Abstract
Introduction
Apathy is a common, troublesome symptom in Parkinson’s disease (PD). However, little is known about its relationship with long-term cognition. We sought to determine if a caregiver-reported apathy measure predicts the development of PD dementia.
Methods
Non-demented PD patients were recruited as part of a longitudinal study of cognition. Demographics, medications, Dementia Rating Scale-2, Unified Parkinson’s Disease Rating Scale, Geriatric Depression Scale and the Neuropsychiatric Inventory-Questionnaire (NPI-Q) ratings were obtained. Apathy was defined as an NPI-Q apathy score ≥ 1. Participants were evaluated annually with cognitive and functional assessments until the end of the study period or a physician consensus diagnosis of dementia was assigned. Cox proportional hazard models were used to assess the effects of baseline apathy on dementia development while controlling for other clinical and demographic factors.
Results
Of 132 PD patients 12.1% (N=16) scored in the apathetic range at baseline. A total of 19.6% (N=26) individuals developed dementia over the course of the study, 8 of whom (30.8% of future dementia patients) had baseline apathy. In bivariate analyses baseline apathy, older age, and worse cognitive, motor, and depressive symptom scores predicted the development of dementia. In a multivariate analysis the predictive effects of baseline apathy were still significant (HR=3.56; 95% CI=1.09–11.62; p=0.04).
Conclusions
A simple, caregiver-reported measure of apathy is an independent predictor of progression to dementia in PD. This highlights the importance of apathy as a clinical characteristic of PD and could prove useful for the prediction of future dementia.
Keywords: Epidemiology, Non-motor symptoms, Cognition, Neuropsychiatric Inventory
Introduction
Apathy is typically characterized by indifference, loss of interest, and lack of motivation[1]. It is a common and disabling feature in Parkinson’s disease (PD), with reported prevalence rates that range from 14–60%[2, 3]. In cross-sectional studies, apathy has been associated with a variety of undesirable consequences in patients, such as poor cognitive performance and dementia[4, 5]. While these findings support the association between apathy and global cognition, little is known about the role of apathy as a risk marker or predictor of future Parkinson’s disease dementia (PDD).
There are several established risk factors for PDD, including older age, greater motor severity and hallucinations[6]. Unfortunately, few longitudinal studies of apathy in PD currently exist. Dujardin and colleagues studied 20 apathetic and 20 non-apathetic PD patients over 18 months and found that apathetic patients had significantly greater cognitive decline as measured by multiple neuropsychiatric batteries compared with non-apathetic patients[7]. We sought to study if apathy as measured by a single caregiver-reported item predicted conversion to clinically diagnosed dementia in a longitudinal cohort study.
Methods
Ethics
This study was approved by the University of Pennsylvania Institutional Review Board and written informed consent was obtained from all participants.
Participants
Participants were patients enrolled in the University of Pennsylvania Morris K. Udall Center for Excellence in Parkinson’s Disease Research cohort. Udall cohort participants were included in this study if they had a diagnosis of idiopathic PD based on UK Brain Bank criteria, a caregiver or study partner, at least one post-baseline study visit, and did not have baseline dementia. Participants were evaluated by a trained research assistant annually for the first four years and biannually thereafter. At each evaluation patients were administered questionnaires, and underwent a cognitive and motor examination.
Measures
All patients received both global and domain specific cognitive tests. Global cognitive tests included the Dementia Rating Scale-2 (DRS-2) for all participants and the Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) for subsets. Functional ability was assessed using the Schwab and England scale and the Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory. In addition, we administered the 15-item Geriatric Depression Scale (GDS-15) and the Unified Parkinson’s Disease Rating Scale (UPDRS). Dopaminergic medication was included in the analysis as a levodopa equivalency daily dose (LED).
Assessment of Apathy
Apathetic symptoms were assessed by the caregiver or study partner using the apathy item of the Neuropsychiatry Inventory-Questionnaire (NPI-Q). The apathy item asks, “Does the patient seem less interested in his/her usual activities or in the activities and plans of others?” If the response is yes, the caregiver is then asked to quantify how severe this symptoms is (1=mild; 2=moderate; 3=severe). A composite score is calculated as presence of apathy X severity (range 0–3). We defined apathy based on any presence of apathy (composite score ≥ 1). While the standard cutoff for the NPI-Q is ≥ 2, we chose a cutoff of 1 to capture all apathetic symptoms.
