Abstract
Background
Clinical, experimental, and ethnographic research suggests that cannabis may be used to help manage pain. Ethnographic research has revealed that some people are using cannabis to temper their illicit opioid use. We seek to learn if there is an association between cannabis use and the frequency of nonmedical opioid use among people who inject drugs (PWID).
Methods
PWID were recruited using targeted sampling methods in Los Angeles and San Francisco, California, 2011–2013. We limited analysis to people who used opioids in past 30 days (N=653). Outcome variable: number of times used any opioids non-medically in past 30 days. Explanatory variable: any cannabis use past 30 days. Statistics: multivariable linear regression with a log-transformed outcome variable.
Results
About half reported cannabis use in the past 30 days. The mean and median number of times using opioids in past 30 days were significantly lower for people who used cannabis than those who did not use cannabis (mean: 58.3 vs. 76.4 times; median: 30 vs 60 times, respectively; p<0.003). In multivariable analysis, people who used cannabis used opioids less often than those who did not use cannabis (Beta: −0.346; 95% confidence interval: −0.575, −0.116; p<0.003).
Conclusions
There is a statistical association between recent cannabis use and lower frequency of nonmedical opioid use among PWID. This may suggest that PWID use cannabis to reduce their pain and/or nonmedical use of opioids. However, more research, including prospective longitudinal studies, is needed to determine the validity of these findings.
Keywords: cannabis, opioids, injection drug use, PWID, epidemiology
1. INTRODUCTION
The therapeutic applications of cannabis were first documented in the oldest known pharmacopeia, written by the Emperor of China, Shen Nung in 2737 BC, where it was recommended for over a wide variety of ailments, from gout to parasitic infections (Li, 1974). Since that time, there has been a stream of medical claims that cannabis eases limb-muscle spasms, is an effective analgesic and has antianxiety and antiemetic properties (Baker et al, 2003), Cannabis was part of the American pharmacopeia for much of the 19th and early 20th centuries, until the US federal government began restricting its use in the late 1930s (Bostwick, 2012), In 1970, the US Congress categorized cannabis as a Schedule I drug under the Controlled Substances Act, declaring it to have high abuse potential and no medical value, thereby rendering its use illegal (Cohen, 2010),
The past two decades has seen an increase in debate about the use of cannabis for medicinal purposes, with California becoming the first U.S. state to authorize medicinal cannabis in 1996 (O'Connell and Bou-Matar, 2007). To date, twenty-three states and the District of Columbia have passed laws that allow adult use of medical cannabis (Portal Labs, 2014). Additionally, as of February, 2014, four states-- Alaska, Colorado, Oregon, Washington-- and the District of Columbia, have legalized possession, manufacture and sale of cannabis for people 21 years of age and older to use recreationally (Merica, 2014).
There is a growing body of literature documenting the therapeutic benefits of cannabis (Bostwick, 2014; Grotenhermen and Muller-Vahl, 2012; Kalant, 2014; Lucas, 2012; Walsh et al, 2013). Reports of improved appetite and reduction in muscle pain, nausea, anxiety, depression and paresthesia have been associated with cannabis use among people with HIV (Woolridge et al, 2005). Cannabis use for pain relief is also common among people living with chronic noncancer pain (Degenhardt et al, 2014). In addition to pain relief, individuals who use cannabis for therapeutic reasons report effective symptom relief for anxiety and sleep disturbances (Walsh et al, 2013). Cannabis may also act to relieve inflammation and has been found to have a useful place in the treatment of rheumatic diseases (Kalant, 2014). Multiple review articles have systematically documented the therapeutic potential of cannabis as treatment for nausea, loss of appetite in HIV and cancer patients, spasticity in multiple sclerosis and spinal cord injuries, neuropathic pain, non-neuropathic pain, Tourette syndrome, and glaucoma (Abrams et al, 2011; Ben Amar, 2006; Grotenhermen and Muller-Vahl, 2012; Kumar et al, 2001; Raby et al, 2009; Robson, 2001).
