Abstract
Background
This study examines the cost-effectiveness of contingency-management (CM) for stimulant dependence among community mental health patients with serious mental illness (SMI).
Methods
Economic evaluation of a 12-week randomized controlled trial investigating the efficacy of CM added to treatment-as-usual (CM+TAU), relative to TAU without CM, for treating stimulant dependence among patients with a SMI. The trial included 176 participants diagnosed with SMI and stimulant dependency who were receiving community mental health and addiction treatment at one community mental health center in Seattle, Washington. Participants were also assessed during a 12-week follow-up period. Positive and negative syndrome scale (PANSS) scores were used to calculate quality-adjusted life-years (QALYs) for the primary economic outcome. The primary clinical outcome, the stimulant-free year (SFY) is a weighted measure of time free from stimulants. Two perspectives were adopted, those of the provider and the payer.
Results
At 12-weeks neither the provider ($2,652, p=.74) nor the payer ($2,611, p=.99) cost differentials were statistically significant. This was also true for the payer at 24-weeks (-$125, p=1.00). QALYs gained were similar across groups, resulting in small, insignificant differences (0.04, p=0.23 at 12-weeks; .01, p=0.70 at 24 weeks). CM+TAU experienced significantly more SFYs, 0.24 (p<0.001) at 12 weeks and 0.20 (p=0.002) at 24 weeks, resulting in at least an 85% chance of being considered cost-effective at a threshold of $200,000/SFY.
Conclusion
Contingency management appears to be a wise investment for both the provider and the payer with regard to the clinical outcome of time free from stimulants.
Keywords: Contingency management, Cost effectiveness, Stimulant use, Serious mental illness
1. INTRODUCTION
Contingency management (CM) is a well-established intervention for drug and alcohol use disorders. CM employs positive reinforcers (e.g., vouchers or prizes) when individuals demonstrate drug or alcohol abstinence. Meta-analyses of CM have found it to be associated with higher rates of treatment retention and abstinence, relative to standard care (Benishek et al., 2014; Dutra et al., 2008; Lussier et al., 2006; Prendergast et al., 2006). CM has demonstrated efficacy as a treatment for stimulants (cocaine, amphetamine, methamphetamine), marijuana, opioids, nicotine, and alcohol use disorders. Importantly, Dutra and colleagues (2008) compared CM approaches to all other psychosocial treatments and found that they had the highest rates of in-treatment abstinence. However, the relatively high in-treatment abstinence rates of CM are not typically sustained (Dutra et al., 2008; Rawson et al., 2002, 2006).
Emerging literature has demonstrated the effectiveness of CM for individuals with substance use disorders (SUDs) who also suffer from severe mental illnesses (SMI; Bellack et al., 2006; McDonell et al., 2013; Roll et al., 2004). Adults with SMI, such as schizophrenia, bipolar and re-occurring major depressive disorders suffer from high rates of SUDs, with lifetime rates as high as 50% (Regier et al., 1990). Relative to people with only one of these conditions, individuals with co-occurring SMIs and SUDs have more severe substance use and psychiatric symptoms (RachBeisel et al., 1999), poorer treatment adherence (Bennett et al., 2001), increased homelessness (Galanter et al., 1998), and higher rates of smoking (de Leon et al., 2007), HIV infection (RachBeisel et al., 1999), psychiatric hospitalization (Haywood et al., 1995), emergency room use (Bartels et al., 1993) and incarceration (Abram and Teplin, 1991). The high rates of SUDs among individuals with SMI, and the consequences of this comorbidity, directly contribute to the high economic cost of SMI in the U.S., which is estimated to be well over $400 billion (2013 USD) annually (Insel, 2008).
Many people with comorbid SUD and SMI do not receive concurrent treatment for the disorders (Substance Abuse and Mental Health Services Administration, 2002; Watkins et al., 2001a), although integrated treatments have been shown to reduce drug use (Baker et al., 2006; Barrowclough et al., 2010; Bellack et al., 2006; Drake et al., 1998; Epstein et al., 2004; Watkins et al., 2001b; Weiss et al., 2009). While the results pertaining to reductions in psychiatric severity associated with many integrated treatments are mixed (Drake et al., 2008), two randomized, controlled trials (RCTs) have shown that CM alone (McDonell et al., 2013), or as part of a cognitive behavioral treatment (Bellack et al., 2006) can reduce drug and alcohol use, improve psychiatric symptoms, and reduce inpatient hospitalizations in adults who suffer from co-occurring SUDs and SMI. Moreover, a recent Cochrane Collaboration review reported that CM is a promising treatment for SUDs in outpatients with SMI (Hunt et al., 2013).
Despite the apparent promise of CM interventions in treating co-occurring SMI and SUD, perceived cost and an inability to bill for urine tests and tangible reinforcers present a significant barrier to implementation (Kirby et al., 1999; McGovern et al., 2004; Petry and Simcic Jr, 2002; Srebnik et al., 2013). Information regarding the cost-effectiveness of CM is needed to inform policymakers who are increasingly making decisions about the availability of such treatments based on their clinical and cost effectiveness (Petry et al., 2014). Previous cost-effectiveness analyses (CEAs) on CM have been favorable, but have focused on its application to the treatment of specific drugs rather than co-occurring SMI and SUD, and have focused solely on clinical measures for the effectiveness outcome, such as abstinence or treatment completion (Olmstead and Petry, 2009; Olmstead et al., 2007a, 2007b, c; Sindelar et al., 2007a, 2007b). No studies to date have investigated the cost-effectiveness of CM for individuals with comorbid SMI and SUD, a particularly costly population.
