Abstract
Background
The nucleus accumbens (NAc) plays a key role in brain reward processes including drug seeking and reinstatement. Several anatomical, behavioral, and neurochemical studies discriminate between the limbic-associated shell and the motor-associated core regions. Less studied is the fact that the shell can be further subdivided into a dorsomedial shell (NAcDMS) and an intermediate zone (NAcINT) based on differential expression of transient c-Fos and long-acting immediate-early gene ΔFosB upon cocaine sensitization. These disparate expression patterns suggest that NAc shell subregions may play distinct roles in reward-seeking behavior. In this study, we examined potential differences in the contributions of the NAcDMS and the NAcINT to reinstatement of reward-seeking behavior after extinction.
Methods
Rats were trained to intravenously self-administer cocaine, extinguished, and subjected to a reinstatement test session consisting of either an intracranial microinfusion of amphetamine or vehicle targeted to the NAcDMS or the NAcINT.
Results
Small amphetamine microinfusions targeted to the NAcDMS resulted in statistically significant reinstatement of lever pressing, whereas no statistical difference was observed for microinfusions targeted to the NAcINT. No significant difference was found for vehicle microinfusions in either case.
Conclusion
These results suggest heterogeneity in the behavioral relevance of NAc shell subregions, a possibility that can be tested in specific neuronal populations in the future with recently developed techniques including optogenetics.
Keywords: nucleus accumbens, nucleus accumbens shell, subregions, cocaine, self-administration, reinstatement
1. INTRODUCTION
The unrelenting desire to obtain drugs of abuse is a persistent and debilitating aspect of addiction. This feeling of motivation—commonly referred to as craving—is mediated by distinct circuits that are believed to precipitate the reinstatement of drug-seeking behavior (Kalivas and McFarland, 2003).
The nucleus accumbens (NAc) is an integral component of the neuronal circuitry underlying drug-seeking behavior in rodents and remains at the center of intense investigation after decades of interrogation. The functional and anatomical heterogeneity of the NAc revealed by past studies resulted in its division into core and shell regions (Deutch and Cameron, 1992; Zaborszky et al., 1985). Despite intense study of the NAc as only two disparate regions, a growing body of research suggests that further partitioning of the NAc shell to account for differences in gene expression, anatomy, and function may be warranted.
The NAc shell is a heterogeneous structure (Jongen-Relo et al., 1993; Meredith et al., 1992; Schilman et al., 2008; Shin et al., 2008; Wright and Groenewegen, 1995). It can be segregated into dorsomedial (NAcDMS), intermediate (NAcINT), and ventrolateral zones based on differences in gross projection patterns (Heimer et al., 1997; Moga et al., 1995; Usuda et al., 1998; Zahm and Brog, 1992) and immunoreactivity to various peptides and neurotransmitters (Heimer et al., 1997; Voorn et al., 1989, 1986; Zahm and Brog, 1992; Zahm et al., 1998). Furthermore, accumulating evidence suggests that each NAc shell subregion is a module that responds to psychostimulants in a manner distinct from the others. Early evidence was provided by differential neurotensin immunoreactivity in response to systemic injections of amphetamine and the dopamine antagonist haloperidol (Heimer et al., 1997). Subsequent experiments revealed that locomotor activity varies in response to cocaine infusions into different NAc shell subregions (Ikemoto, 2002). More recent physiological studies showed sustained changes in neuronal activity during cocaine self-administration that varied across the NAc shell subregions (Fabbricatore et al., 2009).
To further characterize the NAc shell in an addicted state, our group previously sensitized animals to cocaine and subsequently assayed NAc shell subregions for ΔFosB immunoreactivity (Brenhouse and Stellar, 2006). ΔFosB dimerizes with cofactors to form long-lasting complexes that function like a molecular switch. This persisting switch may be involved in the long-term changes that enable reinstatement of drug-seeking behavior even after long periods of extinction (Hope et al., 1994; Nestler et al., 2001). Expression of ΔFosB in the NAcDMS following 14 days withdrawal from cocaine was upregulated, implying that this subregion may be a site of neuronal plasticity that plays a paramount role in the persistence of addiction. In contrast, no significant change was found for NAcINT ΔFosB expression. The aforementioned evidence suggests a functional significance associated with NAc shell subregion segregation. However, studies measuring neurochemical immunoreactivity patterns in response to behavioral sensitization provide only indirect support for their differing roles in addiction. No study has yet demonstrated the consequences of manipulating particular NAc shell subregions on reward-seeking behavior.