Due to potential side effects (including overdose) associated with opioid use (Centers for Disease and Prevention, 2011) and the decrease in analgesic efficacy over time (Lee et al, 2011), there is a need to explore alternative medications to opioids in the management of severe pain. While controversial, cannabis is being explored as a possible complement (Abrams et al, 2011) or alternative to opioids for reducing pain (Carter et al, 2015; Elikkottil et al, 2009; Lucas, 2012). Clinical and pre-clinical studies have documented the synergistic relationship between opioids and cannabis. In a review article, Elikottil and colleagues (2009) assessed the synergistic relationship between opioids and cannabis in both experimental studies with mice and rats and clinical studies with healthy subjects. They conclude that combining smaller doses of cannabis and opioids resulted in positive analgesic effects with fewer side effects than a larger dose of either drug alone. Abrams and colleagues (2011) also found that among chronic pain patients who were treated with opioids, vaporized cannabis augments the analgesic effects of opioids, which may allow for opioid treatment at lower doses with fewer side effects. Similar to clinical and experimental research, data from a community-based study of people who have been prescribed opioids for chronic non-cancer pain found that cannabis use for pain relief purposes was common and that study participants reported greater pain relief in combination with opioids than when opioids were used alone (Degenhardt et al, 2014).
Qualitative studies have recently found that people who use heroin report that they are able to temper or reduce their heroin use by using cannabis. In a sample of street-recruited PWID, study participants reported smoking cannabis to reduce anxiety and cravings experienced while transitioning away from daily heroin use (Wenger et al, 2014). In another qualitative study, Peters found that medical cannabis patients consistently reported using cannabis to substitute or wean off prescription opioids (Peters, 2013). All patients who were taking opioids reported reducing their overall drug use, specifically opioids, by using cannabis. Patients also reported that cannabis was preferred over opioids, eased withdrawal from opioids, and in some cases was more effective in relieving pain.
In this paper, we test whether there is a statistical association between cannabis use and the frequency of nonmedical opioid use in a large cross-sectional sample of street-recruited PWID.
2. METHODS
2.1 Study Procedures
We used targeted sampling methods to recruit PWID in Los Angeles and San Francisco, California, USA (Bluthenthal and Watters, 1995; Kral et al, 2010; Watters and Biernacki, 1989). Eligibility criteria included injection drug use in the past 30 days and being 18 years of age or older. Study staff verified that potential participants had injected drugs by inspecting them for signs of recent venipuncture (“tracks”; Cagle et al, 2002). Each participant went through an informed consent process before enrolling in the study. The study involved a quantitative survey interview which served the dual purposes of collecting quantitative data on a large sample of PWID and providing study staff with information about eligibility into a sub-study that involved a qualitative interview. We only report on results of the quantitative survey in this manuscript. The survey involved a one-on-one, computer-assisted personal interview (CAPI) conducted by a trained interviewer which lasted between 30 and 45 minutes (Questionnaire Development System, NOVA Research, Bethesda, MD). After completion of the survey, participants were remunerated $20. All study procedures were approved by the Institutional Review Boards at the two institutions where the research was carried out: University of Southern California and RTI International.
2.2 Study Sample
The study was conducted between April, 2011 and April, 2013 in Los Angeles and San Francisco, during which time 777 PWID completed the quantitative survey. Because this analysis involves assessing whether the frequency of opioid use among PWID is different from those who use cannabis and those who do not use cannabis, we restricted the sample to the 653 PWID who reported any (a) use of heroin alone or in combination with other drugs (including cocaine or methamphetamine) or (b) nonmedical use of opioid pills or methadone.
2.3 Study Measures
Our outcome variable was the number of times a participant used opioids in the past 30 days (people could use opioids many times per day). This variable was the sum of the answers to questions about the number of times in the past 30 days that the participant reported using heroin (injected and non-injected), “speedball” (mix of heroin and cocaine, injected and non-injected), “goofball” (mix of heroin and methamphetamine, injected and non-injected), non-prescribed methadone (used), and nonmedical use of opiate pills (injected and non-injected). Our explanatory variable was whether the participant responded yes to the question “Have you used marijuana in the last 30 days?” Note that we used the word “marijuana” in the survey instrument, as opposed to cannabis, because this study took place in California, USA, where marijuana is the most common term for cannabis. The following factors were candidate confounding variables: socio-demographic and socioeconomic characteristics, including., age, gender, housing status, income, and sexual orientation, study site (Los Angeles or San Francisco), drug use history (years of injection), recent (last 30 days) crack cocaine, powder cocaine, methamphetamine, alcohol use, and health-related items such as mental health diagnoses, HIV status, health insurance, and drug treatment experience.