Given that substance misuse affects most areas of functioning and SUDs are generally chronic conditions, quality-of-life is increasingly viewed as an important component of long-term recovery (Laudet, 2011); despite that, it is rarely included as an outcome in contingency-management CEAs. A cost-utility analysis (CUA) assesses the relative cost-effectiveness of an intervention; however, the outcome includes a measure of utility (i.e., satisfaction), and is often expressed as quality-adjusted life years (QALYs). QALYs are beneficial as a measure of effectiveness, in that they reflect the combined preference for length and quality of life. The purpose of this study is to conduct an economic evaluation of a CM intervention as an add-on to treatment-as-usual (TAU) for treating stimulant use disorders among 176 outpatients with a SMI.
2. METHODS
2.1. CM intervention
McDonell et al. (2013) conducted a 12-week randomized controlled trial of CM with treatment-as-usual (CM+TAU) relative to TAU with non-contingent rewards for 176 individuals with SMI and stimulant dependence who were receiving community mental health and addiction treatment at one community mental health center in Seattle, Washington. Participants were assessed during the intervention as well as during a 12-week follow-up period. Eligibility criteria for the study included using stimulants in the 30 days prior to the study, and meeting Mini International Neuropsychiatric Interview criteria for methamphetamine, amphetamine or cocaine dependence, and criteria for schizophrenia or schizoaffective (39% of participants), bipolar I or II (34% of participants), or recurrent major depressive disorder (27% of participants). The urn randomization procedure was used to balance the groups according to gender, substance use severity, mood versus psychotic disorder, and psychiatric hospitalization in the year prior to the study.
Participants were randomized to either CM+TAU or TAU with noncontingent rewards for participation. The variable magnitude of reinforcement CM procedure was used. To start, participants in the CM group earned 1 draw from a bowl of tokens for each urine sample that was negative for stimulants (i.e., amphetamine, methamphetamine and cocaine). The tokens varied in value, with 50% simply reading “good job”, and the remainder being associated with a prize valued anywhere between $1 and $80. Urine samples were collected 3 times per week with reward draws at each session. Each full week of continued stimulant abstinence resulted in an additional opportunity to draw a token, as did testing negative for alcohol, opioids and marijuana. If a participant tested positive for stimulants, or missed their session, they were unable to draw a token on that session, and had their number of draws reset to 1. TAU participants were quasi-yoked to those in the CM+TAU group, such that their number of prize draws was equal to the average number for CM participants in the week prior. The number of prize draws for the first week was determined by reassigning the first 5 TAU participants to CM+TAU and calculating their average number of prize draws. These 5 participants were then excluded from the intention-to-treat sample. Noncontingent participants therefore received the same number of prizes as their CM+TAU counterparts, but received prizes for submitting urine samples, instead of for submitting drug-free urine samples. Services provided as part of TAU included: mental health, chemical dependency, housing, and vocational.
For the 12-week intervention period, CM+TAU was associated with significantly fewer days of stimulant use (0.91 versus 4.67, p<0.05) and alcohol use (1.84 versus 4.32, p<0.05), and a significantly lower rate of injection drug use engagement (37% vs. 66%, p<0.05) compared with TAU. Days-of-stimulant-use was also significantly lower for CM+TAU relative to TAU during the follow-up period (1.83 versus 3.65, p<0.05).
2.2. Cost measures
The resource costing method was used to calculate costs. This method consists of multiplying the number of units of each resource utilized by participants, by the respective unit cost. The cost for each participant is then obtained by summing the relevant costs.
Few resources were required for the intervention itself; these included: a case manager, urine analysis (UA) supplies, and reinforcers. The case manager's time (including the time it took to order and manage prizes) was valued using the median annual salary from the Bureau of Labor Statistics’ (BLS) Occupational Outlook Handbook (2014), $28,850, as well as the BLS’ estimated benefit rate of 30.2% for the health care and social assistance industry group. The case manager's estimated total annual compensation was $41,332 ($19.87 per hour). Case managers spent approximately 15 minutes with each client per visit, and 5 hours per week managing prizes for all clients. The values of the (UA) supplies and reinforcers were obtained from the principal investigator and research coordinator. The average cost of UA supplies and reinforcers was $256. Intervention costs varied by individual, by week, depending on session attendance, the number of draws for prizes, and the value of the prizes received. We did not include the cost of the noncontingent prizes received by the control group, as the prizes were not designed to influence the decision-making process of the control group and would not be used in “real-world” applications of the intervention. Therefore, incorporating the value of the noncontingent prizes would bias the costs in favor of the CM group.
The number of non-study outpatient mental-health and chemical-dependency visits, days of inpatient psychiatric and substance abuse treatment, number of detoxification admissions and the number of emergency department (ED) visits were collected from the Washington State Department of Social and Health Services’ (DSHS) databases (McDonell et al., 2013). Mean unit cost estimates from SAMHSA's Alcohol and Drug Services Study (ADSS; Substance Abuse and Mental Health Services Administration, 2003) were used to value outpatient, inpatient and detoxification services. ED visits were valued using mean expenditures for adults aged 18 to 64 years from the Health, United States, 2012 report (National Center for Health Statistics, 2013). All dollar values were converted to 2013 U.S. dollars using the BLS Consumer Price Index for medical care.
2.3. Effectiveness measures
We calculated both a clinical and an economic measure of effectiveness. The clinical outcome, stimulant-free years, is a weighted measure of time free of stimulants. This measure was based on the number of stimulant positive urine samples, measured 3 times a week during the intervention period and monthly during the 12-week follow-up period.
