Abstract
Macrophages undergo a transition from pro-inflammatory to healing-associated phenotypes that is critical for efficient wound healing. However, the regulation of this transition during normal and impaired healing remains to be elucidated. In our studies, the switch in macrophage phenotypes during skin wound healing was associated with upregulation of the peroxisome proliferator-activated receptor (PPAR)-γ and its downstream targets, along with increased mitochondrial content. In the setting of diabetes, upregulation of PPAR-γ activity was impaired by sustained expression of IL-1β in both mouse and human wounds. In addition, experiments with myeloid-specific PPAR-γ knockout mice indicated that loss of PPAR-γ in macrophages is sufficient to prolong wound inflammation and delay healing. Furthermore, PPAR-γ agonists promoted a healing-associated macrophage phenotype both in vitro and in vivo, even in the diabetic wound environment. Importantly, topical administration of PPAR-γ agonists improved healing in diabetic mice, suggesting an appealing strategy for downregulating inflammation and improving healing of chronic wounds.
Keywords: wound healing, diabetes, macrophage, inflammation, resolution of inflammation
INTRODUCTION
Diabetes is associated with serious health complications, including cardiovascular disease, organ and limb ischemia, and impaired wound healing. Importantly, inflammation appears to contribute to the pathophysiology of these complications. Much research has focused on positive regulators of inflammation in the setting of diabetes, with hyperglycemia, hyperlipidemia and damage-associated molecules implicated in sustaining inflammation [1–3]. Recently, pathways involved in the resolution of inflammation have garnered attention for their potential contribution to non-resolving inflammatory responses [4, 5].
Various factors have been implicated in the resolution of inflammation, including lipid mediators, cytokines and nuclear receptors such as the peroxisome proliferator-activated receptors (PPARs). PPARs help to resolve inflammation by binding specific DNA sequences (PPAR response elements), thereby activating expression of pro-resolving genes and/or via binding of other transcriptional modulators and inhibiting pro-inflammatory gene expression [6–8]. In addition, the PPARs influence metabolism through effects on adipogenesis, mitochondrial biogenesis and fat oxidation. PPAR-γ activity in macrophages (Mp) has been reported to promote expression of genes involved in mitochondrial biogenesis, oxidative metabolism and an alternatively activated phenotype [9], although differing results have been reported [10].
During wound healing, Mp undergo a transition from pro-inflammatory to healing-associated phenotypes that is important for the regulation of angiogenesis, granulation tissue formation and wound closure [11–13]. However, in the setting of diabetes, Mp dysfunction contributes to impaired healing [14–19] and we recently identified the NLRP3/IL-1β pathway as a key player in sustaining a destructive pro-inflammatory Mp phenotype in diabetic wounds [20, 21]. In the present study, we test the hypothesis that sustained production of IL-1β downregulates PPAR-γ in diabetic wounds, impairing the switch from pro-inflammatory to pro-healing phenotypes, which in turn, leads to defective healing.
MATERIALS AND METHODS
Animals
Diabetic db/db (DB), non-diabetic db/+ (ND), IL-1R1 knockout (IL-1R1 KO) and C57Bl/6 wild-type (WT) controls were obtained from The Jackson Laboratory. Breeding pairs of Lyz2Cre and PPAR-γflox mice were also obtained from Jackson and bred as described previously [9, 22]. Lyz2Cre/PPAR-γflox mice were myeloid cell-specific PPAR-γ KO mice and littermate PPAR-γfl/fl mice were used as controls. Mice were housed under specific pathogen-free conditions and experiments were performed on 12–16 week-old male mice. Mice were randomly placed into treatment groups and housed singly thereafter. All procedures involving animals were approved by the Animal Care Committee at the University of Illinois at Chicago.
Excisional wounding and treatment
Mice were subjected to excisional wounding with an 8-mm biopsy punch as described previously [16, 20]. As indicated, wounds were treated either with IL-1β blocking antibody or IgG control (20 μg/wound) or with 15-deoxy-Δ-12,14-prostaglandin J2 (15d-PGJ2; 10 μM) or rosiglitazone (50 μM) in F-127 pluronic gel (50 μl of a 25% gel in saline); controls were treated with DMSO vehicle-loaded gel. Doses were chosen based on our in vitro data (cf. Figures 4, S2 and S3). All wounding and treatment procedures took place between 8 a.m. and noon, to minimize circadian interactions with procedures.
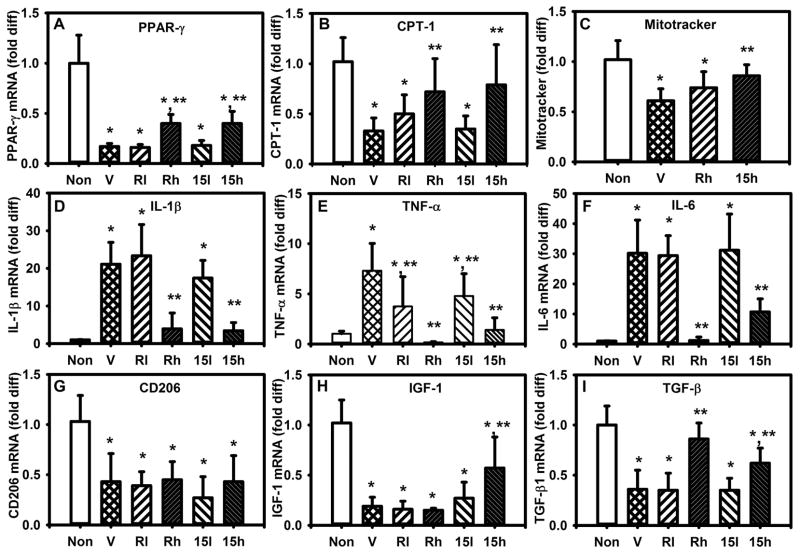
Figure 4. PPAR-γ agonists reverse effects of a simulated diabetic wound environment.