We hypothesized a functional role for the NAcDMS in mediating reinstatement of reward-seeking behavior. We tested this with a self-administration paradigm in which rats were trained to lever-press for intravenous infusions of cocaine. Subsequently, the same lever was paired with saline infusions to extinguish lever pressing. Upon extinction, a microinfusion of amphetamine was targeted to NAc shell subregions to probe reinstatement while animals were freely behaving. To our knowledge, this is the first study to implicate a specific NAc shell subregion in the reinstatement of reward-seeking behavior.
2. MATERIALS AND METHODS
2.1. Animal subjects
Male Sprague-Dawley rats (n=13; Charles River Laboratories) were housed individually in a temperature-controlled, 12hr light:dark cycle facility. Daily food intake was restricted to maintain weights not exceeding 350g. Water was available ad libitum. Animal care was approved by the Northeastern University Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
2.2. Behavioral apparatus and conditions
Training and test sessions were conducted in self-administration chambers inside sound attenuating cubicles (Med Associates) modified for intracranial pump access. Chambers contained two retractable levers, a cue light above each, and one house light. Pressing the active lever resulted in reinforcement and stimulus light illumination followed by a 6sec blackout period. Pressing the inactive lever resulted in no change. The house light was always illuminated during sessions except during reinforcement, when only the cue light was illuminated.
2.3. Food training
Subjects were trained in 1hr daily sessions to lever press for 10sec food reinforcement (Ensure®; Abbott Laboratories) delivered by a motorized dipper. Training was conducted on a fixed ratio (FR) reinforcement schedule increasing between sessions from FR1 to FR5. The FR schedule was increased for the subsequent session upon administration of ≥50 reinforcements. Training ceased when ≥100 reinforcements were administered at FR5 for 2 consecutive sessions. On average, animals required 10 days to complete food training (n=13; mean±SEM: 10.2±1.4d).
2.4. Surgery
After food training, rats were anesthetized with a ketamine/xylazine (90mg×kg−1/10mg×kg−1, i.p.) mixture for surgery. Each was implanted with a chronic indwelling catheter (IVSA28; CamCaths) with silicone tubing inserted into the jugular vein for intravenous self-administration. Catheter patency was determined after surgery by observation of a small blood return resulting from brief application of negative pressure. Catheters were injected pre-session with saline and post-session with heparinized saline (10usp/mL) to maintain patency. In the same surgery, bilateral indwelling guide cannulae (C235G, 26ga, 1.6mm-spread; PlasticsOne) targeting the NAcDMS or NAcINT (Bregma-based coordinates: A-P: +1.6mm; M-L: ±0.8mm; D-VDMS: −5.4mm; D-VINT: −5.6mm) were embedded stereotaxically (Cooley and Vanderwolf, 1990). Cannulae were secured to skull screws with dental acrylic and filled with stylets to prevented occlusion. Recovery was allowed for ≥10d after surgery.
2.5. Cocaine self-administration
Upon recovery, rats were trained in 2hr sessions to self-administer reinforcements consisting of 45μl cocaine hydrochloride (1mg×mL−1 in 0.9% saline; Sigma-Aldrich). Active and inactive lever assignments remained consistent with food training sessions. Reinforcements were administered to the catheter through a swivel (Instech Laboratories) using a syringe pump. Training was conducted on a FR reinforcement schedule increasing between sessions from FR1 to FR5 only upon stabilization of total lever presses and consistent inter-reinforcement-intervals. Stabilization of lever pressing was defined as ≤25% fluctuation in total lever presses across the previous 3 consecutive sessions. Training ceased when ≥15 reinforcements were administered at FR5 for 3 consecutive sessions. On average, animals required 19 days to complete training (n=13; mean±SEM: 18.6±2.9d). Baseline cocaine self-administration responding was calculated as the mean of these 3 last training sessions. Subjects next progressed to an extinction phase.
2.6. Extinction
Extinction sessions mimicked those of FR5 cocaine self-administration training but with saline substituted for cocaine. The cue light was illuminated with saline infusion during all extinction trials. Subjects were considered extinguished when the total number of lever presses was ≤15% of baseline cocaine self-administration responding. On average, animals required 38 days to reach this criterion (n=13; mean±SEM: 38.2±9.2d). Baseline extinction responding was calculated as the mean of these 3 last training sessions.
