Abstract
Objective
There is currently a gap in on-site drug of abuse monitoring. Current detection methods involve invasive sampling of blood and urine specimens, or collection of oral fluid, followed by qualitative screening tests using immunochromatographic cartridges. While remote laboratories then may provide confirmation and quantitative assessment of a presumptive positive, this instrumentation is expensive and decoupled from the initial sampling making the current drug-screening program inefficient and costly. The authors applied a noninvasive oral fluid sampling approach integrated with the in-development chip-based Programmable Bio-Nano-Chip (p-BNC) platform for the detection of drugs of abuse.
Method
The p-BNC assay methodology was applied for the detection of tetrahydrocannabinol, morphine, amphetamine, methamphetamine, cocaine, methadone and benzodiazepines, initially using spiked buffered samples and, ultimately, using oral fluid specimen collected from consented volunteers.
Results
Rapid (~10 minutes), sensitive detection (~ng/ml) and quantitation of 12 drugs of abuse was demonstrated on the p-BNC platform. Furthermore, the system provided visibility to time-course of select drug and metabolite profiles in oral fluids; for the drug cocaine, three regions of slope were observed that, when combined with concentration measurements from this and prior impairment studies, information about cocaine-induced impairment may be revealed.
Conclusions
This chip-based p-BNC detection modality has significant potential to be used in the future by law enforcement officers for roadside drug testing and to serve a variety of other settings, including outpatient and inpatient drug rehabilitation centers, emergency rooms, prisons, schools, and in the workplace.
Keywords: drugs of abuse, programmable bio-nano-chip, oral fluid, multiplexed, on-site testing
1. INTRODUCTION
Driving under the influence of drugs is a problem of growing concern in many countries, including the United States (US) and the United Kingdom (UK). In spite of the most stringent drug policies and punitive laws, in 2008 the US had the highest levels of lifetime illegal cocaine and marijuana use, with these and other drugs and their metabolites being increasingly detected in impaired and injured automobile drivers (http://oas.samhsa.gov/nsduh.htm). Drug use is a contributory factor to a significant percentage of fatal road traffic collisions. In the UK, in 2010/11, an estimated 19% of adult drivers who had taken illegal drugs reported driving at least once or twice within the last 12 months while they were affected by or under the influence of illegal drugs (http://assets.dft.gov.uk/statistics/releases/road-accidents-and-safety-annual-report-2011/rrcgb2011-05.pdf).
Despite these dire facts, neither the US nor UK, nor any other country for that matter, has access to a reliable method analogous to the alcohol breath-alyzer to detect drugged-drivers. Likewise, practical limitations associated with the current sampling approaches to collect forensic specimens, as well as lack of suitable technologies that could efficiently, accurately and sensitively determine drug concentrations outside of the laboratory setting have hindered progress in this area.
Current approaches involve blood sampling, which is invasive, and requires specialized sample collection and preparatory work prior to sample analysis. This process is time-consuming and, in most cases, confined to a laboratory setting. In some situations urine sampling is a better alternative, but associated with privacy issues, concerns with chain of custody and vulnerability to adulteration. Hence, there is a pressing need for alternative diagnostic fluids/matrices, such as hair, sweat and oral fluids for drug testing.
Indeed, in recent reviews saliva is presented as a clinically informative biological fluid that is increasingly shown to be useful for prognosis, laboratory or clinical diagnosis, and monitoring and management of patients with both oral and systemic diseases (Malamud and Rodriguez-Chavez, 2011; Lee et al., 2009; Wong, 2008, Parisi et al., 2009; White et al., 2009).
Oral fluid-based immunochromatographic strip (ICS) tests have been developed with capabilities in various drug testing/screening settings outside the laboratory (Crouch et al., 2008; Vanstechelman et al., 2012). However, these tests are not quantitative, thereby limiting access to potential impairment status, as well as they exhibit only restricted multiplexed capabilities, making them less practical for point-of-need settings (Bosker and Huestis, 2009).
The marriage of noninvasive sampling with portable detection capabilities afforded with lab-on-a-chip systems provides an ideal opportunity to address unmet needs for the drug testing and monitoring areas. Over the past two decades much progress has been made in the direction of development of new chip-based systems. Indeed, pioneering work by Whitesides in the basic sciences defined the ideal coatings, materials and designs for the development of microfluidic channels and manipulations of biological fluids (Whitesides, 2006), while Quake's group advanced the ‘large-scale integration’ of microfluidics, analogous to the electronics field (Thorsen et al., 2002). Other groups, such as those of Mirkin, Wang and Heath, measured diverse sample types and created a variety of assembly types by using precious metal nanoparticles, nanowires, and magnetic techniques, respectively (Goluch et al., 2006; Osterfield et al., 2008; Qin et al., 2009). Advancements by Sia via micro-electromechanical systems and Singh using chip-based separation and quantitation benefited integration of such systems (Srivastava et al., 2009; Chin et al., 2007). Both Singh and Ligler have extended their integrated approaches into the rapid, multiplexed detection of analytes, such as toxins and other bio-threats (Kim et al., 2009), while Walt's work with electronic noses used arrays of optical fibers as the underlying infrastructure for biological sensing systems (Walt, 2005). Finally, researchers in the Toner group have explored a number of novel methods for the isolation and enumeration of lymphocytes, erythrocytes and circulating tumor cells (Cheng et al., 2009; Maheswaran et al., 2008). While such chip-based detection modalities have strong potential for use in clinical and sensor applications, the development of fully integrated and clinically validated structures remains a significant barrier for these structures, including for those intended for drug testing point-of-need applications (Bell and Hanes, 2007; Bruls et al., 2009; Qiang et al., 2009; Zhou et al., 2012). In the area of oral fluid testing, additional challenges are presented with respect to the management and processing of oral fluid samples due to the high viscosity and complex nature of such specimens.
The purpose of this article is to describe recent work that is leading to the development of new oral fluid detection capabilities, whereby multiple drug and metabolites can be detected and quantitated using Programmable Bio-Nano-Chips (p-BNCs; Christodoulides et al., 2002, 2012; Floriano et al., 2009; Goodey et al., 2001; Raamanathan et al., 2012; Rodriguez et al., 2005). These efforts place into the pipeline new options for drug testing that have significant potential to influence measurement of drug samples for a wide variety of settings.
2. METHODS
2.1 Patient recruitment
This study was approved by the Rice University, Baylor College of Medicine (BCM) and Michael De Bakey Veterans Affairs Medical Complex (MEDVAMC) Institutional Review Boards (IRBs). Recruitment of study participants and saliva donors for p-BNC forensic challenge and elucidation of time-course of select drugs experiments was coordinated and carried out at the MEDVAMC, Houston, Texas. All study participants (n=78) were at least 18 years old and provided informed consent after complete description of the study and before engaging in any study procedures. Before admission, each participant underwent a thorough medical and physical examination, including a history of past and recent drug use.