2.4 Statistical Analysis
We used descriptive statistics (e.g., frequencies, means, standard deviations, medians, interquartile ranges) to examine all variables. Given that the outcome variable (number of times used opioids nonmedically) is continuous and not normally distributed, we conducted a standard log transformation for the bivariate and multivariable analyses. Then we conducted bivariate analyses to assess whether the explanatory variable (cannabis use) was associated with our log transformed outcome variable by using t-tests and ANOVA. We also assessed whether the potential confounding variables were associated with the explanatory variable and outcome variable. Statistical significance threshold was predetermined to be at p<0.05. We also evaluated multicollinearity using a diverse range of approaches (e.g., Martingale residuals, model-based correlations) for key variables that are typically found to be highly interrelated in other studies by assessing correlations among potential confounding variables in the same domain (e.g., drug use, demographics, health). In the multivariable analysis, we used linear regression. The final multivariate model included the explanatory variable, as well as whether they had health insurance, were in substance abuse treatment in the past 30 days, and any potential confounding variables significant at the p<0.05 levels. All statistics were computed using SPSS/PASW Statistics 18.0 (released July 30, 2009).
3. RESULTS
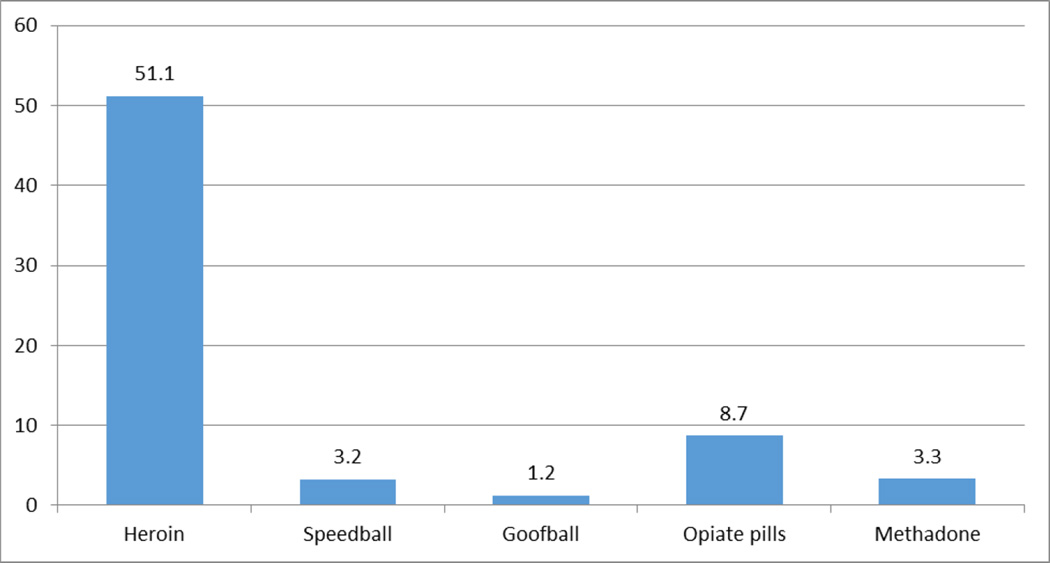
The sample was nearly three-quarters men, one-third African American, one-third Latino, and one-third Caucasian, with the majority being over 50 years old (Table 1). Nearly one half reported having used cannabis in the past 30 days. Nearly all reported having used heroin in the past 30 days (95%), followed by nonmedical use of opioids (38%), methadone (26%), speedball use (20%), and goofball use (15%). The mean (and standard deviation) number of times participants used opioids nonmedically in the past 30 days was 67.5 (78.22) and the median (and interquartile range) was 43 (12.5, 93). Heroin accounted for the vast majority (76% of the number of times opioids were used nonmedically in the past 30 days (mean=51.1 times; see Figure 1).
Table 1.