Dementia Diagnosis
Dementia was diagnosed by experts (either movement disorder specialists or a psychiatrist with specialist knowledge of PD). At each visit two physicians evaluated patient data and classified each participant as normal cognition, mild cognitive impairment (MCI) or dementia based upon cognitive and functional testing according to the MDS task force recommendations for classification of MCI and dementia in PD. Inter-rater agreement between physician teams was outstanding (Kappa = 0.80, 95% CI 0.70–0.90).
Analysis
Baseline clinical characteristics were examined using summary statistics. Means were used to describe normally distributed items and medians were used for items with skewed distributions. We categorized subjects as apathetic or non-apathetic using an NPI-Q cutoff of 1. T-tests (normal data) and Wilcoxon rank sum tests (skewed data) were used to examine baseline clinical, cognitive and behavioral associations between apathetic and non-apathetic subjects. Time to dementia was calculated using Kaplan-Meier survival estimates. Log rank tests were used to examine associations between clinical, demographic, cognitive, and behavioral measures and time to dementia. All reported p-values are two-sided, and we used a standard cut-off of 0.05 for significance.
We used Cox proportional hazard models to determine whether baseline apathy was associated with time to dementia when controlling for demographic and clinical characteristics that were hypothesized to be associated with apathy and/or dementia. We ran four models to ascertain separately the effects of baseline cognitive impairment as measured by a clinical diagnosis of MCI, the two brief cognitive screening measures, and the DRS-2 on the relationship between apathy and development of dementia. All models included covariates that were either an established risk factor for PDD (e.g. hallucinations) or had a p-value < 0.15 in bivariate analysis comparing the covariate and dementia. Model 1 did not adjust for baseline cognitive differences between patients. Model 2 repeated the analysis including overall baseline DRS-2 score. In model 3 we measured cognition using clinical screening measures (either the MoCA or MMSE). Model 4 excluded participants given a consensus diagnosis of “MCI” at baseline and controlled for overall DRS-2 score. We could not control for hallucinations in this model since no one with hallucinations developed dementia.
Results
Baseline Sample Characteristics
Of 314 Udall participants 232 met the study criteria. Due to missing data an additional 100 people were excluded, leaving 132 participants in our final sample. Excluded participants did not differ significantly in terms of major clinical or demographic factors.
Participants in the final sample were an average of 69.4 (SD 7.8) years old, had a median Hoehn and Yahr stage of 2.0 (IQR 2.0–2.5), and a median disease duration of 7 years (IQR 4–11). 68.9% (N=91) of participants were male. The median LED of the sample was 600.0 mg (IQR 375.0–930.3).
At baseline, 12.1% (N=16) had an NPI score ≥ 1 and of those, only 3.1% (N=4) had an NPI score ≥ 2. For subsequent analyses, we categorized all subjects with an NPI score ≥ 1 in the apathetic group. Patients who were apathetic had significantly worse cognition at baseline by DRS (median DRS-2 131.0 vs. 139.0; p<0.01) than those who were non-apathetic. They did not differ in other major clinical characteristics.
Survival analysis
The cohort was followed for an average of 3.13 years (range 0.84–6.02). Average follow up time was 2.06 years (range 0.84–4.26) for those with baseline apathy and 3.28 years (range 0.90–6.02) for those without baseline apathy. A total of 19.7% participants became demented over the course of the study. 50.0% (N=8/16) of the apathetic patients and 15.5% (N=18/116) of the non-apathetic patients developed dementia during follow-up. Average time to dementia was 1.7 years for individuals with baseline apathy and 3.0 years for non-apathetic individuals. Patients with baseline apathy developed dementia at a faster rate than those without apathy (HR= 7.23, 95% CI=3.03–17.25; p<0.01) (Figure 1).
Figure 1.