(A,B) Expression of PPAR-γ and downstream target CPT-1 in cultured bone marrow-derived Mp from non-diabetic (ND) mice either left non-stimulated (Non) or stimulated with day 10 DB wound conditioned medium (CM) plus vehicle (V), CM plus low or high doses of rosiglitazone (Rl, Rh; 10, 50 μM) or CM plus low or high doses of 15d-PGJ2 (15l, 15h; 1, 10 μM). (C) Median fluorescence (MFI) of MitoTracker staining, (D–F) expression of pro-inflammatory cytokines, (G–I) expression of healing-associated factors, with stimulation as indicated. For all graphs, bars = mean ± SD, n = 6. *mean value significantly different from that for Non controls, **mean value significantly different from that for CV + V treated samples, p < 0.05.
Human subjects
Six patients (2 male, 4 female) with chronic wounds provided informed consent. Patients were diagnosed with type 2 diabetes and had non-healing wounds on the sacral region or the lower limb, of at least 3 months’ duration. During a sharp debridement, biopsies were taken from tissue located near the center of the wound. All procedures involving human subjects were approved by the Institutional Review Board at the University of Illinois at Chicago according to Declaration of Helsinki Principles.
Cell isolation
Cells were dissociated from mouse excisional wound tissue and human chronic wound biopsies using an enzymatic digest [16, 20]. Neutrophils, T cells and B cells were marked for depletion by incubating cells with FITC-conjugated anti-Ly6G (1A8), anti-CD3 (17A2) and anti-CD19 (6D5) for mouse cells and FITC-conjugated anti-CD15 (HI98), anti-CD3 (UCHT1) and anti-CD19 (HIB19) for human cells (all from Biolegend), and then depleted from the total cell population using anti-FITC magnetic beads (Miltenyi Biotec). Cells of the monocyte/Mp lineage were then isolated using CD11b magnetic beads. Greater than 90% of these cells expressed Ly6C and/or F4/80, which are markers for cells of the Mo/Mp lineage [16]
Cell culture
To generate cultures of human or mouse Mp, peripheral blood mononuclear cells from normal volunteers (Zen-Bio) or bone marrow cells from wild-type C57Bl/6 mice and IL1R1 KO mice were cultured as described [20, 21]. As indicated, Mp were stimulated with IL-1β (20 ng/ml) or 20% human or mouse wound conditioned medium along with IgG blocking antibody or IgG control, or 15d-PGJ2, rosiglitazone or DMSO vehicle, using doses demonstrated to downregulate the pro-inflammatory Mp phenotype [23–25]. Wound conditioned medium was generated by incubating biopsies in DMEM + 10% FBS (1 ml/100 mg tissue) for 2 hours at 37°C.
RNA analysis
Total RNA was isolated from human or mouse cells using the RNeasy kit (Qiagen). Equal amounts of RNA were reverse-transcribed using the Thermoscript RT-PCR system (Invitrogen). Real-time PCR was performed using the standard run mode in a 7500Fast System (Applied Biosystems) using TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assay primer/probe sets (Applied Biosystems). Relative gene expression was determined using the 2−ΔΔCT method [26], with GAPDH as the endogenous control gene; GAPDH generated the most stable CT values among numerous control genes tested.
Flow cytometry
Cells dissociated from mouse wounds or cultured bone marrow-derived Mp were stained with MitroTracker Green (Life Technologies) to label mitochondria and PE-conjugated anti-F4/80 (BM8) to identify Mp. Mitochondrial content was assessed as the median fluorescence intensity within F4/80 positive cells.
Wound healing assays
Mouse wound healing was assessed on day 10 post-injury, by our published assays of external wound closure, using digital images of the external wound surface and re-epithelialization and granulation tissue formation with hematoxylin and eosin-stained cryosections [16, 20, 21]. Angiogenesis, neutrophil and macrophage accumulation and collagen deposition were measured in CD31-, Ly6G-, F4/80- and Trichrome-stained cryosections, respectively. For histological assays, digital images were obtained using a Nikon Instruments 80i microscope and DS-QI1 digital camera and analyzed using NIS Elements software.
ELISA
Mouse wounds were homogenized in cold PBS (10 μl of PBS per mg wound tissue) supplemented with protease inhibitor cocktail (Sigma). Supernatants of wound homogenates or cell culture medium were used for enzyme-linked immunoassay (ELISA) for IL-1β, TNF-α, IL-6 (eBioscience), IGF-1, TGF-β1, and VEGF (R&D Systems). When wound conditioned medium was used as a cell culture supplement, cytokine release was measured as the difference between levels achieved in wells with cultured cells and levels in blank wells that contained identical medium composition but no cells.
Statistics
Values are reported as means ± standard deviation. Measurements of Mp gene expression, wound closure, re-epithelialization, granulation tissue thickness, Trichrome staining CD31, F4/80 and Ly6G staining data were compared using ANOVA. ANOVA on ranks was used if data sets did not pass tests of normality and equal variance. The Student-Newman-Keuls post hoc test was used when ANOVAs demonstrated significance. Differences between groups were considered significant if P ≤ 0.05.
RESULTS
Impaired PPAR-γ activity in diabetic wound macrophages
We sought to determine whether impaired PPAR-γ activity contributes to a sustained pro-inflammatory Mp phenotype in the setting of diabetes. First, we isolated Mp from chronic wounds of diabetic patients and compared expression of PPAR-γ, PPAR coactivator (PGC)-1β and downstream targets CD36 and carnitine palmitoyl transferase (CPT)-1 in these cells to that of blood-derived Mp from healthy subjects. Expression of PPAR-γ and downstream targets was lower in chronic wound Mp than in non-stimulated blood-derived Mp, but comparable to that of IL-1β-stimulated blood-derived Mp (Figure 1A–D), suggesting that the pro-inflammatory environment of diabetic wounds may downregulate PPAR-γ activity (see also [27]).
Figure 1. Impaired PPAR-γ activity in diabetic wound macrophages.