2.7. Reinstatement test sessions
Animals were tested for reinstatement of lever pressing in sessions that mimicked extinction sessions but included an intracranial microinfusion mid-session (after 60min elapsed from the 120min session). The cue light was not illuminated upon delivery of the microinfusion. Only one test session was carried out per day. Each animal experienced at least 2 test sessions. The first contained a 300nL amphetamine microinfusion (13.5μg×μL−1; Sigma-Aldrich). The second contained a 900nL vehicle control microinfusion (artificial cerebrospinal fluid; Harvard Apparatus). A third session including a 900nL amphetamine microinfusion was given to some animals that previously demonstrated an increase in responding following 300nL amphetamine microinfusion in the first test session. Microinfusions were administered using a hands-free computer-controlled microinjection system without stopping the session or handling the animal (Hildebrand et al., 2009). Amphetamine was chosen over cocaine to increase NAc shell subregion dopamine activity in order to avoid the latter’s local anesthetic and lipid solubility properties. One or more intracranial microinfusion-free (washout) extinction sessions were provided between reinstatement conditions.
2.8. Analysis
Following reinstatement test sessions, animals were deeply anesthetized with a ketamine/xylazine (90mg×kg−1/10mg×kg−1, i.p.) mixture and trans-cardially perfused with 4% formaldehyde. Brains were removed, embedded in paraffin, sectioned, imaged, and analyzed for cannula placements using reference map (Paxinos and Watson, 2006) comparisons by 2 experienced researchers blinded to behavioral results. Placements were divided into NAcDMS and NAcINT groups by referencing NAc shell subregion demarcations from previous work (Heimer et al., 1997; Voorn et al., 1989; Voorn et al., 1986; Zahm and Brog, 1992; Zahm et al., 1998). The behavioral results were then compared across placement groups. All lever-pressing results are presented as mean±SEM.
Comparisons of the number of lever presses in the middle hour of cocaine self-administration and extinction sessions were conducted with Kolmogov-Smirnov tests. To compare epochs with fewer responses (which are more likely to contain ties)—as in the 30min before and 30min after each reinstatement microinfusion—resampling with replacement (bootstrapping) tests were used. In this case, the difference in means was used as the test statistic. The one-sided p-value was calculated as the proportion of 100,000 randomly permuted samples where the difference in means was greater than or equal to the empirically measured difference in means.
3. RESULTS
3.1. Anatomical distribution of cannula placements
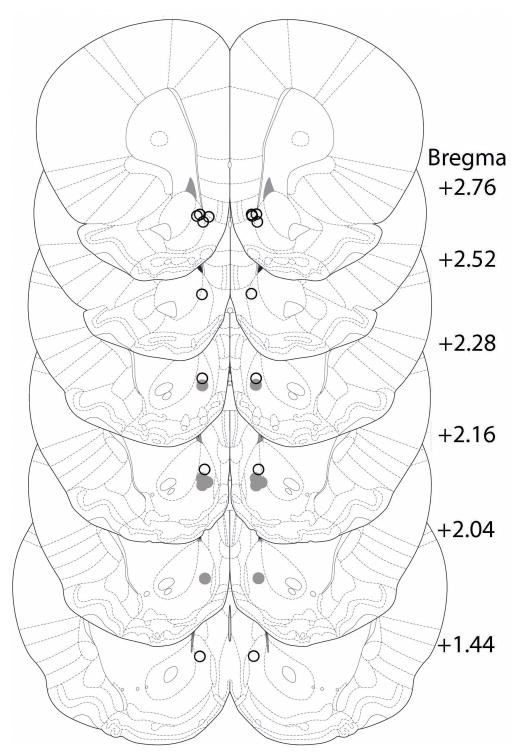
A total of 13 animals completed training, testing, and reinstatement sessions. Among these, 8 cannula placements corresponded to the NAcDMS and 5 to the NAcINT (Figure 1).
Figure 1. Cannula placements targeted to NAc subregions.
Histological analysis shows two anatomically disparate populations of cannula placements: NAcDMS (open circles) and NAcINT (closed circles) placements plotted on serial coronal sections (Paxinos and Watson, 2005).