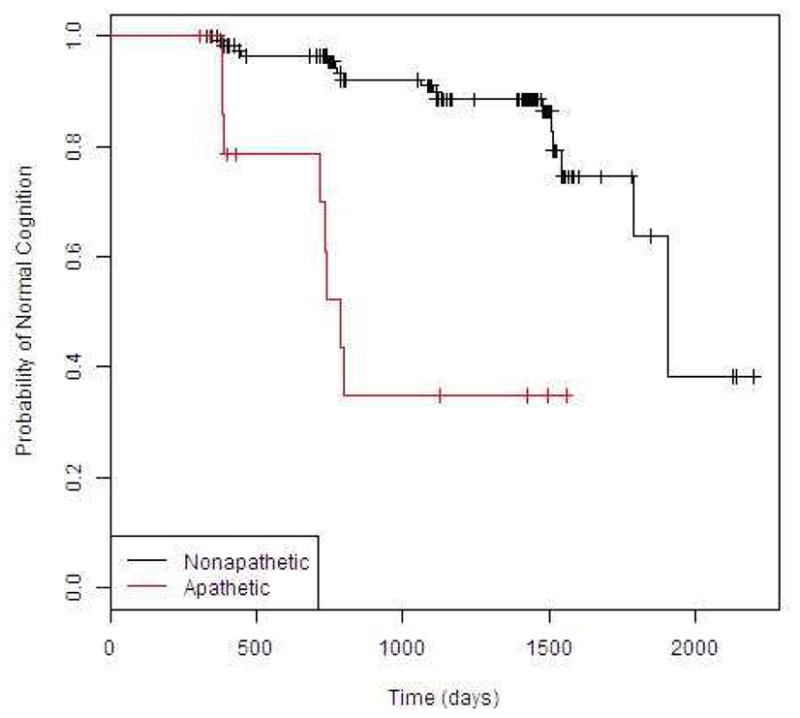
Kaplan Meier curves showing time to dementia between apathetic and non-apathetic patients
Participants who developed dementia were also significantly older (mean age 73.3 vs. 68.5; p<0.01), had significantly higher LED (median dosage in milligrams 820 vs. 600; p=0.004), and had significantly worse motor, (mean UPDRS III 28.2 vs. 18.5; p<0.01), cognitive (median DRS-2 131.0 vs. 139.0; p<0.01) and depression (median GDS 3.5 vs 1.0; p<0.01) scores at baseline than those who did not become demented.
Next, we performed Cox proportional hazards model including age, education, gender, disease duration, apathy, UPDRS-III motor score, GDS score, hallucinations, LED, and antidepressant use as covariates to determine the independent effect of apathy on dementia rates. Presence of apathy, worse motor scores, higher LED, older age, and less education, were all significantly associated with a higher probability of developing dementia (Table 1). When baseline DRS-2 score was included in the model, the effects of apathy remained significant (HR 3.56; 95% CI 1.09–11.62; p=0.04).
Table 1.
Demographic and clinical factors independently associated with conversion to dementia*
| Predictor | Hazard Ratio | 95% Confidence interval | P-value |
|---|---|---|---|
| Apathy | 6.34 | 2.32–11.62 | 0.04 |
| GDS-15 | 1.09 | 0.93–1.28 | 0.23 |
| UPDRS-III | 1.04 | 1.00–1.09 | 0.05 |
| Age | 1.07 | 1.00–1.14 | 0.05 |
| Education | 0.83 | 0.69–1.00 | 0.05 |
| Male | 2.35 | 0.76–7.24 | 0.14 |
| Disease Duration | 0.99 | 0.90–1.10 | 0.91 |
| LED | 2.96 | 1.30–6.75 | 0.01 |
| Antidepressant use | 1.13 | 0.50–2.52 | 0.55 |
| Hallucinations | 0.36 | 0.037–3.67 | 0.39 |
This Cox-regression model adjusts for all listed predictors
We repeated the analysis using brief cognitive screening measures rather than the DRS- 2 to categorize cognition. A total of 14 apathetic and 98 non-apathetic participants, who had an MMSE or a MoCA at baseline, were included. A cutoff of 26/27 was used to denote cognitive impairment for the MMSE and of 25/26 for the MoCA. In this model, the NPI-Q apathy question significantly predicted time to dementia (HR=5.60; 95% CI=1.57–19.93; p<0.01).