(A–D) Macrophages (Mp) were isolated from chronic wound biopsies and expression of PPAR-γ, PGC-1β and downstream targets CD36 and CPT-1 assessed by real time PCR. For comparison, blood-derived Mp from healthy volunteers were either left non-stimulated (Non) or stimulated with IL-1β. (EH) Expression of PPAR-γ, PGC-1β and downstream targets in Mp isolated from wounds of non-diabetic (ND) and diabetic (DB) mice on days 5, 10 and 20 post-injury. (I–L) Expression of PPAR-γ, PGC-1β and downstream targets in bone marrow-derived Mp from wild-type mice stimulated with day 5 or 10 wound conditioned medium (CM) from ND or DB mice. (M) Representative flow cytogram of MitoTracker green labeling in Mp (F4/80+ cells) isolated from day 10 ND and DB wounds. (N) Summary data for median fluorescence intensity (MFI) for MitoTracker green in Mp isolated from day 5 and 10 wounds of ND and DB mice. (O) Representative flow cytogram of MitoTracker green labeling in cultured Mp treated with CM from day 10 wounds of ND or DB mice. (P) Summary data for MFI for MitoTracker green in cultured Mp treated with recombinant IL-1β or with CM from day 10 wounds of ND or DB mice. For all graphs, bars = mean + SD, n = 6 for human data and n = 6–8 for mouse data. *mean value significantly different from that for Non controls or 5d values, **mean value for DB significantly different from that for ND at same time point, p < 0.05.
To determine whether diabetic mice also exhibit impaired Mp PPAR-γ activity following skin wounding, Mp were isolated from wounds of non-diabetic (ND) and diabetic (DB) mice. On day 5 after injury, wound Mp from ND mice expressed low levels of PPAR-γ and downstream targets, but PPAR-γ activity was upregulated on day 10 (Figure 1E–H), coinciding with the switch from pro-inflammatory to pro-healing Mp phenotypes [16, 20]. In contrast, Mp isolated from wounds of DB mice exhibited only low levels PPAR-γ activity through day 10, associated with a sustained pro-inflammatory phenotype and impaired healing. Since IL-4 is known to upregulate PPAR-γ activity in Mp [9, 28], we measured IL-4 and IL-13 (which also signals through the IL-4 receptor) in wound homogenates. However, levels of both IL-4 and IL-13 were below the detection limit of the assay in all wounds of the experiment, including ND day 10 wounds, indicating that the late upregulation of PPAR-γ activity observed in ND wound Mp may be induced by an IL-4/IL-13-independent mechanism.
Diabetic wound environment downregulates macrophage PPAR-γ activity
To determine whether the diabetic wound environment plays a role in blocking the late upregulation in Mp PPAR-γ activity, we cultured bone marrow-derived Mp from WT mice with conditioned medium (CM) from ND or DB wounds. CM from day 5 wounds of both ND and DB mice downregulated expression of PPAR-γ, PGC-1β and downstream targets (Figure 1I–L). Interestingly, CM from day 10 wounds of ND mice downregulated expression of these genes to a lesser degree, indicating the release of inhibition of PPAR-γ activity at this time point. In contrast, inhibition of PPAR-γ activity was sustained by CM from day 10 wounds of DB mice. As with human Mp, recombinant IL-1β downregulated PPAR-γ activity in cultured mouse Mp. Taken together, these data indicate that the diabetic wound environment inhibits Mp PPAR-γ activity during wound healing.
Diabetic wound environment reduces macrophage mitochondrial content
Since PPAR-γ, PGC-1β and downstream target genes are associated with mitochondrial biogenesis, and mitochondrial biogenesis has previously been associated with the “alternatively activated” Mp phenotype [9, 29] we assessed mitochondrial content in wound Mp of ND and DB mice. Mitochondrial content increased from days 5 to 10 after injury in ND Mp (Figure 1M,N), associated with the switch in Mp phenotype. In contrast, DB wound Mp did not exhibit an increase in mitochondrial content, associated with the persistent pro-inflammatory phenotype. In addition, in our in vitro experiments, CM from day 10 wounds of DB mice decreased the mitochondrial content in cultured Mp, to a similar degree as recombinant IL-1β, whereas CM from day 10 wounds of ND mice had little or no effect (Figure 1O,P). Thus, increased PPAR-γ activity in ND wound Mp may lead to mitochondrial biogenesis associated with the healing-associated Mp phenotype and this pathway is impaired in DB wound Mp.
IL-1β in the diabetic wound environment inhibits macrophage PPAR-γ activity
We recently reported that Mp are the dominant producers of IL-1β in wounds of both ND and DB mice [30] and that sustained activity of the NLRP3 inflammasome/IL-1β pathway in Mp contributes to the persistent pro-inflammatory phenotype of wound Mp and impaired healing in diabetic mice [20, 21]. In Mp isolated from chronic wounds of diabetic patients, expression of IL-1β was negatively correlated with expression of PPAR-γ and its downstream target CPT-1 (Figure 2A–D). Furthermore, expression of PGC-1β and CD36 was barely detectable, suggesting that these genes were not induced to an appreciable level in chronic wound Mp. Thus, IL-1β may negatively regulate PPAR-γ activity in diabetic wounds.
Figure 2. IL-1β in the diabetic wound environment inhibits Mp PPAR-γ activity.
(A–D) Correlation between expression of IL-1β and expression of PPAR-γ, PGC-1β or downstream targets in macrophages (Mp) isolated from chronic wound biopsies; expression of each gene assessed by real time PCR. (E–H) Expression of PPAR-γ, PGC-1β and downstream targets in Mp isolated on day 10 after injury from wounds in diabetic (DB) mice treated topically with control IgG (Ig) or IL-1β blocking antibody (ab) compared with non-diabetic (ND) mice. (I–L) Expression of PPAR-γ, PGC-1β and downstream targets in cultured bone marrow-derived Mp from wild-type (WT) mice either left non-stimulated (Non) or stimulated with day 10 DB wound-conditioned medium (CM) along with Ig or ab. (M–P) Expression of PPAR-γ, PGC-1β and downstream targets in bone marrow-derived Mp from WT and IL-1R1 KO mice stimulated with CM from wounds of day 10 wounds of DB mice. For all graphs, bars = mean ± SD, n = 6. *mean value significantly different from that for ND mice for in vivo experiments and for non-stimulated controls for in vitro experiments, **mean value for ab-treated condition significantly different from that for Ig-treated condition. #mean value significantly different from that for Non controls of same strain, ##mean value significantly different from that for CM-treated WT macrophages, p < 0.05.