3.2. Operant behavior during baseline training
Each subject’s baseline responding during cocaine self-administration and extinction sessions was quantified as total lever presses during the 2hr session before reaching the criterion for progression to the next phase. On average, subjects administered 2.63±0.13mg/kg of cocaine during the final three days of cocaine-self administration training. No significant difference in baseline cocaine self-administration total lever presses was observed between subjects with cannula placements in NAcDMS and NAcINT (P= 0.9732, Kolmogorov-Smirnov; NAcDMS: n=8, 100.00±8.84; NAcINT: n=5, 97.00±8.15). The same was found for baseline extinction session responding (P=0.5070, Kolmogorov-Smirnov; NAcDMS: n=8, 11.25±2.78; NAcINT: n=5, 15.60±3.74).
3.3. Reinstatement occurs upon amphetamine microinfusions into NAcDMS
Unlike most previous studies, our mid-session intracranial microinfusions were performed in freely behaving animals without need for interrupting sessions with additional handling (Hildebrand et al., 2009).
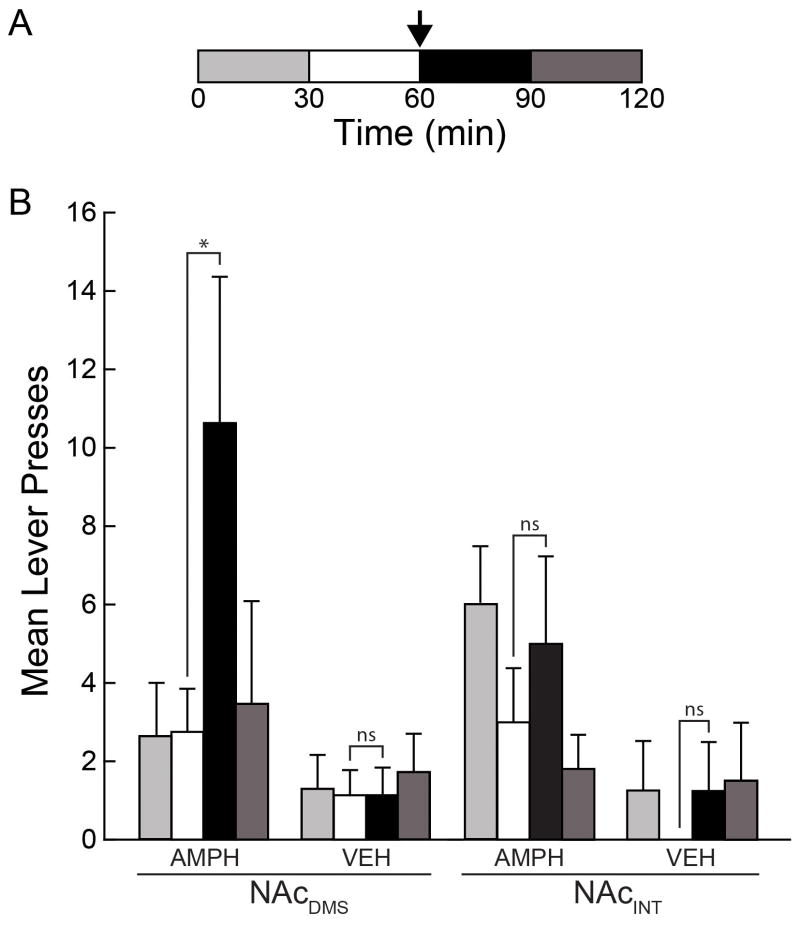
The mean lever presses for subjects with cannula placements in the NAcDMS was significantly higher 30min after 300nL amphetamine microinfusion compared with 30min before (P=0.038, bootstrap; n=8; pre: 2.75±1.10; post: 10.63±3.74) and compared to third quarter of the final extinction session (P=0.036, bootstrap; n=8; extinction: 2.50±1.43; reinstatement: 10.63±3.74). In contrast, no similar significant difference was detected for placements in the NAcINT within sessions (P=0.244, bootstrap; n=5; pre: 3.00±1.38; post: 5.00±2.24) or when compared to the final extinction session (P=0.158, bootstrap; n=8; extinction: 2.00±1.14; reinstatement: 5.00±2.24). Vehicle microinfusions did not have a significant effect on responding whether targeted to the NAcDMS (P=0.508, bootstrap; n=7; pre: 1.14±0.63; post: 1.14±0.71) or NAcINT (P=0.879, bootstrap; n=4; pre: 0.00±0.00, post: 1.25±1.25). These results are summarized in Figure 2. Animals receiving a third test session with a 900nL amphetamine microinfusion also demonstrated increased lever pressing (P<0.001, bootstrap; n=6; pre: 2.67±0.84; post: 23.50±3.88), suggesting that attenuated lever pressing due to additional extinction following the first reinstatement session does not explain the lack of a significantly distinguishable reinstatement response upon vehicle microinfusion.