Our primary economic effectiveness measure is the QALY. QALYs are calculated by multiplying the duration of time spent in a given health state by a preference-weighted health-related quality-of-life (HRQoL) score associated with that state. The weights typically range from 0 to 1, with 0 representing death and 1 representing perfect health. Therefore, 1 additional QALY represents 1 additional year of perfect health. With regard to the health states, it has been suggested that generic HRQoL instruments may not be sensitive enough to measure condition-specific effects on quality of life for patients with SMI (Lenert et al., 2005, 2004; Mavranezouli, 2010). The positive and negative syndrome scale (PANSS; Kay et al., 1987) is one of the most commonly used measures of condition-specific effects for SMIs. The PANSS was originally developed to measure symptom severity among individuals with schizophrenia, but it is also commonly used as a psychotic symptom assessment tool for individuals with mood disorders given that it assesses symptoms associated with such disorders, such as mood lability, depressed mood and hostility. Studies have shown the PANSS to identify similar psychotic symptom domains between patients with schizophrenia and bipolar disorder (Daneluzzo et al., 2002; Lindenmayer et al., 2007, 2004), and between schizophrenia and major depressive disorder (Eisenberg et al., 2009; Milak et al., 2007; Purnine et al., 2000) – the three serious mental disorders with which participants had been diagnosed. However, before the PANSS scores can be used to weight life years, they must be linked to a measure of utility and converted to the aforementioned 0-1 health-utility index. We used the mapping function developed by Mohr et al. (2004), which categorizes each individual into one of 8 disease states based on their PANSS scores. Preference-weighted HRQoL scores were then assigned according to Lenert et al. (2004), who used a visual analog scale followed by the standard gamble method to assign health-utility index values to each of the 8 states. The PANSS was administered at baseline and then monthly during the treatment and follow-up phases.
2.4 Cost effectiveness
The analyses were performed from the perspective of both the provider and the payer. The provider's perspective is important given that is their decision whether or not to adopt the intervention as part of the treatment program. The payer's perspective is important given their obvious role in the reimbursement decisions.
The incremental cost-effectiveness ratios (ICERs) are the primary outcomes of interest. The ICERs calculated from the provider's perspective reflect the 12-week study-provided direct medical costs (the costs of CM and the costs of outpatient mental health and chemical dependency treatment provided by the community mental health center) per QALY and per stimulant-free year. The payer-perspective ICERs reflect the total direct medical costs (all provider costs and the cost of the following non-study medical services – inpatient substance use and psychiatric services, detoxification admissions and ED visits) per QALY and per stimulant-free year over the 12-week intervention period as well as the entire 24-week study period.
2.5 Analysis
Differences in participants’ demographic characteristics, healthcare utilization for the 12-week intervention and follow-up periods, and the total direct medical costs for the 12-weeks prior to randomization, were tested via chi-square tests for categorical variables, t-tests for continuous variables, and Wilcoxon-Mann-Whitney tests for count variables.
All 12-week intervention and 12-week follow-up cost estimates were obtained using individual multivariable generalized linear model (GLM) regressions. The distributions and link functions were chosen according to the fit of the data, and the decisions were guided by the use of the modified Parks test (Manning and Mullahy, 2001; Park, 1966) for the family, and the Pregibon link, Modified Hosmer and Lemeshow, and Pearson's correlation tests for the link functions (Glick et al., 2007). The predicted 12-week intervention and follow-up costs were summed to generate the 24-week estimates. Given that the healthcare resources utilized by each participant were obtained from administrative data, and information on the utilization of intervention resources was recorded at the time of each visit, missing cost data was not an issue.
For the 12-week intervention period, 42% of HRQoL preference weights were missing, as was 53% of stimulant-abstinence information; 57% of the HRQoL and 52% of stimulant-abstinence data was missing for the entire 24-weeks. CM+TAU participants remained in treatment 7.25 (SD=4.25) weeks on average, versus 9.33 (SD=3.98) weeks for TAU participants. The mean number of missing stimulant-abstinence and HRQoL observations was significantly higher among the CM+TAU group; the amount of missingness did not differ between serious mental diagnosis groups. (Information on missingness by period is available from the author upon request.) Multivariable models were used to predict the HRQoL preference weights and the probability of stimulant free urine (Glick et al., 2007). Missing data was addressed using inverse probability weighting (IPW) and weighted-GLM regressions. IPW performs well at removing the bias associated with missing data when the data are missing at random (MAR; Seaman and White, 2013), which it appears to be (McDonell et al., 2013). As with the cost models, the appropriate family and link functions were chosen according to the fit of the data (Manning and Mullahy, 2001; Park, 1966). QALYs and stimulant-free years gained for the intervention period were estimated by calculating the area-under-the-curve of the predicted HRQoL and likelihood of stimulant-free urine values, respectively, for the first 12 weeks. Using the same methodology, the QALYs and stimulant-free years gained were then calculated for the full 24 weeks.
The method of recycled predictions was used to estimate all predicted costs, with the exception of the CM intervention itself, QALYs and stimulant-free years (Glick et al., 2007). To account for sampling uncertainty, p-values and standard errors were derived by performing the above analyses within a nonparametric bootstrap (1,000 iterations). The parameters obtained from the bootstrap were then used to estimate acceptability curves via parametric methods (Glick et al., 2007). Acceptability curves demonstrate the likelihood that the intervention would be considered cost-effective (i.e., a “good value”) at different levels of “willingness-to-pay”.