To determine mechanistically whether IL-1β inhibits upregulation of Mp PPAR-γ activity, we treated wounds of DB mice topically with an IL-1β blocking antibody [20]. Mp isolated from such wounds exhibited increased expression of PPAR-γ and downstream targets, compared to Mp from wounds treated with control IgG (Figure 2E–H). We also performed in vitro experiments to determine whether endogenous IL-1β in CM from DB wounds contributes to the inhibition of PPAR-γ activity observed in Figure 1. Indeed, cultured Mp treated with IL-1β blocking antibody along with CM from DB wounds showed less downregulation in PPAR-γ activity than those treated with control IgG and CM (Figure 2I–L). In addition, CM from day 10 DB wounds induced more pronounced downregulation of PPAR-γ activity in WT Mp than in IL-1R1 KO Mp. The latter data indicate that the lack of IL-1R1 reduced the sensitivity of cultured Mp to downregulation of PPAR-γ activity by wound CM (Figure 2M–P), which parallels the blunted CM-induced pro-inflammatory phenotype we observed previously in IL-1R1 KO Mp [20]. Note that CM still downregulated PPAR-γ and CD36, albeit to a lesser extent in IL-1R1 KO Mp compared to WT Mp, indicating that other factors in the CM, besides IL-1β, act to downregulate PPAR-γ activity. Taken together, these data support the hypothesis that IL-1β in the diabetic wound environment inhibits PPAR-γ activity and the switch to a pro-healing Mp phenotype.
Loss of PPAR-γ in macrophages prolongs inflammation and delays healing
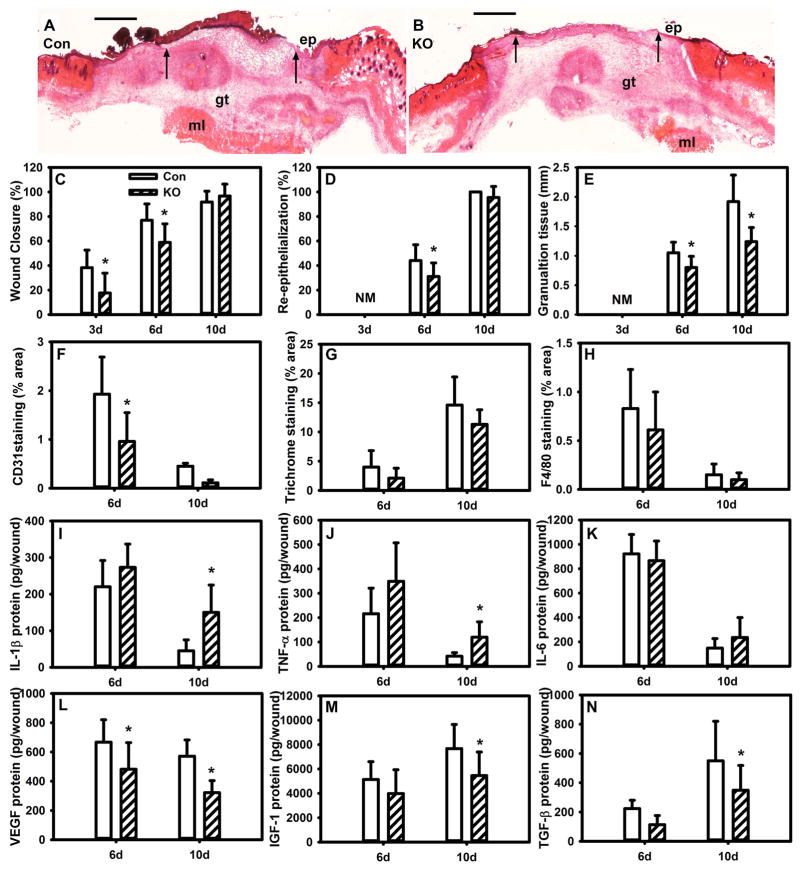
We next sought to determine whether loss of PPAR-γ activity in Mp prolongs inflammation and delays wound healing in the absence of diabetes. Myeloid-specific PPAR-γ KO mice were generated by crossing Lyz2cre mice with PPAR-γflox mice. Wound Mp demonstrated reduced expression of PPAR-γ and target genes in Mp PPAR-γ KO mice, indicating successful reduction of Mp PPAR-γ activity (Figure S1). Wound closure was modestly delayed in Mp PPAR-γ KO mice compared to littermate controls (Figure 3A–D), and angiogenesis and granulation tissue were significantly reduced (Figure 3E,F). In contrast, loss of Mp PPAR-γ activity had no effect on collagen deposition or wound Mp accumulation (Figure 3G,H) or on neutrophil accumulation (data not shown). However, the impairments in wound healing were associated with prolonged accumulation of the pro-inflammatory cytokines IL-1β and TNF-α and reduced levels of pro-healing factors VEGF, IGF-1 and TGF-β (Figure 3I–N). In short, these data indicate that loss of Mp PPAR-γ activity is sufficient to prolong inflammation, reduce growth factors and granulation tissue and induce a modest delay in wound closure, in the absence of diabetes. These impairments are less severe than those observed in DB mice, indicating that additional factors likely contribute to impaired healing in diabetes.
Figure 3. Myeloid cell-specific knockout of PPAR-γ impairs resolution of inflammation and wound healing in non-diabetic mice.
(A,B) Excisional wounds in control (Con) PPAR-γflox mice and Lyz2Cre/PPAR-γflox knockout (KO) mice were harvested on day 6 after injury, sectioned and stained with H&E. Note the reduced re-epithelialization and granulation tissue formation in the KO mice. ep: epithelium, gt: granulation tissue, ml: muscle layer; arrows indicate ends of epithelial tongues, scale bar = 0.5 mm. (C) Wound closure assessed in digital images of wound surface on days 3, 6 and 10 after injury; (D,E) re-epithelialization and granulation tissue thickness measured in H&E stained cryosections; (F–H) CD31 staining, Trichrome staining and F4/80 staining measured in cryosections as % area stained. (I–N) Levels of cytokines in wound homogenates measured using ELISA, including pro-inflammatory cytokines IL-1β, TNF-α, IL-6 and healing-associated cytokines VEGF, IGF-1, TGF-β. For all graphs, bars = mean ± SD, n = 8. *mean value significantly different from that for Con at same time point, p < 0.05.