Figure 2. Subregion-dependent reinstatement of reward seeking.
(A) The time course of the reinstatement test session. Lever presses were separated into quarter-session bins. The reinstatement challenge consisted of an intracranial microinfusion of either amphetamine or vehicle given mid-session (arrow). (B) The mean number of lever presses across subjects in each quarter session before (white) and after (black) microinfusion of amphetamine (AMPH) or vehicle (VEH) targeted to the NAcDMS or NAcINT (*P<0.038, bootstrap).
4. DISCUSSION
In this study, we found that animals with placements in the NAcDMS—unlike those in the NAcINT—show significantly elevated lever-pressing behavior following an intracranial amphetamine challenge during the extinguished state. These results suggest that increasing dopamine levels in the NAcDMS subregion alone is sufficient to induce reinstatement of reward-seeking behavior. This may be due to disparities in dopamine availability caused by heterogeneous tyrosine hydroxylase expression across the NAc shell subregions (Todtenkopf and Stellar, 2000). Subregion-specific differences in dopamine receptor distribution could also contribute to the anatomical specificity of reinstatement induction we observe (Gangarossa et al., 2013).
It is important to note that previous studies show reinstatement to cocaine microinfusions with similar NAc cannula placements to our NAcINT placements (Bachtell et al., 2005; Cornish and Kalivas, 2000; Schmidt et al., 2006). We believe that this can be explained either by the comparatively small injection volume used to elicit reinstatement in our study or the type of pharmacological agent administered. In addition, the contribution of food training to observed reinstatement values cannot be excluded using our methodology, as we performed food training over a period of several days. Subsequent studies will further elucidate whether the functional heterogeneity of the NAc shell affects reward-seeking behavior broadly or is specific to drug seeking.
Our findings indicate that the NAcDMS may be involved in maintaining long-term changes associated with addiction that lead to persistent reward-seeking behavior. This concept is consistent with increased expression of the transcription factor ΔFosB in the NAcDMS but not the NAcINT following 14-day withdrawal from cocaine exposure (Brenhouse and Stellar, 2006). ΔFosB heterodymerizes with Jun family proteins to form complexes that regulate gene transcription (Hope et al., 1994). It has been shown to play a powerful role in mediating the rewarding effects of cocaine (Nestler, 2008). Furthermore, ΔFosB accumulates and persists in the brain for several weeks following cocaine administration due to its long half-life (Chen et al., 1997). This long timescale of action allows ΔFosB to function as a molecular switch, making it a prime candidate for mediating long-lasting changes associated with drug exposure.
Changes in transcription through ΔFosB are thought to mediate the behavioral effects of cocaine (Taylor et al., 2007), in part through changes in glutamatergic (Kelz et al., 1999) and dopaminergic signaling (Bibb et al., 2001). Chronic cocaine exposure induces ΔFosB expression preferentially in D1-expressing medium spiny neurons (MSNs) in the NAc. Additionally, optogenetic stimulation of NAc afferents induces ΔFosB expression preferentially in D1-expressing MSNs in the NAc shell (Lobo et al., 2013). ΔFosB is required for NAc dendritic spine formation following repeated cocaine exposure (Maze et al., 2010). Recent evidence also suggests that ΔFosB-mediated spine formation occurs in a cell-type specific manner, as specific overexpression of ΔFosB in D1-expressing MSNs increases immature spines on D1-expressing neurons and enhances behavioral responses to cocaine. In contrast, overexpression of ΔFosB in D2-expressing MSNs decreases silent synapses (Grueter et al., 2013). Interestingly, D1 and D2 receptor-expressing neurons are thought to reflect separate pathways, with reward-related behaviors mediated through the direct pathway and aversive-related behaviors through the indirect pathway, respectively (Hikida et al., 2013; Kravitz et al., 2012). Combined with the fact that these receptors have subregion-specific expression patterns (Gangarossa et al., 2013), these studies support our results, which suggest that distinct NAc subregions mediate different behaviors. Taken together with previous findings, our study suggests cocaine may exert its long-term effects through subregion-specific and pathway-specific structural modifications in NAc shell neurons mediated by ΔFosB.