3. RESULTS
Descriptive statistics for patients’ demographic information, healthcare utilization (both intervention and follow-up) and direct medical costs for the 12-weeks prior to randomization, by study group, can be viewed in Table 1. The only significant difference between the two treatment groups was the number of inpatient days in the follow-up period, with the noncontingent-control group experiencing 4 and the CM group 0.
Table 1.
Descriptive Statistics
| CM + TAU | TAU | |||
|---|---|---|---|---|
| Variable | n | Mean | n | Mean |
| Age (S.D.) | 91 | 43.01 (9.27) | 84 | 42.96 (8.87) |
| Female | 91 | 34% | 85 | 35% |
| White/Caucasian | 91 | 51% | 85 | 58% |
| Black/African American | 91 | 34% | 85 | 26% |
| Other Race | 91 | 15% | 85 | 16% |
| 12-Week Intervention Counts (S.D.) | ||||
| Emergency Department Visits | 91 | 0.87 (1.90) | 85 | 0.78 (1.64) |
| Outpatient Visits | 91 | 32.23 (43.87) | 85 | 31.24 (29.61) |
| Detoxification Visits | 91 | 0.07 (0.29) | 85 | 0.05 (0.21) |
| Inpatient Days | 91 | 0.13 (0.99) | 85 | 0.58 (2.88) |
| 12-Week Follow-Up Counts (S.D.) | ||||
| Emergency Department Visits | 91 | 0.77 (1.99) | 85 | 0.61 (1.48) |
| Outpatient Visits | 91 | 23.78 (25.55) | 85 | 27.19 (33.17) |
| Detoxification Visits | 91 | 0.07 (0.29) | 85 | 0.02 (0.15) |
| Inpatient Days | 91 | 0 (0.00) | 85 | 0.73 (3.69) |
| Total Direct Medical Costs 12-Weeks Priora | 91 | $58,478 (58,685) | 85 | $65,077 ($103,077) |
Unadjusted
3.1. Cost
Table 2 contains the 12-week provider's-perspective cost results, while Table 3 contains the 12- and 24-week cost results presented from the payer's perspective. The predicted mean cost of CM was $396 (SE=41) for the 12-week intervention period.
Table 2.
Adjusted Cost and Effectiveness Outcomes - Provider Perspective
| 12-Weeks |
||||
|---|---|---|---|---|
| Variable | CM+TAU | TAU | Diff (SE) | P-value |
| Costs | ||||
| Contingency management | 396 | 0 | 396 (41) | <0.001 |
| Outpatient services | 62,274 | 60,018 | 2,256 (8,099) | 0.78 |
| Total Costs | 62,670 | 60,018 | 2,652 (8,097) | 0.74 |
| Outcomes | ||||
| Stimulant-free yearsa | 0.85 | 0.61 | 0.24 (0.04) | <0.001 |
| QALYsa | 0.85 | 0.81 | 0.04 (0.04) | 0.23 |
Annualized
Table 3.
Adjusted Cost and Effectiveness Outcomes - Payer Perspective
| 12-Weeks | 24-Weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | CM+TAU | TAU | Diff (SE) | P-value | CM+TAU | TAU | Diff (SE) | P-value |
| Costs | ||||||||
| Contingency management | 396 | 0 | 396 (41) | <0.001 | 396 | 0 | 396 (41) | <0.001 |
| Outpatient services | 62,274 | 60,018 | 2,256 (8,099) | 0.78 | 113,896 | 114,540 | −645 (13,249) | 0.96 |
| Nonstudy services | 797 | 838 | −41 (272,902) | 1.00 | 1,487 | 1,364 | 124 (368,214) | 1.00 |
| Total Costs | 63,467 | 60,856 | 2,611 (272,807) | 0.99 | 115,779 | 115,904 | −125 (368,360) | 1.00 |
| Outcomes | ||||||||
| Stimulant-free yearsa | 0.85 | 0.61 | 0.24 (0.04) | <0.001 | 0.77 | 0.58 | 0.20 (0.07) | 0.002 |
| QALYsa | 0.85 | 0.81 | 0.04 (0.04) | 0.23 | 0.83 | 0.82 | 0.01 (0.02) | 0.70 |
Annualized
The 12-week total direct medical cost differentials for CM+TAU relative to TAU were not significantly different for either the provider ($2,652; SE=8,097, p=0.74) or the payer ($2,611; SE=272,807) following the 12-week intervention; this was also true for the payer over the full 24-week time horizon (-$125; SE=368,360, p=1.00). The high levels of insignificance are due to the sizeable standard error of the outpatient variable and, to a larger extent, the non-study services variable. The variability in outpatient and non-study services limits our ability to draw inferences about the direct-medical costs of CM+TAU relative to TAU, particularly from the payer's perspective.
3.2. Effectiveness
The 12-week annualized results from the QALY and stimulant-free year analyses can be viewed in Tables 2 and 3; the 24-week results are available in Table 3. Over the 12-week intervention period, the annualized stimulant-free years gained by CM+TAU relative to TAU alone were .24 (SE=0.04, p<0.001). The differential narrowed slightly after 24 weeks, but remained in favor of CM+TAU at 0.20 (SE=0.07, p=0.002) stimulant-free years (annualized). The QALYs gained by CM+TAU also exceeded those of TAU alone at 12 and 24 weeks; however, the differences were not statistically significant. Over the first 12-weeks, CM+TAU experienced 0.85 QALYs (annualized) compared to 0.81 QALYs (annualized) for TAU alone (SE=0.04, p=0.23). The QALYs experienced by CM+TAU and TAU over the 24-week time horizon were also very similar at 0.83 (annualized) and 0.82 (annualized), respectively (SE=0.02, p=0.70).