PPAR-γ agonists reverse pro-inflammatory effects of diabetic wound environment
Since the diabetic wound environment downregulates PPAR-γ activity (Figures 1 and 2) and induces a pro-inflammatory Mp phenotype [20], we sought to determine whether the PPAR-γ agonists 15d-PGJ2 and rosiglitazone could reverse this process and promote a pro-healing Mp phenotype. We first performed in vitro experiments, in which CM from DB wounds again downregulated PPAR-γ activity and mitochondrial content (Figure 4A–C), increased the expression of pro-inflammatory cytokines (Figure 4D–F) and decreased that of healing-associated genes (Figure 4G–I) in cultured Mp of WT mice. However, the PPAR-γ agonists dose-dependently increased the expression of PPAR-γ and its downstream targets, increased the mitochondrial content, decreased the expression of pro-inflammatory cytokines and increased that of healing-associated genes. Since PPAR-γ agonists can exert both PPAR-γ-dependent and PPAR-γ-independent effects, we performed additional experiments comparing responses of PPAR-γ KO Mp to control Mp. PPAR-γ KO Mp were less responsive to PPAR-γ agonists (Figure S2), indicating that at least part of the upregulation of PPAR-γ target genes, downregulation of IL-1β and TNF-α, and upregulation of VEGF was PPAR-γ-dependent. Furthermore, to determine whether PPAR-γ agonists could also promote a pro-healing phenotype in human Mp, blood-derived Mp of normal human subjects were stimulated with CM from chronic wound biopsies along with PPAR-γ agonists. As with mouse Mp, PPAR-γ agonists downregulated the CM-induced pro-inflammatory Mp phenotype and promoted a healing-associated Mp phenotype (Figure S3).
Previous studies have reported that inhibition of oxidative metabolism prevents full expression of the alternatively activated Mp phenotype [29, 31]. As in these previous studies, we used etomoxir to inhibit CPT-1 activity, which catalyzes a rate-limiting step in fatty acid transport into mitochondria for oxidation. However, etomoxir did not influence expression of pro-inflammatory genes in cultured mouse Mp, either alone or in combination with wound conditioned medium and PPAR-γ agonists (Figure S4). Likewise, etomoxir did not affect expression of the pro-healing genes VEGF or TGF-β1, but did downregulate IGF-1. Thus, inhibiting CPT-1 activity had only limited effects on Mp phenotype in a simulated diabetic wound environment.
PPAR-γ agonists promote wound healing in diabetic mice
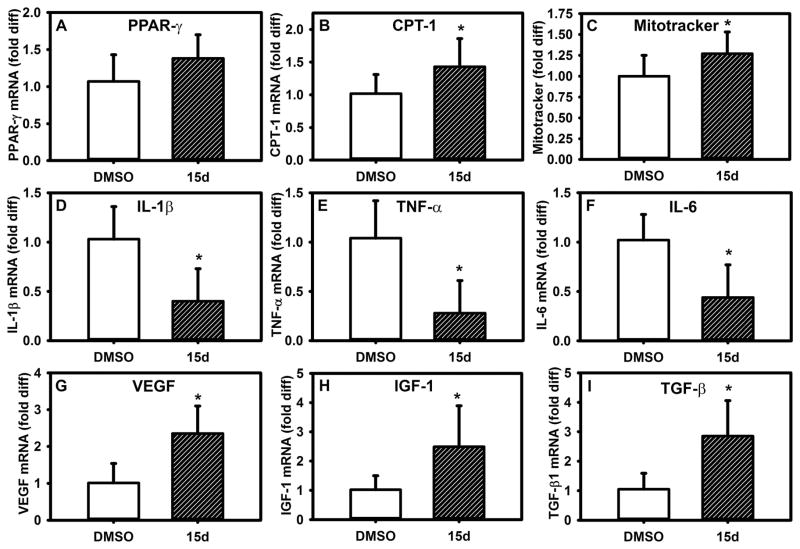
To determine whether PPAR-γ agonists also promote a pro-healing wound Mp phenotype in vivo, we treated wounds of DB mice topically with PPAR-γ agonists. Indeed, treatment with 15d-PGJ2 increased PPAR-γ activity and mitochondrial content in wound Mp (Figure 5A–C), decreased the expression of pro-inflammatory cytokines (Figure 5D–F) and increased that of pro-healing genes (Figure 5G–I). Thus, PPAR-γ agonists can reverse the diabetic environment-induced pro-inflammatory phenotype and induce a healing-associated phenotype in vivo.
Figure 5. PPAR-γ agonists induce switch to healing-associated wound Mp phenotype in diabetic mice.
Excisional wounds in diabetic (DB) mice were treated topically with 15d-PGJ2 (10 μM) or DMSO vehicle and Mp isolated from day 10 wounds. (A,B) Wound Mp expression of PPAR-γ and downstream target CPT-1, (C) median fluorescence (MFI) of MitoTracker staining, (D–F) expression of pro-inflammatory cytokines, (G–I) expression of healing-associated factors, with stimulation as indicated. For all graphs, bars = mean ± SD, n = 8. *mean value significantly different from that for DMSO controls, p < 0.05.