In summary, we demonstrate that increased dopaminergic activity in the NAcDMS is sufficient to induce reward-seeking behavior in extinguished animals, while no significant changes are observed for the NAcINT. Our work is a preliminary result that provides behavioral evidence for the anatomical differentiation of subregions within the NAc shell and could be followed up using recently developed methods with the ability to more cleanly segregate neuronal populations. For example, future experiments could make use of dopamine receptor-specific pharmacological manipulations or optogenetic manipulations of specific neuron classes that appear to segregate into the NAc shell subregions. These methods would allow more precise control of neuronal activity in a behavioral testing paradigm similar to ours, therefore permitting further disambiguation of the role each NAc shell subregions plays in reward-seeking behavior.
We explored functional heterogeneity across nucleus accumbens shell subregions.
Rats were trained to self-administer cocaine and subsequently extinguished.
Amphetamine microinfusions were targeted to subregions as reinstatement tests.
Targeting the dorsomedial shell led to significant reinstatement.
No significant reinstatement was observed when targeting the intermediate zone.
Acknowledgments
Role of Funding Source: Funding for this study was provided by the Whitehall Foundation and NIH grant DA019946. Neither funding source had a role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank J.L. Morgan for discussions regarding statistical analysis.
Footnotes
Contributions: M.D.R. and D.G.C.H. collected data, performed analyses, and wrote the paper. M.F. and J.R.S. provided advice, guidance, and resources. All other authors collected data.
Conflict of Interest: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachtell R, Whisler K, Karanian D, Self D. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology. 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Stellar JR. c-Fos and deltaFosB expression are differentially altered in distinct subregions of the nucleus accumbens shell in cocaine-sensitized rats. Neuroscience. 2006;137:773–780. doi: 10.1016/j.neuroscience.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley RK, Vanderwolf CH. The Rat: A Photographic Series. A.J. Kirby Co; London, Ontario: 1990. Stereotaxic Surgery. [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus-accumbens core and shell. Neuroscience. 1992;46:49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat accumbens during cocaine self-administration: tonic firing patterns. Eur J Neurosci. 2009;30:2387–2400. doi: 10.1111/j.1460-9568.2009.07033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d’Exaerde A, El Mestikawy S, Gerfen CR, Herve D, Girault JA, Valjent E. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits. 2013;7:22. doi: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Hikida T, Yawata S, Yamaguchi T, Danjo T, Sasaoka T, Wang Y, Nakanishi S. Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc Natl Acad Sci U S A. 2013;110:342–347. doi: 10.1073/pnas.1220358110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand DG, Knudsen DP, Hesse GW, Stellar JR. A flexible system for hands-free intracranial microinjection. J Neurosci Methods. 2009;185:62–65. doi: 10.1016/j.jneumeth.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neurosciencem. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo AL, Groenewegen HJ, Voorn P. Evidence for a multi-compartmental histochemical organization of the nucleus accumbens in the rat. J Comp Neurol. 1993;337:267–276. doi: 10.1002/cne.903370207. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, Dinieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Agolia R, Arts MPM, Groenewegen HJ, Zahm DS. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience. 1992;50:149–162. doi: 10.1016/0306-4522(92)90389-j. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Stereotaxic Coordinates. Academic Press; San Diego: 2006. The Rat Brain. [Google Scholar]
- Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci U S A. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Stellar JR. Assessment of tyrosine hydroxylase immunoreactive innervation in five subregions of the nucleus accumbens shell in rats treated with repeated cocaine. Synapse. 2000;38:261–270. doi: 10.1002/1098-2396(20001201)38:3<261::AID-SYN5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- Voorn P, Jorritsma-Byham B, Van Dijk C, Buijs RM. The dopaminergic innervation of the ventral striatum in the rat: a light- and electron-microscopical study with antibodies against dopamine. J Comp Neurol. 1986;251:84–99. doi: 10.1002/cne.902510106. [DOI] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol. 1995;361:383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M. Cholecystokinin Innervation of the Ventral Striatum - a morphological and radioimmunological study. Neuroscience. 1985;14:427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Williams ES, Krause JE, Welch MA, Grosu DS. Distinct and interactive effects of d-amphetamine and haloperidol on levels of neurotensin and its mRNA in subterritories in the dorsal and ventral striatum of the rat. J Comp Neurol. 1998;400:487–503. doi: 10.1002/(sici)1096-9861(19981102)400:4<487::aid-cne4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]