3.3 Cost-effectiveness
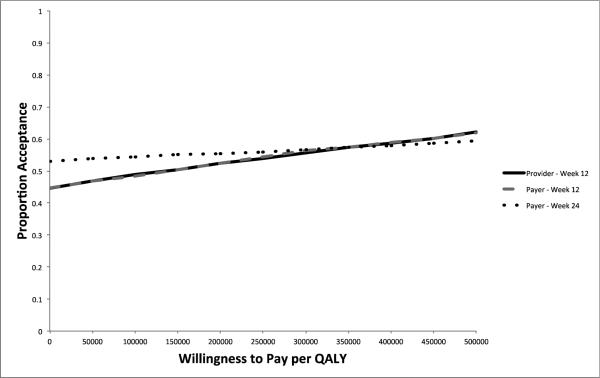
Table 4 contains the ICERs for the provider and the payer. The small QALY differentials contribute to large point estimates for both the provider and payer at 12-weeks; the point estimate for the payer at 24-weeks indicates that CM+TAU dominates TAU. However, the variability in costs and the small, insignificant, differences in QALYs result in wide confidence intervals around the point estimates and low levels of certainty regarding CM+TAU being considered cost-effective at common willingness-to-pay (WTP) threshold values (see Figure 1).
Table 4.
Incremental cost-effectiveness ratios
| 12-Weeks | 24-Weeks | |||||
|---|---|---|---|---|---|---|
| Point Estimate | Lower Interval | Upper Interval | Point Estimate | Lower Interval | Upper Interval | |
| Provider costs/QALY | 308,665 | 2,681,341a | Dominated | NA | NA | NA |
| Payer costs/QALY | 303,900 | 1,965,838a | Dominated | Dominates | Dominated | Dominated |
| Provider costs/Stimulant-free year | 48,133 | Dominates | 370,968 | NA | NA | NA |
| Payer costs/Stimulant-free year | 47,390 | Dominates | 407,435 | Dominates | Dominates | 487,888 |
CM costs and QALYs are < TAU.
Figure 1.
Cost per QALY Acceptability Curves
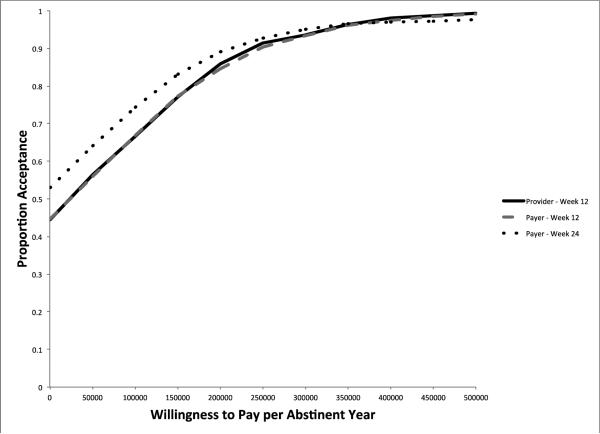
The provider point estimate for cost per stimulant-free year over the first 12 weeks is $48,133. The cost per stimulant-free year for the payer over the 12-week intervention period is very similar at $47,390. Using a 24-week time horizon, the point estimate indicates that CM+TAU dominates TAU from a payer perspective when using stimulant-free years as the effectiveness measure. As is reflected in the acceptability curves displayed in Figure 2, CM+TAU has a higher likelihood of being considered a “good value” when stimulant-free years are used as the measure of effectiveness. At a WTP threshold of $200,000 per stimulant-free year, CM+TAU has approximately an 85% chance of being deemed cost-effective at 12-weeks for both the provider and the payer; at 24-weeks the likelihood climbs to 89% for the payer.
Figure 2.
Cost per Stimulant-Free Year Acceptability Curves
4. DISCUSSION
The contingency management add-on to treatment as usual for patients with comorbid substance-use and serious-mental disorders costs an estimated $396 per individual over a 12-week treatment episode. Although the total direct medical cost differentials are all highly insignificant, due to the variability in the outpatient and non-study service variables, the results highlight some points worth of consideration for future CM studies. After adding outpatient mental-health and chemical-dependency treatment services offered by the provider (i.e., the community mental health center) to CM, the direct medical cost for CM+TAU is $2,652 higher than TAU. Similarly, from the perspective of the payer, the inclusion of outpatient and non-study services results in CM+TAU costing $2,611 more than TAU at 12 weeks. At 24 weeks CM+TAU is $125 less than TAU from the payer's perspective. The predicted means reflect the raw counts of utilization displayed in Table 1. At 12-weeks the outpatient treatment services account for approximately 85% of the total direct medical costs for both the provider and the payer. Although the standard error of the non-study services cost variable is very large, these services account for very little of the total payer cost at 12 and 24 weeks. There is a small, but insignificant, cost offset in outpatient services at 24 weeks. While a decrease in utilization of mental health services could potentially be problematic, there was a sustained significant increase in time free from stimulants for the CM+TAU group relative to TAU, with a small, but insignificant, increase in HRQoL. Moreover, the QALY findings give us an idea of the general HRQoL levels of this very ill population, and how they changed over time following engagement with treatment services.