Our final model controlled for baseline DRS-2 score, but excluded participants with a baseline cognitive categorization of MCI. A total of 9 apathetic and 98 non-apathetic individuals met this criterion. In this subsample apathy still significantly predicted time to dementia development (HR=46.36; 95% CI=3.72–576.99; p=0<0.01).
Discussion
This study showed that a single informant-based apathy item significantly predicted the subsequent development of PDD. This result was robust when controlling for a number of different baseline cognitive measures, including the DRS-2, and brief cognitive screening measures such as the MoCA and MMSE, and when only examining cognitively normal patients. Furthermore, the single apathy item was found to predict development of dementia with a similar effect size as standard cognitive screening measures.
Our findings are consistent with the existing literature. Dujardin and colleagues also found that apathy predicted subsequent cognitive decline in a parkinsonian population [7]. Our analysis differed from Dujardin and colleagues in several ways. First, we included depressed participants in our analysis. Thus, while we did not look at the effects of pure apathy, we were able to examine the independent effects of depression and apathy on dementia risk which increases the generalizability of our results.
Second, Dujardin and colleagues used the Lille Apathy Rating Scale and Starkstein Apathy Scale, while we used a single-question apathy item from the NPI-Q to measure apathy. While the NPI-Q may be a less sensitive measure of apathy, the brevity of the scale and inclusion of other neuropsychiatric symptoms also makes it especially relevant to clinical practice. The NPI-Q may have other advantages as a measurement tool. It could overcome limitations of self-reported or physician interview-based measures that rely on patient insight into apathy symptoms. As many PD patients develop cognitive impairment and depression, accuracy of self-reported apathy may be affected[8]. On the other hand, caregiver reported measures of apathy may also introduce biases. One report showed larger discrepancies between patient and caregiver reports of apathy when caregivers report greater levels of depression or burden[9]. It is unclear exactly how these factors affect overall reports of apathy. Studies have found both that caregivers over-report and under-report apathetic symptoms, compared to patient-reported measures[10, 11]. Ultimately, more objective measures will help to accurately identify apathetic patients.
Unfortunately, there are currently no gold standard criteria for the diagnosis of apathy. However, the NPI is a suggested scale by a Movement Disorders Society task force on rating scales to determine if apathy is present[12]. The prevalence of apathetic symptoms reported among our cohort was lower than that commonly reported for in PD samples[2, 3]. This suggests that apathy was under-reported in our study. We attempted to overcome this limitation by including all subjects with apathetic symptoms using a lower cutoff.
Nevertheless, our findings have pragmatic clinical implications. Specifically, the presence of even mild apathetic symptoms predicted subsequent development of dementia equally as well as performance on the clinical cognitive screening measures typically used. As the apathy item was a single question, it would be much easier to assess on a routine clinical visit. This makes it a promising, simple screening measure to determine which patients might be at risk for developing dementia. When patients with MCI were excluded from the sample, our finding remained unchanged. Thus, the single apathy item seems to provide a powerful potential screening measure even in cognitively normal PD patients.
There are several limitations of our study. Though we had a large overall sample (n=132), our apathetic sample was small (n=16), even when using a lower threshold for apathetic symptoms. Despite this small sample, the association between apathy and dementia was robust when controlling for a number of different variables and when using different measures of cognition in the model. Additional studies with larger samples will help validate these findings. Second, the apathy scale we chose, which was only a single item, was a non-specific measure of apathy. Moreover, because of our small apathetic sample we used a lower cutoff than is traditionally used for this scale. However, since the NPI-Q apathy item was significantly associated with the subsequent development of dementia it is clear that the type of “apathy” captured by this scale is clinically meaningful. Finally, the nature of the study design makes it susceptible to potential selection biases. Using a caregiver report measure for apathy meant that we could not include individuals without care partners. Additionally the rigorous testing required might only be completed by highly motivated people without cognitive impairment.