Demographics of people who inject opioids in Los Angeles and San Francisco, 2011-2013 (N=653)
| Demographics | n |
Cannabis Yes (n=321) |
Cannabis No (n=332) |
|
|---|---|---|---|---|
| City | Los Angeles | 365 | 36% | 64% |
| San Francisco | 288 | 66% | 34% | |
| Sex | Male | 468 | 54% | 46% |
| Female | 184 | 38% | 62% | |
| Race | Black | 208 | 48% | 52% |
| White | 195 | 59% | 41% | |
| Hispanic | 183 | 34% | 66% | |
| Other race/ethnicity | 63 | 65% | 35% | |
| Age | 18 to 29 years | 62 | 69% | 31% |
| 30 to 39 years | 66 | 70% | 30% | |
| 40 to 49 years | 175 | 49% | 51% | |
| 50 years or older | 350 | 42% | 58% | |
| Sexual orientation | Heterosexual | 582 | 48% | 52% |
| Gay/lesbian/bisexual | 71 | 61% | 39% | |
| Considers self homeless | Yes | 406 | 51% | 49% |
| No | 247 | 46% | 54% | |
| HIV antibody status self-reported (n=619) | Negative | 586 | 50% | 50% |
| Positive | 33 | 55% | 45% | |
| Drug use | n | % | ||
| Age at first injection | 9 to 17 years | 248 | 46% | 54% |
| 18 to 29 years | 300 | 55% | 45% | |
| 30 years or older | 107 | 39% | 61% | |
| Number days used alcohol (n=390) | No alcohol use | 162 | 41% | 59% |
| 1 to 4 days | 90 | 52% | 48% | |
| 5 to 29 days | 68 | 69% | 31% | |
| Every day | 70 | 59% | 41% | |
| Crack cocaine past 30 days | 310 | 58% | 42% | |
| Powder cocaine past 30 days | 106 | 59% | 41% | |
| Methamphetamine past 30 days | 220 | 65% | 35% | |
| Heroin past 30 days | 619 | 48% | 52% | |
| Speedball (heroin plus cocaine) past 30 days | 131 | 56% | 46% | |
| Goofball (heroin plus methamphetamine) past 30 days | 95 | 67% | 33% | |
| Opioid pills past 30 days | 245 | 63% | 37% | |
| Illicit methadone | 162 | 49% | 51% | |
| Any substance abuse treatment past 30 days | 261 | 50% | 50% | |
Figure 1.
Mean opioid episodes by type of opioid used in past 30 days
(Total=67.5 opioid episodes in past 30 days)
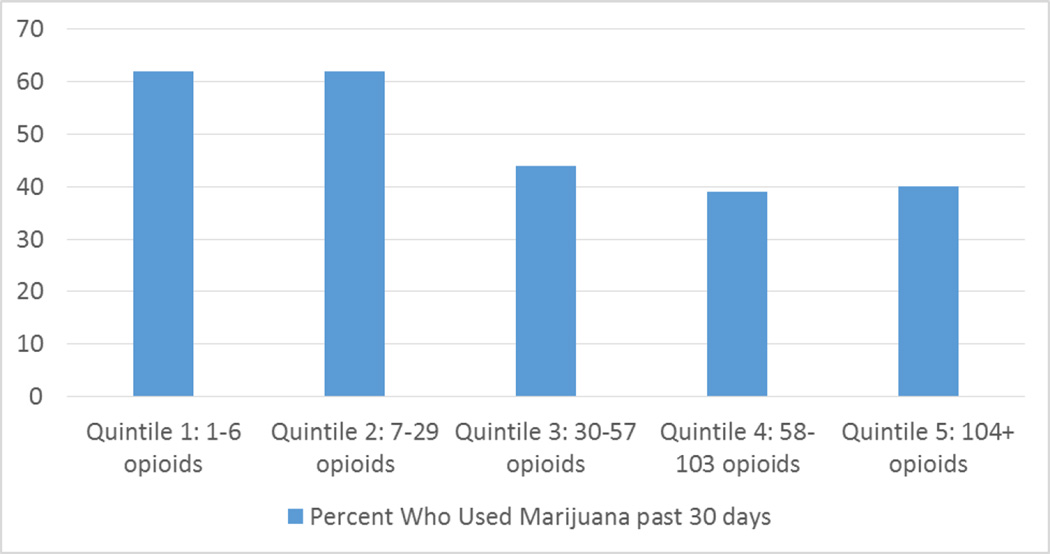
The mean and median number of times opioids were used in past 30 days were significantly lower for people who used cannabis than those who did not use cannabis in the past 30 days (mean= 58.3 times [standard deviation=79.4] vs. mean=76.4 times [standard deviation=76.1]; median: 30 vs 60 times, respectively; p<0.003). We created a variable that consisted of quintiles of the number of times opioids were used in the past 30 days. The quintile cut-offs were 1 to 6 times, 7 to 29 times, 30 to 57 times, 58 to 103 times, and 104 or more times. Cannabis use was more prevalent among the lower quintiles of opioid use than the higher quintiles (Figure 2; chisquare value=27.923; degrees of freedom=4; p<0.001). In multivariable analysis, cannabis use in the past 30 days was highly significantly negatively associated (Beta= −0.346; 95% confidence interval=−0.575, −0.116; degrees of freedom=7; p<0.003) with the log transformed variable for number of times used opioids in past 30 days, while controlling for age, age at first injection, being Latino, recruited in Los Angeles, having no health insurance, and no methadone treatment in past month (Table 2).