As mentioned above, the variability in costs and the small, insignificant, difference in QALYs introduces a great deal of uncertainty into the cost per QALY estimates with regard to assessing “value”. Therefore, we focus more on our clinical effectiveness measure of time free from stimulants. Unfortunately, unlike QALYs, there is not a generally accepted range for which cost per stimulant-free years would be considered cost-effective. For QALYs the range is generally considered to be $50,000 to $200,000 (Hirth et al., 2000). Using the 12-week time horizon, the cost per stimulant-free year point estimates for the provider and payer are under $50,000, and at 24-weeks CM+TAU dominates TAU from a payer perspective. Moreover, even with the variability in costs, CM+TAU has roughly an 85% chance of being accepted as cost-effective from both the provider's and payer's perspective at 12-weeks using a threshold of $200,000 per stimulant-free year. Using the same measure of “value”, there is an 89% chance that CM+TAU would be considered cost-effective at 24-weeks for the payer.
4.1. Strengths and limitations
To the best of our knowledge, this is the first study to assess the cost-effectiveness of CM for SUDs among individuals with an SMI. Moreover, studies on CM interventions seldom incorporate preference-weighted HRQoL and calculate QALYs. Even though the QALYs gained by CM relative to TAU were not statistically significant, this is an important addition to the literature. As mentioned above, the belief that quality-of-life should be a core component of long-term recovery from SUDs is becoming the consensus. Furthermore, we were able to use the condition-specific PANSS to measure preference-weighted QoL and calculate QALYs, which is important given that generic HRQoL instruments are likely not sensitive enough to fully capture changes in quality of life for this population. However, given that 39% of participants had a primary diagnosis of schizophrenia, while 34% had a diagnosis of bipolar disorder and 27% had a diagnosis of major depressive disorder, the fact that the HRQoL preference weights were developed for schizophrenic health states identified via the PANSS is a limitation, as is the fact that, to the best of our knowledge, the mapping function has only been applied to and tested on individuals with a primary diagnosis of schizophrenia (Heeg et al., 2008; Järbrink et al., 2009; Lenert et al., 2005; Rabinowitz et al., 2013). Although, as discussed above, studies have shown similarities between schizophrenia and bipolar and major depressive disorders with regard to the psychotic symptom domains identified by the PANSS (Daneluzzo et al., 2002; Eisenberg et al., 2009; Lindenmayer et al., 2007, 2004; Milak et al., 2007; Purnine et al., 2000). Moreover, we only observed significant group differences on PANSS ratings related to mood lability, rather than psychotic symptoms, in a sample that was primarily mood disordered. With regard to the HRQoL preference weights, the only significant group difference over time was between participants with diagnosed schizophrenia and those diagnosed with major depressive disorder, with schizophrenics scoring lower. Additionally, the fact that HRQoL was not also measured using a generic instrument limits our ability to generalize the QALY findings.
Another limitation of the study is that, aside from ED use, we do not have healthcare utilization data on non-psychiatric/chemical-dependency care. The missing preference-weighted HRQoL scores, which were used to calculate QALYs, and the missing stimulant-abstinence data also serve as limitations. We addressed the missingness with an approach shown to perform well with regard to correcting bias when the data is missing at random (Seaman and White, 2013), which it appears to be. In addition, we applied a missing-completely-at–random (MCAR) assumption and found no discernible difference in the results. Still, the missing data reduces the power of our effectiveness findings.
4.2 Conclusion
CM added to TAU appears to be a wise investment for providers and payers for treating SUDs among the very costly and difficult-to-manage population of individuals with a co-occurring SMI. CM plus TAU significantly improved time free from stimulants relative to TAU, an effect that was sustained over the follow-up period, with no significant difference in direct-medical costs or health-related quality-of-life.
Highlights.
We conducted an economic evaluation of a contingency management (CM) intervention.
Evaluated CM as treatment for stimulant use among those with severe mental illness.
CM increased stimulant-free time with no significant difference in costs or QoL.
CM appears to be a wise investment for treating those with comorbid SUD and SMI.
Acknowledgments
Role of Funding Source
Supported by National Institute on Drug Abuse grant R01 DA022476-01 (principal investigator, Dr. Ries). The funding agency had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Authors SMM, MGM and SM conceived the study, and managed the literature searches and summaries of previous related work. MGM, SM and FA were responsible for obtaining and cleaning the data. SMM performed the statistical analyses and wrote the first draft of the manuscript. All authors provided input on the statistical approach, performed critical reviews and collaborated with SMM on manuscript revisions. All authors have approved the final manuscript.
Conflict of Interest (mandatory)
Drs. McPherson and Roll have received research funding from the Bristol-Myers Squibb Foundation. Dr. Ries has been on the speakers bureaus of Alkermes and Janssen. The other authors report no financial relationships with commercial interests.
REFERENCES
- Abram KM, Teplin LA. Co-occurring disorders among mentally ill jail detainees. Implications for public policy. Am. Psychol. 1991;46:1036–1045. doi: 10.1037//0003-066x.46.10.1036. [DOI] [PubMed] [Google Scholar]
- Baker A, Bucci S, Lewin TJ, Kay-Lambkin F, Constable PM, Carr VJ. Cognitive-behavioural therapy for substance use disorders in people with psychotic disorders: Randomised controlled trial. Br. J. Psychiatry 188 439-448. 2006 doi: 10.1192/bjp.188.5.439. [DOI] [PubMed] [Google Scholar]
- Barrowclough C, Haddock G, Wykes T, Beardmore R, Conrod P, Craig T, Davies L, Dunn G, Eisner E, Lewis S, Moring J, Steel C, Tarrier N. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ. 2010 doi: 10.1136/bmj.c6325. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels SJ, T.G.B., Drake RE, Clark RE, Bush PW, Noordsy DL. Substance abuse in schizophrenia: service utilization and costs. J. Nerv. Ment. Dis. 1993;181:227–232. doi: 10.1097/00005053-199304000-00003. [DOI] [PubMed] [Google Scholar]
- Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y. A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Arch. Gen. Psychiatry. 2006;63:426–432. doi: 10.1001/archpsyc.63.4.426. [DOI] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, Festinger DS. Prize based contingency management for the treatment of substance abusers: a meta analysis. Addiction. 2014;109:1426–1436. doi: 10.1111/add.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ME, Bellack AS, Gearon JS. Treating substance abuse in schizophrenia. An initial report. J. Subst. Abuse Treat. 2001;20:163–175. doi: 10.1016/s0740-5472(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics Occupational Outlook Handbook. 2014 http://www.bls.gov/ooh/home.htm.