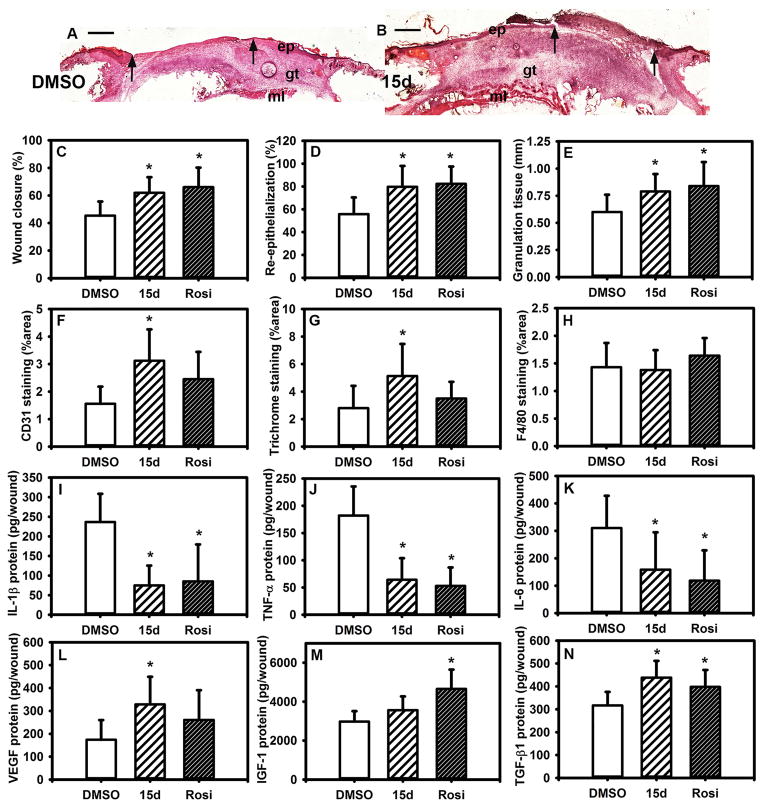
Finally, we determined whether PPAR-γ agonists downregulate inflammation and promote healing of wounds in DB mice. Topical treatment with PPAR-γ agonists accelerated wound closure, as assessed both externally and histologically (Figure 6A–D). The PPAR-γ agonists also tended to increase granulation tissue formation (Figure 6A,B,E), angiogenesis (Figure 6F) and collagen deposition (Figure 6G), but had no effect on Mp accumulation (Figure 6H) or neutrophil accumulation (not shown). The effects on angiogenesis and collagen deposition were significant for 15d-PGJ2 but not for rosiglitazone, indicating that these drugs may have somewhat different mechanisms of action. Both the PPAR-γ agonists reduced the levels of pro-inflammatory cytokines in the wound (Figure 6I–K) and tended to increase the levels of pro-healing growth factors (Figure 6L–N), although there were again differences between drugs, with 15d-PGJ2 increasing VEGF levels and rosiglitazone increasing IGF-1 levels. To determine whether the effects of PPAR-γ agonists were indeed mediated by PPAR-γ, we treated wounds of Mp PPAR-γ KO mice and controls with topical rosiglitazone. Whereas rosiglitazone induced a modest acceleration of wound closure in control mice, this effect was absent in KO mice (Figure S5), indicating that Mp PPAR-γ is needed for the full positive effect of rosiglitazone. Taken together with the data on the changes in Mp phenotype, these data indicate that PPAR-γ agonists promote healing in part by altering Mp production of cytokines and growth factors.
Figure 6. PPAR-γ agonists improve wound healing in diabetic mice.
Excisional wounds in diabetic (DB) mice were treated topically with 15d-PGJ2 (10 μM), rosiglitazone (50 μM) or DMSO vehicle and harvested on day 10 after injury. (A,B) Examples of wounds treated with 15d-PGJ2 and assessed by H&E staining. Note the increased re-epithelialization and granulation tissue formation in the PPAR-γ agonist treated mice. ep: epithelium, gt: granulation tissue, ml: muscle layer; arrows indicate ends of epithelial tongues, scale bar = 0.5 mm. (C) Wound closure assessed in digital images of wound surface, (D,E) re-epithelialization and granulation tissue thickness measured in H&E-stained cryosections (F–H) CD31 staining, Trichrome staining and F4/80 staining measured in cryosections as % area stained. (I–N) Levels of cytokines in wound homogenates measured using ELISA, including pro-inflammatory cytokines IL-1β, TNF-α, IL-6 and healing-associated cytokines VEGF, IGF-1, TGF-β. For all graphs, bars = mean ± SD, n = 8. *mean value significantly different from that for DMSO treatment, p < 0.05.
DISCUSSION
Chronic diabetic wounds are often described as being “stuck” in the inflammatory phase of wound healing, but much remains to be learned about the mechanisms that contribute to this persistent inflammatory response. The major finding of this study is that PPAR-γ plays a vital role in the switch from pro-inflammatory to pro-healing Mp phenotypes during normal wound healing, and that the diabetic wound environment impairs the upregulation of PPAR-γ activity and the switch to a healing-associated Mp phenotype (Figure S6). In particular, sustained production of IL-1β in the diabetic wound environment inhibits PPAR-γ activity in both mouse and human Mp. Importantly, local administration of PPAR-γ agonists promoted a healing-associated macrophage phenotype leading to improved healing, suggesting an appealing strategy for downregulating inflammation and improving healing of chronic wounds.
Our previous studies demonstrated that sustained production of IL-1β by the NLRP3 inflammasome in diabetic wounds blocks the induction of a pro-healing wound Mp phenotype [20, 21]. In the present study, we provide a mechanism by which IL-1β impairs the induction of pro-healing Mp, namely by blocking the upregulation of PPAR-γ activity. Another group reported that IL-1β inhibits PPAR-γ expression in articular chondrocytes and may be involved in the pathophysiology of osteoarthritis [32]. In addition, the pro-inflammatory cytokine IFN-γ has been shown to downregulate expression of PPAR-γ and target genes in human blood monocyte-derived Mp [27]. Thus, the inhibition of PPAR-γ activity may represent a common feedback mechanism by which inflammatory stimuli impede the resolution of inflammation. However, our data do not exclude the possibility that IL-1β and PPAR-γ each may play roles in wound inflammation and healing that are independent of each other.
Although IL-4 can upregulate Mp PPAR-γ activity and an alternatively activated Mp phenotype [9, 28], our data indicate that the upregulation of PPAR-γ activity in ND wound Mp occurred through an IL-4/IL-13-independent mechanism. These data corroborate those of our previous study [16] and others [33] that also reported a transition in wound Mp phenotype in the absence of IL-4 and IL-13. An alternative mechanism underlying the upregulation of Mp PPAR-γ activity is the phagocytosis of apoptotic cells, which has been shown to both induce PPAR-γ and a phenotypic switch consistent with that observed in the present study [34]. In addition, impaired phagocytosis of apoptotic cells has been proposed as a mechanism by which wound healing is impaired in diabetes [15]. Thus, the role of phagocytosis in the upregulation on PPAR-γ activity in wound Mp may deserve future study.