This study provides good evidence that caregiver report of apathetic symptoms is a predictor of future development of dementia in PD. This combined with the brevity of the apathy question on the NPI-Q could facilitate screening and interventions for those at high risk for developing PDD.
Highlights.
20% of Parkinson’s disease subjects developed dementia over an average of 3 years
We measured apathetic symptoms using a single-item completed by the caregiver
Baseline apathetic symptoms significantly predicted the onset of future dementia in Parkinson’s disease
A simple, caregiver-reported measure of apathy could facilitate screening and interventions for dementia
Acknowledgments
Funding sources for study: This study received support from the NIH (P50 NS053488, U01 NS082134). Alice Chen-Plotkin is also supported by the Doris Duke Charitable Foundation Clinician-Scientist Development Award, the Burroughs Wellcome Fund Career Award for Medical Scientists, and the Benaroya Fund. Lauren Massimo is supported by NINR F32 NR014777. Nabila Dahodwala is supported by NIA K23 AG034236.
Funding sources had no role in the study design, data collection, analysis or interpretation, writing of the report or decision to submit for publication.
Data contributed to this project by the Morris K. Udall Center at the Perelman School of Medicine at the University of Pennsylvania (P50 NS053488, Trojanowski JQ-PI; Hurtig HICore B Leader; Weintraub D-Core B Co-Leader; Chen-Plotkin A-Investigator and Project 1 PI, Chahine L-Investigator; Duda JE-Investigator; Rick J-Project-Project Manager).
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–54. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 2.Oguru M, Tachibana H, Toda K, Okuda B, Oka N. Apathy and depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2010;23(1):35–41. doi: 10.1177/0891988709351834. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen KF, Larsen JP, Alves G, Aarsland D. Prevalence and clinical correlates of apathy in Parkinson’s disease: a community-based study. Parkinsonism Relat Disord. 2009;15(4):295–9. doi: 10.1016/j.parkreldis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134–9. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 5.Dujardin K, Sockeel P, Devos D, Delliaux M, Krystkowiak P, Destee A, et al. Characteristics of apathy in Parkinson’s disease. Mov Disord. 2007;22(6):778–84. doi: 10.1002/mds.21316. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289(1–2):18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Dujardin K, Sockeel P, Delliaux M, Destee A, Defebvre L. Apathy may herald cognitive decline and dementia in Parkinson’s disease. Mov Disord. 2009;24(16):2391–7. doi: 10.1002/mds.22843. [DOI] [PubMed] [Google Scholar]
- 8.Leritz E, Loftis C, Crucian G, Friedman W, Bowers D. Self-awareness of deficits in Parkinson disease. Clin Neuropsychol. 2004;18(3):352–61. doi: 10.1080/1385404049052412. [DOI] [PubMed] [Google Scholar]
- 9.Schiehser DM, Liu L, Lessig SL, Song DD, Obtera KM, Burke MM, III, et al. Predictors of discrepancies in Parkinson’s disease patient and caregiver ratings of apathy, disinhibition, and executive dysfunction before and after diagnosis. J Int Neuropsychol Soc: JINS. 2013;19(3):295–304. doi: 10.1017/s1355617712001385. [DOI] [PubMed] [Google Scholar]
- 10.McKinlay A, Grace RC, Dalrymple-Alford JC, Anderson TJ, Fink J, Roger D. Neuropsychiatric problems in Parkinson’s disease: comparisons between self and caregiver report. Aging Ment Health. 2008;12(5):647–53. doi: 10.1080/13607860802343225. [DOI] [PubMed] [Google Scholar]
- 11.Dujardin K, Sockeel P, Delliaux M, Destee A, Defebvre L. The Lille Apathy Rating Scale: validation of a caregiver-based version. Mov Disord. 2008;23(6):845–9. doi: 10.1002/mds.21968. [DOI] [PubMed] [Google Scholar]
- 12.Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, et al. Apathy and anhedonia rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2008;23(14):2004–14. doi: 10.1002/mds.22229. [DOI] [PubMed] [Google Scholar]