- Daneluzzo E, Arduini L, Rinaldi O, Di Domenico M, Petruzzi C, Kalyvoka A, Rossi A. PANSS factors and scores in schizophrenic and bipolar disorders during an index acute episode: a further analysis of the cognitive component. Schizophr Res. 2002;56:129–136. doi: 10.1016/s0920-9964(01)00277-8. [DOI] [PubMed] [Google Scholar]
- de Leon J, Gurpegui M, Diaz FJ. Epidemiology of comorbid tobacco use and schizophrenia: thinking about risks and protective factors. J. Dual Diagn. 2007;3:9–25. [Google Scholar]
- Drake RE, McHugo GJ, Clark RE, Teague GB, Xie H, Miles K, Ackerson TH. Assertive community treatment for patients with co-occurring severe mental illness and substance use disorder: a clinical trial. Am. J. Orthopsychiatry. 1998;68:201. doi: 10.1037/h0080330. [DOI] [PubMed] [Google Scholar]
- Drake RE, O'Neal EL, Wallach MA. A systematic review of psychosocial research on psychosocial interventions for people with co-occurring severe mental and substance use disorders. J. Subst. Abuse Treat. 2008;34:123–138. doi: 10.1016/j.jsat.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden S, Leyro T, Powers M, Otto M. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Eisenberg DP, Aniskin DB, White L, Stein JA, Harvey PD, Galynker II. Structural differences within negative and depressive syndrome dimensions in schizophrenia, organic brain disease, and major depression: a confirmatory factor analysis of the positive and negative syndrome scale. Psychopathology. 2009;42:242. doi: 10.1159/000218522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JF, Hourani LL, Heller DC. Predictors of treatment receipt among adults with a drug use disorder. Am. J. Drug Alcohol Abuse. 2004;30:841–869. doi: 10.1081/ada-200037550. [DOI] [PubMed] [Google Scholar]
- Galanter M, Dermatis H, Egelko S, De Leon G. Homelessness and mental illness in a professional- and peer-led cocaine treatment clinic. Psychiatr. Serv. 1998;49:533–535. doi: 10.1176/ps.49.4.533. [DOI] [PubMed] [Google Scholar]
- Glick H, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation In Clinical Trials. Oxford University Press; 2007. [Google Scholar]
- Heeg B, Buskens E, Botteman M, Caleo S, Ingham M, Damen J, De Charro F, Van Hout B. The Cost‐Effectiveness of Atypicals in the UK. Value Health. 2008;11:1007–1021. doi: 10.1111/j.1524-4733.2008.00344.x. [DOI] [PubMed] [Google Scholar]
- Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to Pay for a Quality-adjusted Life Year: In Search of a Standard. Medical Decision Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL, Jr., Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am. J. Psychiatry. 1995;152:856–861. doi: 10.1176/ajp.152.6.856. [DOI] [PubMed] [Google Scholar]
- Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Schizophr. Bull. 2013;40:18–20. doi: 10.1093/schbul/sbt160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T. Assessing the economic costs of serious mental illness. Am. J. Psychiatry. 2008;165:663–665. doi: 10.1176/appi.ajp.2008.08030366. [DOI] [PubMed] [Google Scholar]
- Järbrink K, Kreif N, Benedict A, Locklear J. Quality of life and drug costs associated with switching antipsychotic medication to once-daily extended release quetiapine fumarate in patients with schizophrenia. Curr Med Res Opin. 2009;25:709–716. doi: 10.1185/03007990902738810. [DOI] [PubMed] [Google Scholar]
- Kay SR, Flszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Amass L, McLellan AT. Disseminating contingency management research to drug abuse treatment practitioners. 1999 [Google Scholar]
- Laudet AB. The case for considering quality of life in addiction research and clinical practice. Addict. Sci. Clin. Pract. 2011;6:44. [PMC free article] [PubMed] [Google Scholar]
- Lenert LA, Rupnow MF, Elnitsky C. Application of a disease-specific mapping function to estimate utility gains with effective treatment of schizophrenia. Health Qual. Life Outcomes. 2005;3:57. doi: 10.1186/1477-7525-3-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenert LA, Sturley AP, Rapaport MH, Chavez S, Mohr PE, Rupnow M. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophr. Res. 2004;71:155–165. doi: 10.1016/j.schres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P, Bossie CA, Kujawa M, Zhu Y, Canuso CM. Dimensions of psychosis in patients with bipolar mania as measured by the positive and negative syndrome scale. Psychopathology. 2007;41:264–270. doi: 10.1159/000128325. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P, Brown E, Baker RW, Schuh LM, Shao L, Tohen M, Ahmed S, Stauffer VL. An excitement subscale of the Positive and Negative Syndrome Scale. Schizophr Res. 2004;68:331–337. doi: 10.1016/S0920-9964(03)00087-2. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta analysis of voucher based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J. Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Mavranezouli I. A review and critique of studies reporting utility values for schizophrenia-related health states. Pharmacoeconomics. 2010;28:1109–1121. doi: 10.2165/11537300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, Short RA, Roll JM, Ries RK. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. Am. J. Psychiatry. 2013;170:94–101. doi: 10.1176/appi.ajp.2012.11121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. J. Subst. Abuse Treat. 2004;26:305–312. doi: 10.1016/j.jsat.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Milak MS, Aniskin DB, Eisenberg DP, Prikhojan A, Cohen LJ, Yard SS, Galynker II. The negative syndrome as a dimension: factor analyses of PANSS in major depressive disorder and organic brain disease compared with negative syndrome structures found in the schizophrenia literature. Cognitive and behavioral neurology. 2007;20:113–120. doi: 10.1097/WNN.0b013e3180653c35. [DOI] [PubMed] [Google Scholar]