Our data showing that upregulation of Mp PPAR-γ activity promotes the resolution of inflammation and wound healing in DB mice are consistent with in vitro data indicating that PPAR-γ activity downregulates the expression of pro-inflammatory molecules in cultured Mp [23, 24], enhances pro-angiogenic potential of endothelial cells and bone marrow cells [35] and migration and TGF-β production of fibroblasts [36]. Our data are also consistent with in vivo data indicating that the resolution of inflammation during incisional wound healing is associated with increased PPAR-γ expression [37], that resolution of inflammation in acute peritoneal inflammation is impaired in myeloid cell-specific PPAR-γ knockout mice [38] and that PPAR-γ agonists induce a switch from a pro-inflammatory to a pro-healing Mp phenotype during wound healing [39]. In contrast, a recent study reported that rosiglitazone induced a destructive wound Mp phenotype and delayed healing in IL-6 deficient mice treated with UV radiation [40], indicating that the effects of rosiglitazone on Mp and on wound healing may depend on the wound environment. Our data do not exclude possible effects of the agonists used on other PPAR isoforms (e.g. PPAR-β/δ), other transcription factors (e.g. NF-κB) and other wound cells (e.g. keratinocytes and fibroblasts) [25, 41–44]. Nonetheless, our data indicate that in the setting of diabetes, PPAR-γ agonists induce a pro-healing Mp phenotype, the resolution of inflammation and better wound healing.
Recent studies have emphasized the importance of metabolic pathways in regulating Mp phenotypes, with glycolytic metabolism dominating in pro-inflammatory Mp and oxidative metabolism in healing-associated Mp [45–47]. Our data indicate that, as wound healing progresses, genes associated with lipid transport (CD36, CPT-1) and mitochondrial biogenesis (PPAR-γ, PGC-1β) are upregulated in wound Mp along with increased mitochondrial content. Although CPT-1 knockdown or inhibition with etomoxir has been reported to promote expression of pro-inflammatory genes [29, 31], our initial experiments have not corroborated these findings. A potential reason for the differing results is the different types of stimulation used in each experiment, with etomoxir enhancing palmitate-induced pro-inflammatory gene expression in THP-1 cells [31] and blocking IL-4-induced downregulation of pro-inflammatory genes in IFN-γ/LPS-stimulated mouse bone marrow-derived Mp [29], but not influencing PPAR-γ agonist-induced pro-inflammatory gene downregulation in diabetic wound CM treated mouse Mp (present study).
Limitations of this study include use of a single mouse model of type 2 diabetes, the leptin receptor mutant DB mouse. However, we and others have demonstrated similarities between the healing responses in DB mice and diabetic humans, including prolonged accumulation of Mp and pro-inflammatory cytokines and proteases, reduced levels of growth factors, delayed closure, and reduced angiogenesis and matrix deposition [16, 17, 20, 21, 48–56]. Also, the present study demonstrates similarities in the regulation of Mp phenotypes by IL-1β and PPAR-γ in both DB mouse and diabetic human Mp. Another limitation is the small number of human diabetic participants and the lack of data on wound macrophages from non-diabetic participants; ongoing studies will address these issues. Finally, assessments of wound Mp mitochondria were limited to expression of genes associated with mitochondrial biogenesis or function and incorporation of a dye to indicate mitochondrial content. An in-depth study of Mp substrate utilization and mitochondrial function during normal and impaired wound healing is warranted.
In conclusion, PPAR-γ appears to be a central regulatory factor in the switch of Mp phenotypes from pro-inflammatory to pro-healing and the downregulation of inflammation that are required for efficient wound healing. Since there is uncertainty about the effects of PPAR-γ agonists on the risk of cardiovascular events in humans [57, 58], our data is important because it demonstrates that topical administration of PPAR-γ agonists downregulated inflammation and improved angiogenesis, granulation tissue formation and wound closure. Future study will reveal whether similar treatment with PPAR-γ agonists can downregulate inflammation and improve healing in human diabetic wounds.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (R01GM092850 to TK). The authors thank Dr. Giamila Fantuzzi, University of Illinois at Chicago, for critical comments on a previous draft of this manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Footnotes
Conflict of Interest Statement: The authors have no conflicting financial interests.
AUTHOR CONTRIBUTIONS
RM contributed to study design, researched data, and wrote the manuscript. MF researched data, reviewed and edited the manuscript. MN researched data, reviewed and edited the manuscript, NU researched data, reviewed and edited the manuscript, AS researched data, reviewed and edited manuscript. WE contributed to study design, reviewed and edited the manuscript. TK designed the study, and wrote the manuscript.
NOTE
While our manuscript was in review, another group published findings demonstrating significantly reduced angiogenesis and granulation tissue formation and modestly delayed wound closure in Lyz2Cre/PPAR-γflox (Mp PPAR-γ KO) compared to control mice [59]. As in our study, impaired healing was associated with elevated expression of TNF-α and reduced expression of VEGF. These newly published data also support a role for impaired apoptotic cell clearance in the impaired healing of Mp PPAR-γ KO mice.