Figure 2.
Percent Who Used Cannabis past 30 days by Quintiles of Number of Opioid Episodes in past 30 days
Table 2.
Association between cannabis use and number of times used opioids in past 30 days, among people who inject opioids in Los Angeles and San Francisco, 2011–2013 (N=652)
| Independent Variables | Beta | 95% C.I. |
|---|---|---|
| Explanatory Variable | ||
| Cannabis use past 30 days | −0.346 | (−0.575, −0.116)* |
| Confounding Variables | ||
| Latino | 0.350 | (0.087, 0.613)* |
| Los Angeles | 0.636 | (0.377, 0.895)* |
| 30 to 39 years old | 0.628 | (0.264, 0.991)* |
| Age at first injection | −0.015 | (−0.028, −0.002)* |
| No health insurance | 0.113 | (−0.143, 0.369) |
| In substance abuse treatment past 30 days | −0.197 | (−0.443, 0.048) |
p-value <0.05.
Beta coefficients were derived from OLS estimation procedure on the log-transformed variable
4. DISCUSSION
We found that in this sample of street-recruited PWID who use opioids in Los Angeles and San Francisco, people who use cannabis used opioids less frequently. A number of possible explanations exist for this phenomenon. It is possible that PWIDs who use cannabis have a qualitatively different set of motivations than those who do not, or that PWIDs who use cannabis have a less severe form of opioid use disorder than those who do not. Alternatively, it may be that cannabis is deliberately or unconsciously used by PWIDs to decrease or manage opioid use. Given previous research on the potential for both substances to be used to reduce pain (Abrams et al, 2011; Ben Amar, 2006; Bostwick, 2014; Degenhardt et al, 2014; Grotenhermen and Muller-Vahl, 2012; Kalant, 2014; Kumar et al, 2001; Lucas, 2012; Robson, 2001; Walsh et al, 2013; Woolridge et al, 2005), it is also feasible that cannabis is being used by street-recruited PWID to self-treat pain. Reductions in opioid use might be deliberately achieved by substituting cannabis to treat pain, psychic distress, cravings, or withdrawal. Conversely, cannabis might incidentally reduce opioid use by satisfying some of the same needs that are satisfied by opioids, leading PWID to unintentionally reduce their frequency of opioid use. In addition, it is interesting that none of the other substance use variables (cocaine, methamphetamine, alcohol, etc.) were significantly associated with frequency of opioid use, suggesting there may be something unique about the relationship between cannabis and opioid use. While this study cannot prove a causal connection or elucidate a mechanism, increased access to and decreased stigma associated with cannabis in many U.S. states provides new opportunities to conduct observational studies on the various therapeutic uses of cannabis, including its use to reduce pain or opioid use. Such studies should include various opioid using populations, including pain patients, people who use heroin or other illicit opioids, and patients on buprenorphine or methadone for maintenance therapy of opioid use disorders, and should employ a range of methods from epidemiological cohorts to qualitative and ethnographic studies.
It is noteworthy that about one-half of PWID who use opioids reported having used cannabis in the past month in these two California cities. Medicinal cannabis has been legal in California since state proposition 215 passed in 1996. In this study, we did not assess whether the participants had obtained cannabis legally, and if so, for what therapeutic purpose. For the purposes of this study, it does not necessarily matter how the study participants obtained their cannabis or whether or not it was used according to medical prescription.