2.2 Sample collection
Collection of oral fluids was completed using an oral fluid collection kit (Aware Messenger, Calypte Inc., Portland, OR), as recommended by the manufacturer. Briefly, use of this kit involves brushing the entire upper and lower gum line starting with each side of the swab and then inserting the swab into the specimen collection tube and plunging the swab up and down 8 times. Once the plunging of the swab process is completed, the swab is removed and gently pressed against the walls of the vial to optimize the extraction of the sample into the collection buffer, to be processed with the p-BNC.
2.2 Forensic challenge experiment and methods comparison study
Adult consented volunteer study subjects were hospitalized for up to 3 days of stabilization and detoxification, after which they received either cocaine or methamphetamine, depending on their prior drug use history, or placebo. Drug administration was performed using a single-blind design. Cocaine (40 mg) or matched placebo were administered intravenously (IV) over 1 min. Methamphetamine (50 mg) or matched placebo were administered IV over 2 min.
Up to 5 oral fluid samples from each subject who was administered morning and afternoon infusions of either a placebo or the drug of interest in a controlled fashion, were collected and transported from the clinical site to the Rice lab for testing with the p-BNC system. Matched samples were also shipped to the Center of Human Toxicology, at the University of Utah for testing by liquid chromatography tandem mass spectrometry (LC-MS/MS).
Samples were tested in a blinded fashion to ensure non-biased assessment, as well as de-identified to ensure privacy of the donor. Upon completion of the p-BNC tests, results were evaluated to identify a) the samples containing the drug and b) the time of drug administration.
2.3 Drug time-course in oral fluids
The time course of drugs cocaine and methamphetamine in oral fluids were assessed in separate experiments involving one study volunteer in each case. Duplicate samples were collected ~10-15 minutes prior to IV administration of the drug (40 mg cocaine or 50 mg methamphetamine) and at various time-points between 10 minutes and 48 hours post administration. Samples were then processed with p-BNC and LC-MS/MS thereby serving both the time-course as well as the methods comparison studies for the two drugs.
2.4 Methods comparison studies
For the methods comparison study, samples were collected from each consented adult volunteer enrolled for each drug component (n=7 for MDA, n=8 for MDMA, n=12 morphine, n=41 for methamphetamine, n=20 for cocaine). Samples collected were aliquoted in two vials, one destined for p-BNC testing and the other for LC-MS/MS analysis.
2.5 The LC-MS/MS analysis
Separate LC-MS/MS target assays were used for the following drugs: methamphetamine, cocaine/benzoylecgonine (BZE), MDMA and MDA, and morphine. The methods were adapted to oral fluid by use of Synthetic Negative Saliva (Immunalysis, Pomona, CA) to prepare calibrators and controls. All reference material and deuterated isotopomers of each drug used as internal standards were from Cerilliant (Round Rock, TX). The methamphetamine method was adapted from the method for selegiline and metabolites (Slawson et al., 2002). Changes included exclusion of selegiline and norselegiline, reduction of aliquot size to 0.5 mL and change of calibration range from 2.0-100 and 1.0-50 ng/mL for methamphetamine and amphetamine. A similar liquid-liquid extraction procedure (minus addition of 0.1 % hydrochloric acid to dried residue) was used for the MDMA and MDA assay (1.0 mL aliquot, calibration range 1-1000 ng/mL). Cocaine and BZE (1.0 mL aliquot, calibration range 2.5-750 ng/mL), were determined using our previously described assay that employs Clean Screen ® ZSDA020 solid phase columns (United Chemical Technologies, Bristol, PA) for extraction (SPE) (Lin et al., 2001).
The unpublished morphine assay (0.5 mL aliquot, calibration range 1-1000 ng/mL) also used the same SPE columns. Samples were prepared by the addition of 1 mL 50 % (w/v) potassium phosphate. The SPE columns were conditioned by successive addition of 3 mL methanol and 3 mL deionized water. Samples were then added to the columns; the columns were washed with successive addition of 3 mL of deionized water, 2 mL potassium acetate (pH 4), and 3 mL of methanol; then dried and eluted as described for cocaine. The extracts were reconstituted with 0.1 mL of 0.1 % formic acid: methanol (96:4). The extracts were analyzed on a 1100 HPLC (Agilent, Palo Alto, CA) and TSQ 7000 MS/MS (Thermo, San Jose, CA), except for cocaine and BZE, which used a Surveyor HPLC (Thermo) and TSQ Quantum (Thermo) MS/MS system. The liquid chromatographic conditions for the unpublished assays were as follows: MDMA and MDA, 3μm, 100×3 mm Metabasil 3 Basic column (Varian, Lake Forest, CA) with isocratic elution using 0.1% formic acid:methanol (80:20); morhine, 3 μm, 50×2 mm YMC-ODS AQ column (Waters, Milford, MA) with isocratic elution using 0.1% formic acid: methanol (96:4). The ionization mode was positive ion electrospray.
2.6 The p-BNC (macro vs micro)
For the purpose of these studies the majority of the initial development work on the chip-based drug detection was completed using ‘macro’ laboratory prototypes, as shown in Supplementary Figure 1A1. At the same time, parallel efforts have been directed toward the establishment of ‘micro’ instrumentation and disposable labcards that in the future have potential to be used at the roadside and other settings. The ‘micro’ instrumentation and the associated disposable labcards that are under development are illustrated in Supplementary Figure 1B2.
Agarose bead sensors with a size-tunable network of nanometer-scale fibers (a ‘nano-net’) and a fluorescent signal arising from nano-scale reagents are used to isolate and quantify soluble targets in both the ‘macro’ laboratory and ‘micro’ portable p-BNC systems. Using this instrumentation, disposable and microassay sequence combination, it is possible to capture and quantitate small molecules, oligonucleotides, and proteins from complex biological matrices, such as whole blood, urine or oral fluid, within a closed miniaturized microchip platform. As such, the bead-based p-BNC sensor has a demonstrated capacity to host diverse classes (genomic, and proteomic) of analytes, as well as a diverse list of analytes within each class, including the screening of chest pain patients for acute myocardial infarction in the emergency room setting (Christodoulides et al., 2012; Floriano et al., 2009) as well as ovarian cancer screening test (Raamanathan et al., 2012).
2.7 P-BNC assay sequence, image capture and data processing
All assays on the p-BNC system were performed at room temperature under continuous fluid flow conditions using the ‘macro’ laboratory flow cell with a research grade microscope (See Supplementary Figure 1A3). The total assay time for the p-BNC drugs of abuse tests is ~10 minutes. This includes the sequential priming of the microfluidic lines, delivery of the tracer/sample mixture to the array hosting bead sensors, followed by a final washing step with phosphate buffered saline (PBS). After each assay run, photomicrographs of the bead array are captured at various charged coupled device exposure settings.