- Mohr PE, Cheng CM, Claxton K, Conley RR, Feldman JJ, Hargreaves WA, Lehman AF, Lenert LA, Mahmoud R, Marder SR. The heterogeneity of schizophrenia in disease states. Schizophr. Res. 2004;71:83–95. doi: 10.1016/j.schres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics . Health, United States, 2012: With Special Feature On Emergency Care. Hyattsville, MD.: 2013. [PubMed] [Google Scholar]
- Olmstead TA, Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine-or opioid-dependent outpatients. Drug Alcohol Depend. 2009;102:108–115. doi: 10.1016/j.drugalcdep.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, Easton CJ, Carroll KM. The cost effectiveness of four treatments for marijuana dependence. Addiction. 2007a;102:1443–1453. doi: 10.1111/j.1360-0443.2007.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, Petry NM. Clinic variation in the cost effectiveness of contingency management. Am. J. Addict. 2007b;16:457–460. doi: 10.1080/10550490701643062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, Petry NM. Cost-effectiveness of prize-based incentives for stimulant abusers in outpatient psychosocial treatment programs. Drug Alcohol Depend. 2007c;87:175–182. doi: 10.1016/j.drugalcdep.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park RE. Estimation with heteroscedastic error terms. Econometrica. 1966;34:888. [Google Scholar]
- Petry NM, DePhilippis D, Rash CJ, Drapkin M, McKay JR. Nationwide dissemination of contingency management: the Veterans Administration Initiative. Am. J. Addict. 2014;23:205–210. doi: 10.1111/j.1521-0391.2014.12092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Simcic F., Jr. Recent advances in the dissemination of contingency management techniques: clinical and research perspectives. J. Subst. Abuse Treat. 2002;23:81–86. doi: 10.1016/s0740-5472(02)00251-9. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Purnine DM, Carey KB, Maisto SA, Carey MP. Assessing positive and negative symptoms in outpatients with schizophrenia and mood disorders. The J Nerv Ment Dis. 2000;188:653–661. doi: 10.1097/00005053-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Berardo CG, Bugarski-Kirola D, Marder S. Association of prominent positive and prominent negative symptoms and functional health, well-being, healthcare-related quality of life and family burden: a CATIE analysis. Schizophr Res. 2013;50:339–342. doi: 10.1016/j.schres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- RachBeisel J, Scott J, Dixon L. Co-occurring severe mental illness and substance use disorders: a review of recent research. Psychiatr. Serv. 1999;50:1427–1434. doi: 10.1176/ps.50.11.1427. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch. Gen. Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Roll JM, Chermack ST, Chudzynski JE. Investigating the use of contingency management in the treatment of cocaine abuse among individuals with schizophrenia: a feasibility study. Psychiatry Res. 2004;125:61–64. doi: 10.1016/j.psychres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat. Methods Med. Res. 2013;22:278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- Sindelar J, Elbel B, Petry NM. What do we get for our money? Cost effectiveness of adding contingency management. Addiction. 2007a;102:309–316. doi: 10.1111/j.1360-0443.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Olmstead TA, Peirce JM. Cost effectiveness of prize based contingency management in methadone maintenance treatment programs. Addiction. 2007b;102:1463–1471. doi: 10.1111/j.1360-0443.2007.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebnik D, Sugar A, Coblentz P, McDonell MG, Angelo F, Lowe JM, Ries RK, Roll J. Acceptability of contingency management among clinicians and clients within a cooccurring mental health and substance use treatment program. Am. J. Addict. 2013;22:432–436. doi: 10.1111/j.1521-0391.2013.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Report To Congress On The Prevention And Treatment Of Co-Occuring Substance Abuse Disorders And Mental Disorders: Executive Summary. SAMHSA; Rockville, MD.: 2002. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . The ADSS Cost Study: Costs Of Substance Abuse Treatment In The Specialty Sector. DHHS; Rockville, MD.: 2003. [Google Scholar]
- Watkins KE, Burnam A, Kung F-Y, Paddock S. A national survey of care for persons with co-occurring mental and substance use disorders. Psychiatr. Serv. 2001a;52:1062–1068. doi: 10.1176/appi.ps.52.8.1062. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Burnam AB, Kung F, Paddock S. A national survey of care for persons with co-occurring mental and substance use disorders. Psychiatr. Serv. 2001b;52:1062–1068. doi: 10.1176/appi.ps.52.8.1062. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Jaffee WB, Bender RE, Graff FS, Gallop RJ, Fitzmaurice GM. A “community-friendly” version of integrated group therapy for patients with bipolar disorder and substance dependence: a randomized controlled trial. Drug Alcohol Depend. 2009;104:212–219. doi: 10.1016/j.drugalcdep.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]