References
- 1.Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill MJ, Metcalfe D, McTernan PG. Obesity and diabetes: lipids, ‘nowhere to run to’. Clin Sci (Lond) 2009;116:113–123. doi: 10.1042/CS20080050. CS20080050 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Jin C, Flavell RA. Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol. 2013;132:287–294. doi: 10.1016/j.jaci.2013.06.022. S0091-6749(13)00990-1 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Maskrey BH, Megson IL, Whitfield PD, et al. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:1001–1006. doi: 10.1161/ATVBAHA.110.213850. 31/5/1001 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. S1550-4131(13)00418-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. 106/10/1559 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. S1471-4906(07)00244-X [pii] [DOI] [PubMed] [Google Scholar]
- 8.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. S0092-8674(05)01277-8 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marathe C, Bradley MN, Hong C, et al. Preserved glucose tolerance in high-fat-fed C57BL/6 mice transplanted with PPARgamma−/−, PPARdelta−/−, PPARgammadelta−/−, or LXRalphabeta−/− bone marrow. J Lipid Res. 2009;50:214–224. doi: 10.1194/jlr.M800189-JLR200. M800189-JLR200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goren I, Allmann N, Yogev N, et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. ajpath.2009.081002 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. jimmunol.0903356 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. ajpath.2009.090248 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bannon P, Wood S, Restivo T, et al. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis Model Mech. 2013;6:1434–1447. doi: 10.1242/dmm.012237. dmm.012237 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56:256–264. doi: 10.1016/j.cyto.2011.06.016. S1043-4666(11)00208-0 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Wetzler C, Kampfer H, Stallmeyer B, et al. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. jid029 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Tian H, Lu Y, Shah SP, et al. Autacoid 14S,21R-dihydroxy-docosahexaenoic acid counteracts diabetic impairment of macrophage prohealing functions. Am J Pathol. 2011;179:1780–1791. doi: 10.1016/j.ajpath.2011.06.026. S0002-9440(11)00644-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodero MP, Hodgson SS, Hollier B, et al. Reduced Il17a Expression Distinguishes a Ly6c(lo)MHCII(hi) Macrophage Population Promoting Wound Healing. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.368. jid2012368 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Mirza RE, Fang MM, Ennis WJ, et al. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–2587. doi: 10.2337/db12-1450. db12-1450 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirza RE, Fang MM, Weinheimer-Haus EM, et al. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63:1103–1114. doi: 10.2337/db13-0927. db13-0927 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama TE, Sakai S, Lambert G, et al. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–2619. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 24.Ricote M, Li AC, Willson TM, et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 25.Welch JS, Ricote M, Akiyama TE, et al. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. S1046-2023(01)91262-9 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Szanto A, Balint BL, Nagy ZS, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. S1074-7613(10)00415-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 29.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. S1550-4131(06)00202-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirza RE, Koh TJ. Contributions of cell subsets to cytokine production during normal and impaired wound healing. Cytokine. 2015;71:409–412. doi: 10.1016/j.cyto.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namgaladze D, Lips S, Leiker TJ, et al. Inhibition of macrophage fatty acid beta-oxidation exacerbates palmitate-induced inflammatory and endoplasmic reticulum stress responses. Diabetologia. 2014;57:1067–1077. doi: 10.1007/s00125-014-3173-4. [DOI] [PubMed] [Google Scholar]
- 32.Afif H, Benderdour M, Mfuna-Endam L, et al. Peroxisome proliferator-activated receptor gamma1 expression is diminished in human osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes. Arthritis Res Ther. 2007;9:R31. doi: 10.1186/ar2151. ar2151 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daley JM, Brancato SK, Thomay AA, et al. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freire-de-Lima CG, Xiao YQ, Gardai SJ, et al. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. M605146200 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Kotlinowski J, Grochot-Przeczek A, Taha H, et al. PPARgamma activation but not PPARgamma haplodeficiency affects proangiogenic potential of endothelial cells and bone marrow-derived progenitors. Cardiovasc Diabetol. 2014;13:150. doi: 10.1186/s12933-014-0150-7. s12933-014-0150-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao H, Pastar I, Chen W. Rosiglitazone modulates the behaviors of diabetic host-derived fibroblasts in a carboxymethyllysine-modified collagen model. Wound Repair Regen. 2012;20:435–443. doi: 10.1111/j.1524-475X.2012.00795.x. [DOI] [PubMed] [Google Scholar]
- 37.Kapoor M, Kojima F, Yang L, et al. Sequential induction of pro- and anti-inflammatory prostaglandins and peroxisome proliferators-activated receptor-gamma during normal wound healing: a time course study. Prostaglandins Leukot Essent Fatty Acids. 2007;76:103–112. doi: 10.1016/j.plefa.2006.11.006. S0952-3278(06)00185-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautier EL, Chow A, Spanbroek R, et al. Systemic analysis of PPARgamma in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. J Immunol. 2012;189:2614–2624. doi: 10.4049/jimmunol.1200495. jimmunol.1200495 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasegawa-Moriyama M, Ohnou T, Godai K, et al. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates postincisional pain by regulating macrophage polarization. Biochem Biophys Res Commun. 2012;426:76–82. doi: 10.1016/j.bbrc.2012.08.039. S0006-291X(12)01549-5 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Das LM, Rosenjack J, Au L, et al. Hyper-Inflammation and Skin Destruction Mediated by Rosiglitazone Activation of Macrophages in IL-6 Deficiency. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.375. jid2014375 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao-Qiang M, Fowler AJ, Schmuth M, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004;123:305–312. doi: 10.1111/j.0022-202X.2004.23235.x. JID23235 [pii] [DOI] [PubMed] [Google Scholar]
- 42.Chong HC, Tan MJ, Philippe V, et al. Regulation of epithelial-mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J Cell Biol. 2009;184:817–831. doi: 10.1083/jcb.200809028. jcb.200809028 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michalik L, Desvergne B, Tan NS, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPARbeta mutant mice. J Cell Biol. 2001;154:799–814. doi: 10.1083/jcb.200011148. 154/4/799 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straus DS, Pascual G, Li M, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro H, Lutaty A, Ariel A. Macrophages, meta-inflammation, and immuno-metabolism. ScientificWorldJournal. 2011;11:2509–2529. doi: 10.1100/2011/397971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. nature11862 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Mounier R, Theret M, Arnold L, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. S1550-4131(13)00287-8 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23:594–608. doi: 10.1111/j.1464-5491.2006.01773.x. DME1773 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Greenhalgh DG, Sprugel KH, Murray MJ, et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 50.Lobmann R, Ambrosch A, Schultz G, et al. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 51.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4:411–420. doi: 10.1046/j.1524-475X.1996.40404.x. WRRwrr_040404 [pii] [DOI] [PubMed] [Google Scholar]
- 52.Mirza RE, Koh TJ. Contributions of cell subsets to cytokine production during normal and impaired wound healing. Cytokine. 2014 doi: 10.1016/j.cyto.2014.09.005. S1043-4666(14)00533-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodgers KE, Ellefson DD, Espinoza T, et al. Expression of intracellular filament, collagen, and collagenase genes in diabetic and normal skin after injury. Wound Repair Regen. 2006;14:298–305. doi: 10.1111/j.1743-6109.2006.00124.x. WRR124 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. 44490 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 56.Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen. 1996;4:234–239. doi: 10.1046/j.1524-475X.1996.40211.x. WRRwrr_040211 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. NEJMoa072761 [pii] [DOI] [PubMed] [Google Scholar]
- 58.Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med. 2007;147:578–581. doi: 10.7326/0003-4819-147-8-200710160-00182. [DOI] [PubMed] [Google Scholar]
- 59.Chen H, Shi R, Luo B, et al. Macrophage peroxisome proliferator-activated receptor gamma deficiency delays skin wound healing through impairing apoptotic cell clearance in mice. Cell death & disease. 2015;6:e1597. doi: 10.1038/cddis.2014.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.