Customized macros developed and optimized for the automated analysis of drug-specific bead-based assays served to determine the exact bead location, followed by their respective bead-specific assignments and to extract bead data using “regional pixel extraction-analysis” strategies that were automatically applied for the generation of dose response curves, as well as used for the measurement of the various drug concentrations in unknown samples. Analysis of each bead within the group, for each of the drug tests reported here, at each exposure time, was initiated within the macro through line profile (LP) and circular area of interest (cAOI) routines for data extraction.
The algorithm compiles results for each bead, statistical analysis with exclusion of outliers within each group of beads and outputs log files with the average, standard deviation and coefficient of variance for each group that can be automatically parsed and read by a MATLAB application. Intensity versus concentration calibration curves were constructed in MATLAB with a best-fit non-linear 5-parameter logistic regression analysis for determination of the optimal integration time, using limit of detection (3 standard deviations below zero antigen control), assay range and precision as the criteria for the decision. Data from the best integration time was then exported to Sigma Plot to obtain a dose response curve. The dose response data as well as data obtained from the testing of samples were entered into unknown prediction equations according to standard curves obtained for each analyte on the system to determine the drug concentrations.
2.8 Statistical analyses
Limits of detection (LOD) of the p-BNC assays (at 99.5% CI) were derived using Sigma Plot software; the first step of the process involves calculation of a “Threshold Signal Intensity (TSI)” value, derived from the mean signal intensity (SI) minus 3 standard deviations, as achieved on at least 4 redundant drug bead sensors in the array for the zero antigen control run, and subsequent treatment of TSI value as an unknown that would be processed/interpolated from the dose curve to provide a solution with the LOD value. In the case that a “No Solution” indication was provided by the software, a practical LOD was defined for the assay as the lowest drug standard concentration detected generating a mean bead signal intensity lower than the TSI. Similarly, limits of quantitation (LOQ) (at 99.5% CI) of the p-BNC assays were determined using the mean signal intensity (SI) minus 10 standard deviations. For all p-BNCs and reference assays, precision values were determined by calculating the mean, standard deviation at 95% CI. For determination of accuracy, due to sample size and composition and effects on distribution, Pearson and Spearman correlations were performed to measure the degree of association between measurements by p-BNC and reference methods.
3. RESULTS
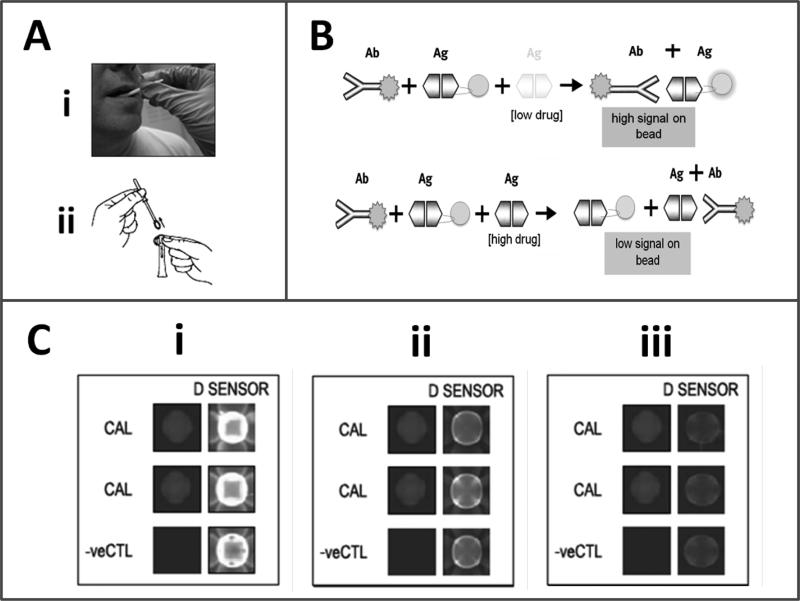
The bead-based p-BNC drug tests reported here function with non-invasive oral fluid sampling, that involves use of an oral swab to brush the entire upper and lower gum line (Figure 1A), and then insertion of the swab into a specimen collection tube to extract the sample into the assay fluid that includes the tracer antibody used in bead/chip-based competitive immunoassay (Figure 1B). The ‘macro’ laboratory-based iteration of the p-BNC reveals if there are specific drugs (i.e., drug immune-equivalents) present in the body within ~10-minutes (Figure 1B). In this setup, the test is initiated with the mixing of the salivary sample (1 mL) with the tracer. The mixture is then delivered to the p-BNC flow cell equipped with a bead-array platform. The test sequence is completed with a final washing step with PBS. In the absence of drug in the sample, the tracer antibody specifically recognizes its corresponding drug sensor(s) and, thereby, produces a strong signal on the surface, as well as within the interior of the porous bead in the array (Figure 1C for proof of concept for the assay targeting drug D-amphetamine and Supplementary Material Video4: Low_Level_Drug Animation.mov and High_Level_Drug_Animation.mov). In the presence of drug in the sample the binding of the tracer is reduced in a drug-specific, dose-dependent manner.
Figure 1.
Programmable BNC/oral fluid-based drug tests. Oral fluid sample collected by a swab (Ai) is extracted in assay buffer (Aii) and then delivered to the microfluidic cell hosting bead sensors arrayed on a microchip. In (B) shown are the schematic decoding the immuno-components of this competitive assay approach. In (C) shown are charge-coupled device (CCD)-captured images of a concentration-dependent response for the competitive-type p-BNC-based amphetamine test (i- control 0, ii- 10 and iii- 100 ng/mL amphetamine. Noted are a) the decrease in signal acquired on the drug sensors (D sensor) in response to the drug, b) the absence of signal on negative control beads (-ve CTL) and c) the consistency in signal intensity on the calibrator beads upon completion of three independent assay runs.
The array format of the p-BNC allows for redundancy of bead sensors per drug target, the inclusion of calibrator beads that allow for the baseline calibration of this optical sensor, negative control beads that indicate specificity of the immune-based reactions, as well as positive control beads that confirm consistent delivery of tracer antibody within the array. All of these features serve for a more robust quality control, which translates into higher accuracy and more precise measurements.
