radiation dose dependent.19, 20 These changes in the acute phase of RC may appear in 4 to 6 weeks after radiotherapy and are characterized by inflammatory changes in the bladder wall caused by the breakdown of the urothelial barrier.20 Stromal and urothelial changes such as edema with acute inflammation, hemorrhage and vascular dilation with fibrin thrombi, endothelial swelling and vessel wall thickening with light necrosis distinguish the acute from the chronic phase of RC. Localized urothelial damage seen at lower doses (Fig. 3B&C) progresses to extensive denudation and mucosal ulceration at higher doses (Fig. 3D). The group irradiated with 40 Gy showed evidence of urothelial hyperplasia, with nests and cords that extend into the lamina propria and around ectatic blood vessels. This pseudoneoplastic condition, mimicking invasive urothelial carcinoma, is referred as pseudocarcinomatous urothelial hyperplasia (Fig. 3D). This histological feature is used as a signature of pathological aberrations seen in the bladder after ischemia and chronic radiation.21
Our results suggest that utilization of the SARRP for targeted radiation delivery provides advanced irradiation, imaging and planning capabilities that are suitably downsized for small animal radiation research and appropriate for the development of a RC rat model. The IMI results suggest that changes in IMI with different doses of radiation measures a change in the voiding pattern and bladder function. Lipo-tacrolimus treatment had a significant effect in increasing the IMI distribution back to baseline conditions and may be viewed as a potential drug candidate for RC treatment.
CONCLUSION
3-beam SAARP is an effective method to induce radiation cystitis in settings that attempt to model the clinical RT for pelvic cancer. The RC rat model demonstrated a dose-dependent decrease in inter-micturition intervals without inducing short-term skin or gastrointestinal damage. This study also demonstrated that lipo-tacrolimus may be a promising new intravesical therapy for acute radiation cystitis.
Acknowledgements
This work was supported by the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R44DK102247. Bharathi Raja is an American Urological Association Research Scholars Fellow and is highly grateful to the American Urological Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to especially thank Bernadette Zwaans, Laura Lamb and Barb Pruetz for their help with the tissue samples and histology.
Footnotes
Conflict of Interest
The authors declare no other conflicts of interest except Janicki is an employee of Lipella Pharmaceuticals and Chancellor is the Founder and Chief Scientific Officer of Lipella Pharmaceuticals.
References
- 1.Rajaganapathy BR, J N, Tyagi P, Kaufman J, Chancellor MB. Advances in therapeutic development for radiation cystitis. Lower Urinary Tract Symptoms. 2014;6:1. doi: 10.1111/luts.12045. [DOI] [PubMed] [Google Scholar]
- 2.Veerasarn V, Boonnuch W, Kakanaporn C. A phase II study to evaluate WF10 in patients with late hemorrhagic radiation cystitis and proctitis. Gynecol Oncol. 2006;100:179. doi: 10.1016/j.ygyno.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Echols RM, Tosiello RL, Haverstock DC, et al. Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin Infect Dis. 1999;29:113. doi: 10.1086/520138. [DOI] [PubMed] [Google Scholar]
- 4.Wong J, Armour E, Kazanzides P, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys. 2008;71:1591. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dilworth JT, Krueger SA, Wilson GD, et al. Preclinical models for translational research should maintain pace with modern clinical practice. Int J Radiat Oncol Biol Phys. 2014;88:540. doi: 10.1016/j.ijrobp.2013.11.209. [DOI] [PubMed] [Google Scholar]
- 6.Smit SG, Heyns CF. Management of radiation cystitis. Nat Rev Urol. 2010;7:206. doi: 10.1038/nrurol.2010.23. [DOI] [PubMed] [Google Scholar]
- 7.Rico MJ, Lawrence I. Tacrolimus ointment for the treatment of atopic dermatitis: clinical and pharmacologic effects. Allergy Asthma Proc. 2002;23:191. [PubMed] [Google Scholar]
- 8.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 9.Akar Y, Yucel G, Durukan A, et al. Systemic toxicity of tacrolimus given by various routes and the response to dose reduction. Clin Experiment Ophthalmol. 2005;33:53. doi: 10.1111/j.1442-9071.2005.00942.x. [DOI] [PubMed] [Google Scholar]
- 10.Ebert AK, Rosch WH, Vogt T. Safety and tolerability of adjuvant topical tacrolimus treatment in boys with lichen sclerosus: a prospective phase 2 study. Eur Urol. 2008;54:932. doi: 10.1016/j.eururo.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Patel P, Patel H, Panchal S, et al. Formulation strategies for drug delivery of tacrolimus: An overview. Int J Pharm Investig. 2012;2:169. doi: 10.4103/2230-973X.106981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nirmal J, Tyagi P, Chancellor MB, et al. Development of potential orphan drug therapy of intravesical liposomal tacrolimus for hemorrhagic cystitis due to increased local drug exposure. J Urol. 2013;189:1553. doi: 10.1016/j.juro.2012.10.123. [DOI] [PubMed] [Google Scholar]
- 13.Chuang YC, Tyagi P, Huang HY, et al. Intravesical immune suppression by liposomal tacrolimus in cyclophosphamide-induced inflammatory cystitis. Neurourol Urodyn. 2011;30:421. doi: 10.1002/nau.20981. [DOI] [PubMed] [Google Scholar]
- 14.Dunn OJ. Multiple Comparisons Among Means. Journal of the American Statistical Association. 1961;56:52. [Google Scholar]
- 15.Rossi P. CancerQuest: Benefits of RT. 2010 [Google Scholar]
- 16.Brenner DJ. Induced second cancers after prostate-cancer radiotherapy: no cause for concern? Int J Radiat Oncol Biol Phys. 2006;65:637. doi: 10.1016/j.ijrobp.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 17.Manikandan R, Kumar S, Dorairajan LN. Hemorrhagic cystitis: A challenge to the urologist. Indian J Urol. 2010;26:159. doi: 10.4103/0970-1591.65380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanai AJ, Zeidel ML, Lavelle JP, et al. Manganese superoxide dismutase gene therapy protects against irradiation-induced cystitis. Am J Physiol Renal Physiol. 2002;283:F1304. doi: 10.1152/ajprenal.00228.2002. [DOI] [PubMed] [Google Scholar]
- 19.Stewart FA. Mechanism of bladder damage and repair after treatment with radiation and cytostatic drugs. Br J Cancer Suppl. 1986;7:280. [PMC free article] [PubMed] [Google Scholar]
- 20.Soler R, Vianello A, Fullhase C, et al. Vascular therapy for radiation cystitis. Neurourol Urodyn. 2011;30:428. doi: 10.1002/nau.21002. [DOI] [PubMed] [Google Scholar]
- 21.Lane Z, Epstein JI. Pseudocarcinomatous epithelial hyperplasia in the bladder unassociated with prior irradiation or chemotherapy. Am J Surg Pathol. 2008;32:92. doi: 10.1097/PAS.0b013e3180eaa1dc. [DOI] [PubMed] [Google Scholar]