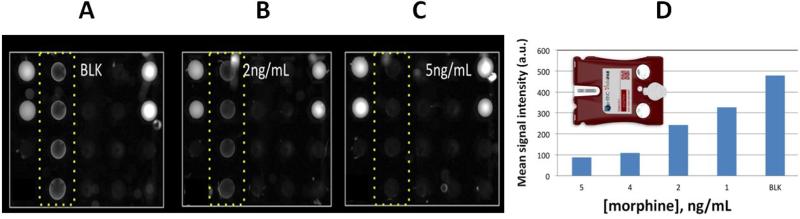
Supplementary Table 15 lists the drug tests fully developed as single-analyte tests on the ‘macro’ laboratory based p-BNC platform, while Figure 2 showcases select recent measurements of drug testing on the ‘micro’ prototype instrumentation using the injection-molded labcards.
Figure 2.
Morphine concentration-dependent data recorded within the ‘micro’ portable instrumentation using the injection molded labcards. Panels A), B), and C) reveal the raw images recorded for the various trials. The bead configuration used for these trials (that utilized morphine tracer alone) moving in columns from left to right is as follows. Left-most column includes at the top two calibrator beads and at the bottom two negative control beads. Next in sequence is a column of morphine detection beads (i.e. within highlighted dot box). Then is the cocaine detection column, followed by the methadone column. The right-most column includes additional calibrators and negative controls at the top two and bottom two positions, respectively. A) Shows the behavior observed for the blank condition where no drugs are present. Under this condition there is no competition and the morphine tracer is captured efficiently resulting in a bright column of beads next to the controls. B) A slight decrease in the tracer signal is noted for the next condition where 2 ng/ml of morphine is introduced. C) Further decrease in signal is noted as the morphine concentration is increased to 5 ng/ml. D) The dose dependence of the morphine signals is shown in the bottom panel. The inset shows a rendered image of the injection molded labcard that was used to collect the data here shown with the portable instrumentation featured in SF1.
Collectively, results from this study show the p-BNC drug testing platform compares favorably to those of LC-MS/MS and ICS counterparts, considered as the current gold standards (or reference methods) for the laboratory and the point-of-need settings, respectively (see Supplementary Table 16 for the performance characteristics of LOD and LOQ of the twelve drug tests in development on the p-BNC platform and Supplementary Table 27 for a comparison of the features associated with each of the three methodologies). Unlike ICS tests, which are qualitative (Yes/No) type of tests, both LC-MS/MS and p-BNC-based drug tests are fully quantitative, albeit p-BNC are highly-dependent on the specificity of the antibody reagents that they employ. Likewise, with LODs ranging from 0.14-7.4 ng/mL, the majority of the p-BNC tests developed exhibit a capacity to detect as low concentrations of the drugs as those measurable by LC-MS/MS and, for the most part, lower amounts than those detectable in oral fluids by their ICS test counterparts. Furthermore, unlike the laboratory-confined LC-MS/MS, p-BNC tests require no tedious and time-consuming sample processing, and they are amenable to settings outside the laboratory, while still offering the capacity to multiplex, or to test for more than one analyte (drug) concurrently using microliters of sample.
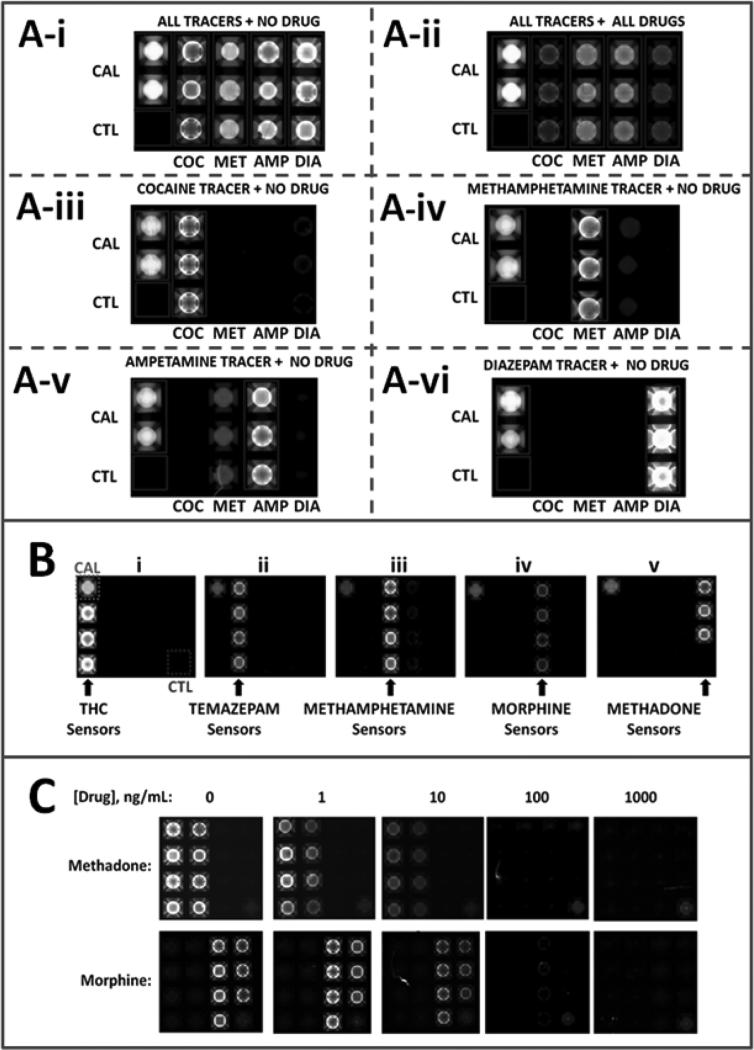
Likewise, various drugs have been combined as panels or multiplexed tests, with demonstrated specific interactions between their fluorescently-labeled tracer antibody reagents and corresponding bead sensors (Figure 3). It should be noted the antibody used for the p-BNC assay for cocaine recognizes both the parent drug as well as its metabolite- BZE. Future iterations of the same assay will implement reagents specific for each of the two for enhanced specificity and coverage.
Figure 3.
Multiplexed drug-specific p-BNC panels recorded with the ‘macro’ laboratory prototype. (A) Panel for drugs cocaine, methamphetamine, amphetamine and diazepam; (B) demonstration of the specificity of tracers for their respective drug targets tetrahydrocannabinol (THC), temazepam, methamphetamine, morphine and methadone; (C) quantitative demonstration of specificity of duplex p-BNC assay for the drugs methadone and morphine with evidence of dose dependent reduction of signal, i.e. competition.
CAL: beads coupled to a fixed amount of fluor serve as calibrators of the p-BNC system
CTL: beads coupled to bovine serum albumin alone, serve as negative controls and further indicators of the specificity of the reactions that take place within the flow cell.
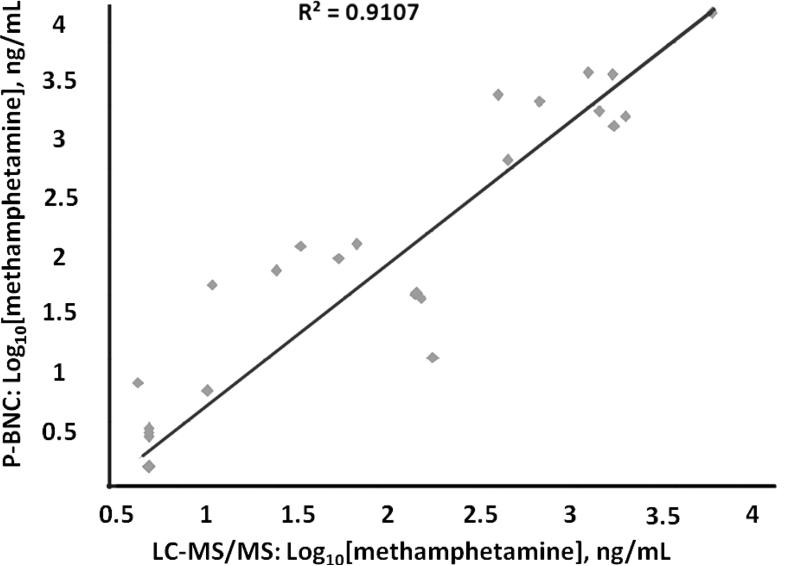
Furthermore, select p-BNC drug tests have been validated in methods comparison studies, in which matched oral fluid clinical samples collected from users of 5 distinct illicit drugs were tested by both p-BNCs and LC-MS/MS. Results showed highly positive correlation between the two methods with R2 values from 0.911 or greater at 95% CI (Figure 4 and Supplementary Table 38).
Figure 4.
Validation of p-BNC test for the drug methamphetamine via a methods comparison study. The p-BNC measurements of the drug methamphetamine in oral fluids correlate well with those achieved with LC-MS/MS. (R2=0.91, n=41)
With the new chip-based drug detection capability in hand, it has become possible to use this mini-sensor ensemble to explore its capabilities in forensic challenge experiments, as well as used so to elucidate the time-course of select drugs in oral fluids.
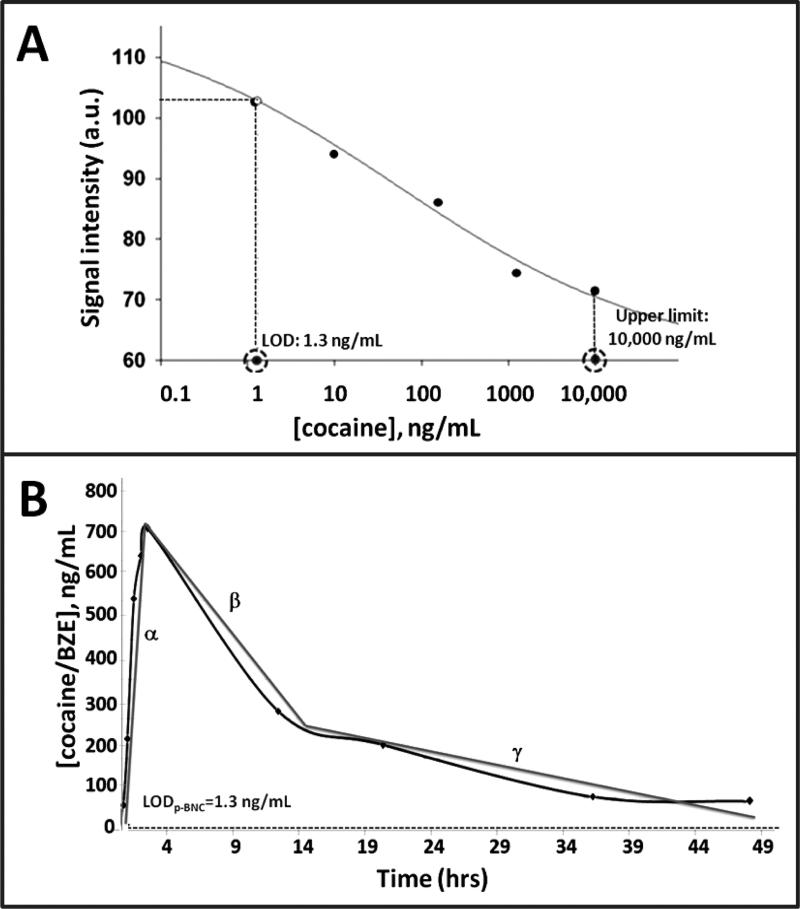
For example, the high-sensitivity test for cocaine and BZE (LOD=1.3 ng/mL, Figure 5A) allowed for the detection of the drug and its metabolite 10 minutes post its IV administration in a forensic challenge experiment (Supplementary Figure 29). The drug was consistently detected 10 minutes and up to ~49 hours post its administration in the timecourse experiment (Figure 5B). More specifically, ~50 ng/mL of the drug were detected with the p-BNC at the 10 minute timepoint, followed by a significant 15-fold increase of the drug level in the 3 hour timepoint, after which levels begun to decline to reach 50 ng/mL by the 49 hour and last timepoint of the experiment.
Figure 5.
High-sensitivity, wide range p-BNC assay for cocaine/BZE and elucidation of the time- course of the drug in oral fluids. A) Dose curve, LOD and assay range for p-BNC assay for cocaine and its metabolite BZE. B) P-BNC-based measurements of cocaine/BZE levels in oral fluid samples collected from a study volunteer that was administered a 40 mg intravenous (i.v.) dosage of cocaine. Samples were collected ~10 minutes pre-drug administration and at 9 distinct time points between 10 minutes and 49 hours post drug administration.
The time-course of cocaine in oral fluids, as elucidated with the p-BNC, is analogous with that reported previously whereby measurable levels of the same drug/metabolite were detected in oral fluids 0.08 to 24 hours and 0.08 to 32 hours after low and high doses, respectively (Cone et al., 1997; Jufer et al., 2006; Scheidweiler et al., 2010).
In contrast, in a similar experiment, application of the p-BNC test for the drug methamphetamine elucidated a slightly different time-course for this specific drug, with a demonstrated peak in levels 60 minutes post administration and ultimate clearing from oral fluid 3 hours later (Supplementary Figure 310).
Interestingly, from the observed time-course for the drug cocaine (Figure 5B) it is clear that in addition to the magnitude of concentration that varies in a systematic way, there are also three regions of slope that are observed in the time-course data (labeled as α, β or γ). These quantitation measurements when combined with prior impairment studies reveal that it is not only the concentration, but also the slope that can be used to estimate the time of exposure. Previously, it has been shown that the time frame of 1-3 hours post use is most strongly associated with cocaine-induced impairment (Ellinwood and Nikaido, 1987).
As such, the ease and convenience of oral fluid sample collection when combined with a portable, quantitative 10-minute p-BNC test, provides a testing solution amenable to frequent, short interval sampling and testing, that when used in conjunction with a clinical examination, promise to offer a unique point-of-need capability to distinguish between impaired and non- impaired drivers in near real-time.
4. DISCUSSION
Currently, police officers stop suspect motorists at the roadside and impairment tests are then conducted, followed by an alcohol breath test. If there is evidence, after clinical evaluation, that the driver is impaired, the individual is taken to the police station to be examined by a forensic practitioner who rules whether the suspect has a condition due to drugs. In both the UK and US, if the forensic practitioner decides there is basis for suspicion for drug use, a blood sample is taken for confirmatory analysis. The process requires a trained phlebotomist and use of two physically different techniques (immunoassay and LC-MS/MS) completed at the remote laboratory with results reported days/week(s) after the traffic stop.
Despite the fact that current national and local laws of many countries in the world provide the underpinnings of drug-testing programs, only but a few of them (e.g., in the US within Florida state law, Germany, Ireland, Poland and the Czech Republic) have specific statutes that mention use of alternative biological matrices, such as oral fluid (Cone, 2001). Likewise, while there is still a clear need for parallel development of guidelines governing the use of this alternative drug testing matrix, scientific technology utilized in oral fluid-based drug testing is indeed advancing rapidly.
Initial evidence of unique capabilities of p-BNCs for the measurement of drugs of abuse in oral fluids has been presented here. The process involving a saliva roadside or police station-based screening device, such as the envisioned ‘micro’ portable p-BNC, could replace the requirement of a consent by forensic practitioner for a blood sample to be taken, leading to time and cost savings for the police forces. Likewise, the roadside specification of the ‘micro’ portable p-BNC device will be as a screening oral fluid test, as well as be associated with much more stringent requirements (definitely in terms of robustness and possibly in terms of number of drugs and concentrations), a challenge promised to be met by this powerful new technology.
With these new capabilities in mind, it is projected the p-BNC could impact drug testing and law enforcement moving forward in other settings, including outpatient and inpatient rehab centers, schools, prison facilities, emergency rooms and the workplace.
Indeed, relevant to the later setting, drugs can significantly impair a person's work performance, thereby lowering productivity. Employees who use drugs have double the rate of absenteeism, higher job turnover rates, and cost three times as much in terms of medical benefits as those who don't use drugs (http://www.scu.edu/ethics/publications/iie/v1n1/test.html). Further, drug abuse in the workplace constitutes a serious hazard to others, as employees who use drugs have three times the accident rate as non-users. In some cases, such as in nuclear plants and air-traffic control settings, such accidents could have horrifying consequences. Conversely, it is claimed, routine and random drug testing subject employees to humiliation and violate their right to privacy. Employers cannot intrude on this privacy without serious cause and in a manner that is reasonable. It is further claimed, drug testing is not an effective means for screening out employees whose on-the-job performance is being impaired by drugs. Therefore, drug-testing in the workplace, as well as in other settings, presents a difficult moral challenge where safety and fundamental right to privacy are balanced and respected.
Currently, there is no comprehensive federal law in the US that regulates drug testing in the private sector (http://www.testcountry.com/StateLaws/#sthash). The Drug-Free Workplace Act does impose certain employee education requirements on companies that do business with the government, but it does not require testing, nor does it restrict testing in any way. Drug testing is allowed under the Americans with Disabilities Act (ADA) because the ADA does not consider drug abuse a disability, but the law does not regulate or prohibit testing. Instead of a comprehensive regulatory system, Federal law provides for specific agencies to adopt drug testing regulations for employers under their jurisdiction. Since there is no comprehensive federal drug testing law, this leaves the field open to state regulation, and many states have enacted provisions imposing drug testing restrictions of various kinds. Some limit testing to “reasonable suspicion” or “probable cause” situations, while others explicitly authorize random testing under certain circumstances.
The p-BNC in conjunction with oral fluid sampling provides for a powerful drug screening tool that is non-invasive, sensitive, accurate and reliable, offering quick (real time) turn-around of test results, whilst significantly reducing the risk of sample adulteration. The availability of technologies as such promises to help resolve at least some of the ethical dilemmas associated with drug testing and to positively influence drafting of new laws and guidelines for drug testing.
Rapid (~10 minutes), sensitive detection (~ng/ml) and quantitation of 12 drugs of abuse is demonstrated on the p-BNC platform. This medical microdevice system demonstrates simultaneous multi-drug detection capabilities, as well as it providing key information related to time-course of select drug and metabolite profiles as extracted from oral fluids. These new tools have potential to aid in the creation of safer workplace environments as well as to have a significant impact on drug-impaired driving thereby saving lives.
Importantly, also derived from the time course analyses is information on the rate of change in drug concentrations detected over time, which when combined with concentration measurements from prior studies, yields information about cocaine-induced impairment. Clearly, much additional work is needed to confirm the association between the time-course of specific drugs in oral fluids and level of impairment for the driver, yet the new approach provides an avenue to explore these associations.
It should also be emphasized that these findings related to drug of abuse testing need to be evaluated in the context of the person's mental health status examination, in order to obtain a clear picture on the level of impairment. Further, due to the fast metabolism of some drugs of abuse, the clinical examination has to be performed as soon as possible and results acquired should be evaluated with the understanding that, as in alcohol dependence due to tolerance, a dependent person could have high drug concentration without any behavioral or cognitive disturbance whilst, in contrast, an occasional user could show cognitive and behavioral disturbance even after low level consumption.
Supplementary Material
Highlights.
First application of p-BNC technology for the detection of drugs of abuse in oral fluids
Rapid (~10 minutes), sensitive detection (~ng/ml) and quantitation of 12 drugs of abuse was demonstrated on the p-BNC platform.
Demonstration of simultaneous detection and measurement of multiple drugs
Strong correlation of p-BNC test against reference method
Furthermore, the p-BNC system provided visibility to time-course of drug and metabolite profiles in oral fluids and demonstrated its potential to reveal information related to level of impairment.
Acknowledgements
Acknowledged here is the funding support and guidance provided for this work by the United Kingdom (UK) Home Office Centre of Applied Science and Technology (CAST).
Also acknowledged is the support for the p-BNC core instrumentation work as provided by the National Institutes of Health (NIH) through the National Institute of Dental and Craniofacial Research (Award Number 5U01 DE017793).
Part of this work is the result of work facilitated with resources and the use of the infrastructure at the Michael E. DeBakey VA Medical Center, Houston, Texas.
The content is solely the responsibility of the authors and does not necessarily represent or reflect the official views of CAST, NIH nor the UK or US governments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
AUTHOR DISCLOSURES
Affiliations
J.T. McDevitt, N. Christodoulides, J. Wong, P. Floriano and G. W. Simmons are affiliated with the Department of Bioengineering and the Department of Chemistry at Rice University in Houston, Texas. M. McRae, R. Smith, J. Hohenstein, S. Gomez, H. Talavera, D. J. Sloan and A. Haque are affiliated with the Department of Bioengineering at Rice University. R. De La Garza II is affiliated with the Menninger Department of Psychiatry & Behavioral Sciences, the Department of Pharmacology and the Department of Neuroscience, Baylor College of Medicine, Houston TX. T. Newton is affiliated with the Menninger Department of Psychiatry & Behavioral Sciences, the Department of Pharmacology, and Department of Neuroscience at Baylor College of Medicine and the Veterans Affairs Medical Center, Houston, TX. J. Mahoney, III is affiliated with the Menninger Department of Psychiatry & Behavioral Sciences at Baylor College of Medicine. T. Kosten is affiliated with the Menninger Department of Psychiatry & Behavioral Sciences, the Department of Pharmacology and the Department of Neuroscience, Baylor College of Medicine, and the Veterans Affairs Medical Center, Houston TX. D. Moody and D. Andrenyak are affiliated with the Center for Human Toxicology, Department of Pharmacology and Toxicology, University of Utah, Salt Lake City, UT.
Conflict of Interest
Principal Investigator, John T. McDevitt, has an equity interest in SensoDX, LLC. Inc, and also serves on Scientific Advisory Board. The terms of this arrangement have been reviewed by Rice University and he is currently under a management plan in accordance with its conflict of interest policies.
Contributions
Dr. J. T. McDevitt served as overall Principal Investigator (PI) of this study. As such, he was involved in this study by providing oversight for the p-BNC group at Rice University. Dr. R. De La Garza served as the lead clinical PI from Baylor College of Medicine, the recruitment site at which this addiction portion of the study was conducted. Dr. N. Christodoulides has served as the director of assay development for the p-BNC drug tests. Dr. P. Floriano served as the director of data and image analysis for the p-BNC drug tests. R. Smith was responsible for the development of p-BNC tests for methamphetamine, amphetamine, MDA, MDMA, oxazepam, diazepam, temazepam and nordiazepam. J. Hohenstein developed p-BNC tests for THC, methadone and morphine assays. S. Gomez developed p-BNC tests for THC and cocaine. Dr. J. Wong was involved in collection of data in the integrated injection molded labcard and the portable instrumentation. Dr. Wong was also responsible for creating renderings on the instrumentation and labcards as well as producing the molecular simulations of drug and metabolite capture and signaling at the agarose nano-nets. These simulations are included as supplementary materials. M. P. McRae has lead the effort along with A. Haque for the development of portable instrumentation. G. W. Simmons has provided oversight for the design, fabrication and testing of integrated lab cards. D. J. Sloan served as the initial program manager for the p-BNC program. Dr. J. J. Mahoney, III served as the clinical coordinator at the Baylor College of Medicine clinical site. Dr. T. Newton and Dr. T. Kosten served as the clinical co-PIs at Baylor College of Medicine. H. Talavera assisted in the development of automated macros for the analysis of images and data gathered on p-BNCs. Dr. D.E. Moody and Dr. D. M. Andrenyak from the University of Utah analyzed oral fluid samples by LC-MS/MS for the methods comparison study.
All authors have read and approved of the submission of this manuscript to Drug and Alcohol Dependence.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:..
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
REFERENCES
- Bell SC, Hanes RD. A Microfluidic device for presumptive testing of controlled substances. J. Forensic Sci. 2007;52:884–888. doi: 10.1111/j.1556-4029.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin. Chem. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruls DM, Evers TH, Kahlman JAH, van Lankvelt PJW, Ovsyanko M, Pelssers EGM, Schleipen JJHB, de Theije FK, Verschuren CA, van der Wijk T, van Zon JBA, Dittmer WU, Immink AHJ, Nieuwenhuis JH, Prins MWJ. Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip. 2009;9:3504–3510. doi: 10.1039/b913960e. [DOI] [PubMed] [Google Scholar]
- Cheng X, Gupta A, Chen C, Tompkins RG, Rodriguez W, Toner M. Enhancing the performance of a point-of-care CD4+ T-cell counting microchip through monocyte depletion for HIV/AIDS diagnostics. Lab Chip. 2009;9:1357–1384. doi: 10.1039/b818813k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CD, Linder V, Sia SK. Lab-on-a-chip devices for global health: past studies and future opportunities. Lab Chip. 2007;7:41–57. doi: 10.1039/b611455e. [DOI] [PubMed] [Google Scholar]
- Christodoulides N, Tran M, Floriano PN, Rodriguez M, Goodey A, Ali M, Neikirk D, McDevitt JT. A microchip-based multianalyte assay system for the assessment of cardiac risk. Anal. Chem. 2002;74:3030–3036. doi: 10.1021/ac011150a. [DOI] [PubMed] [Google Scholar]
- Christodoulides N, Floriano PN, Sanchez X, Li L, Hocquard K, Patton A, Muldoon R, Miller CS, Ebersole JL, Redding S, Yeh C-K, Furmaga WB, Wampler DA, Bozkurt B, Ballantyne CM, McDevitt JT. Programmable bio-nanochip technology for the diagnosis of cardiovascular disease at the point of care. Methodist Debakey Cardiovasc. J. 2012;1:6–12. doi: 10.14797/mdcj-8-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ, Oyler J, Darwin WD. Cocaine disposition in saliva following intravenous, intranasal, and smoked administration. J. Anal. Toxicol. 1997;21:465–475. doi: 10.1093/jat/21.6.465. [DOI] [PubMed] [Google Scholar]
- Cone EJ. Legal, workplace, and treatment drug testing with alternate biological matrices on a global scale. Forens. Sci Intern. 2001;121:7–15. doi: 10.1016/s0379-0738(01)00446-7. [DOI] [PubMed] [Google Scholar]
- Crouch DJ, Walsh JM, Cangianelli L, Quintela O. Laboratory evaluation and field application of roadside oral fluid collectors and drug testing devices. Ther. Drug Monit. 2008;30:188–195. doi: 10.1097/FTD.0b013e3181679249. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Nikaido AM. Stimulant induced impairment: a perspective across dose and duration of use. Alcohol Drugs Driving. 1987;3:19–24. [Google Scholar]
- Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus J, Rose BG, Novak MJ, Steinhubl S, Acosta S, Mohanty S, Dharshan P, Yeh C-K, Redding S, Furmaga W, McDevitt JT. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin. Chem. 2009;55:1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodey A, Lavigne JJ, Savoy SM, Rodriguez MD, Curey T, Tsao A, Simmons G, Wright J, Yoo S-J, Sohn Y, Anslyn EV, Shear JB, Neikirk DP, McDevitt JT. Development of multianalyte sensor arrays composed of chemically derivatized polymeric microspheres localized in micromachined cavities. J. Am. Chem. Soc. 2001;123:2559–2570. doi: 10.1021/ja003341l. [DOI] [PubMed] [Google Scholar]
- Goluch ED, Nam J-M, Georganopolou DG, Chiesl TN, Shaikh KA, Ryu KS, Barron AE, Mirkin CA, Liu C. A bio-barcode assay for on-chip attomolar-sensitivity protein detection. Lab Chip. 2006;6:1293–1299. doi: 10.1039/b606294f. [DOI] [PubMed] [Google Scholar]
- Jufer RA, Walsh SL, Cone EJ, Sampson-Cone A. Effect of repeated cocaine administration on detection times in oral fluid and urine. J. Anal. Toxicol. 2006;30:458–462. doi: 10.1093/jat/30.7.458. [DOI] [PubMed] [Google Scholar]
- Kim JS, Anderson GP, Erickson JS, Golden JP, Nasir M, Ligler FS. Multiplexed detection of bacteria and toxins using a microflow cytometer. Anal. Chem. 2009;81:5426–5432. doi: 10.1021/ac9005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Garon E, Wong DT. Salivary diagnostics. Orthod. Craniofac. Res. 2009;12:206–211. doi: 10.1111/j.1601-6343.2009.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-N, Moody DE, Bigelow GE, Foltz RL. A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometric method for quantitation of cocaine and benzoylecgonine in human plasma. J. Anal. Toxicol. 2001;25:497–503. doi: 10.1093/jat/25.7.497. [DOI] [PubMed] [Google Scholar]
- Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs SA, Lafrate J, Bell DW, Digumarthy S, Muzikansky A, Irimia D, Settleman J, Tompkins RJ, Lynch TJ, Toner M, Haber DA. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud D, Rodriguez-Chavez IR. Saliva as a diagnostic fluid. Dent. Clin. North Am. 2011;55:159–178. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterfield SJ, Yu H, Gaster RS, Caramuta S, Xu L, Han S-J, Hall DA, Wilson RJ, Sun S, White RL, Davis RW, Pourmand N, Wang SX. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA. 2008;105:20637–20640. doi: 10.1073/pnas.0810822105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MR, Soldini L, Di Perri G, Tiberi S, Lazzarin A, Lillo FB. Offer of rapid testing and alternative biological samples as practical tools to implement HIV screening programs. New Microbiol. 2009;32:391–396. [PubMed] [Google Scholar]
- Qiang W, Zhai C, Lei J, Song C, Zhang D, Sheng J, Xu D. Disposable microfluidic device with ultraviolet detection for highly resolved screening of illicit drugs. Analyst. 2009;134:1834–1839. doi: 10.1039/b906434f. [DOI] [PubMed] [Google Scholar]
- Qin L, Vermesh O, Shi Q, Heath JR. Self-powered microfluidic chips for multiplexed protein assays from whole blood. Lab Chip. 2009;9:2016–2020. doi: 10.1039/b821247c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raamanathan A, Simmons GW, Christodoulides N, Floriano PN, Furmaga WB, Redding SW, Lu KH, Bast RC, Jr, McDevitt JT. Programmable bio-nano-chip systems for serum CA125 quantification: towards ovarian cancer diagnostics at the point-of-care. Cancer Prev. Res. 2012;5:706–716. doi: 10.1158/1940-6207.CAPR-11-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez WR, Christodoulides N, Floriano PN, Graham S, Mohanty S, Dixon M, Hsiang M, Peter T, Zavahir S, Thior I, Romanovicz D, Bernard B, Goodey AP, Walker DW, McDevitt JT. A microchip CD4 counting method for HIV Monitoring in resource-poor settings. PloS Med. 2005;2:663–672. doi: 10.1371/journal.pmed.0020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidweiler KB, Kolbrich-Spargo EA, Kelly TL, Cone EJ, Barnes AJ, Huestis MA. Pharmacokinetics of cocaine and metabolites in human oral fluid and correlation with plasma concentrations after controlled administration. Ther. Drug Monit. 2010;32:628–637. doi: 10.1097/FTD.0b013e3181f2b729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson MH, Taccogno J, Foltz RL, Moody DE. Quantitative analysis of selegiline and three metabolites (N-desmethylselegiline, methamphetamine and amphetamine) by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J. Anal. Toxicol. 2002;26:430–437. doi: 10.1093/jat/26.7.430. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Brennan JS, Renzi RF, Wu M, Branda SS, Singh AK, Herr AE. Fully integrated microfluidic platform enabling automated phosphoprofiling of macrophage response. Anal. Chem. 2009;81:3261–3269. doi: 10.1021/ac8024224. [DOI] [PubMed] [Google Scholar]
- Thorsen T, Maerkl SJ, Quake SR. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- Vanstechelman S, Isalberti C, Van der Linden T, Pil K, Legrand S-A, Verstraete AG. Analytical evaluation of four on-site oral fluid drug testing devices. J. Anal. Toxicol. 2012;36:136–140. doi: 10.1093/jat/bkr016. [DOI] [PubMed] [Google Scholar]
- Walt DR. Chemistry: miniature analytical methods for medical diagnostics. Science. 2005;308:217–219. doi: 10.1126/science.1108161. [DOI] [PubMed] [Google Scholar]
- White DA, Scribner AN, Huang JV. A comparison of patient acceptance of fingerstick whole blood and oral fluid rapid HIV screening in an emergency department. J. Acquir. Immune Defic. Syndr. 2009;52:75–78. doi: 10.1097/QAI.0b013e3181afd33d. [DOI] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Wong D. Salivary Diagnostics. Wiley-Blackwell; Ames, Iowa: 2008. [Google Scholar]
- Zhou J, Ellis AV, Kobus H, Voelcker NH. Aptamer sensor for cocaine using minor groove binder based energy transfer. Anal. Chim. Acta. 2012;719:76–81. doi: 10.1016/j.aca.2012.01.011. [DOI] [PubMed] [Google Scholar]
- http://oas.samhsa.gov/nsduh.htm. (10/06/2014)
- http://assets.dft.gov.uk/statistics/releases/road-accidents-and-safety-annual-repor2011/rrcgb2011-05.pdf. (10/06/2014)
- http://www.scu.edu/ethics/publications/iie/v1n1/test.html)
- http://www.testcountry.com/StateLaws/#sthash (01/09/2015)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.