While there is a statistical association in our study between cannabis use and the number of times opioids were used nonmedically, we do not want to imply that there is necessarily causation. Though socio-demographic and socio-economic factors were accounted for and corrected for in our statistical analysis, it remains possible that the differences between groups are due to other, unexamined differences between the cannabis using and non-using cohorts. The average age of our cross-section was higher than many studies of PWID, which may have contributed to a higher burden of pain and therefore magnification of the impact of cannabis on opioid use. Age and age of initiation were included in our multivariable analysis of the main effect to help control for age-related influences. Other socio-demographic and socio-economic factors significantly impacted opioid use, including Latino ethnicity, residence in Los Angeles rather than San Francisco, young age, and age at first use. The impact of cannabis remained significant even after controlling for these variables, but in order to better assess whether there is causation, we would suggest prospective longitudinal observational studies and experimental studies that assess whether changes in cannabis use are associated with changes in opioid use. We also want to point out that our outcome variable- number of times used opioids – is only a proxy for amount of opioids that were used. Heroin accounted for the majority of the number of times opioids were used and it is not possible to determine how much heroin was used each time, nor the potency of the heroin used. In the clinical literature, there is a conversion methodology to aid clinicians in dosing patients. (Svendsen et al, 2011) The “morphine equivalent” standard helps calibrate to a common metric for comparison based on potency. Obviously, the active pharmaceutical ingredients involving cannabis and opioids are different, so there is no direct and objective way to compare potency and therefore, symptom relief. It is also not feasible to derive a morphine equivalent from self-reported use of heroin (or drugs used with heroin) because the potency of heroin varies greatly.
The study is also limited in that it did not collect much data with respect to the explanatory variable (cannabis use). For example, we do not have any dose-related information, including standard units of cannabis consumed per episode. We also did not collect data on types of cannabis, different routes of administration (smoking, eating, and topical application), amount of cannabis used, or cannabidiol and tetrahydrocannabinol levels. A prospective study needs to better assess cannabis use, enabling us to assess whether the amount, frequency, and type of cannabis use is associated with number of times opioids are used. Finally, we did not assess directly whether people were consciously using cannabis to either manage pain or to moderate their opioid use. Future studies of the association among cannabis use and opioid use should specifically ask about motivations for use and whether people use cannabis in these ways. They should also assess whether cannabis is used to try to reduce use of other illicit drugs, or whether this is an unintended but possibly helpful consequence of cannabis use.
Despite these limitations, the present study should stimulate more research about the relationship between cannabis and opioid use among PWID. It is among the first epidemiological observational studies, of which we are aware, to examine the association between cannabis and opioid use among nonmedical opioid users recruited in street settings. Nonetheless, we need to learn more about whether this is a meaningful association. This may be achieved through more rigorous prospective studies that are designed to study this association using longitudinal epidemiological and qualitative research methods.
Highlights.
People who inject drugs (PWID) were recruited using targeted sampling in Los Angeles and San Francisco, CA
About one-half of PWID reported cannabis use in the past 30 days
PWID who use cannabis used opioids less often than those who did not use cannabis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alex H. Kral, Email: akral@rti.org.
Karen F. Corsi, Email: Karen.Corsi@ucdenver.edu.
Ricky N. Bluthenthal, Email: rbluthen@usc.edu.
REFERENCES
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. Clin. Pharmacol. Ther. 2011;90:844–851. doi: 10.1038/clpt.2011.188. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Giovannoni G, Thompson AJ. The therapeutic potential of cannabis. Lancet Neurol. 2003;2:291–298. doi: 10.1016/s1474-4422(03)00381-8. [DOI] [PubMed] [Google Scholar]
- Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J. Ethnopharmacol. 2006;105:1–25. doi: 10.1016/j.jep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bluthenthal RN, Watters JK. Multimethod research from targeted sampling to HIV risk environments. NIDA Res. Monogr. 1995;157:212–230. [PubMed] [Google Scholar]
- Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin. Proc. 2012;87:172–186. doi: 10.1016/j.mayocp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick JM. The use of cannabis for management of chronic pain. Gen. Hosp. Psychiatry. 2014;36:2–3. doi: 10.1016/j.genhosppsych.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Cagle HH, Fisher DG, Senter TP, Thurmond RD, Kaster AJ. In: Classifying Skin Lesions Of Injection Drug Users: A Method For Corroborating Disease Risk. Substance Abuse and Mental Health Services Administratin, Treatment CfSA, editor. Rockville, MD: 2002. [Google Scholar]
- Carter GT, Javaher SP, Nguyen MH, Garret S, Carlini BH. Re-branding cannabis: the next generation of chronic pain medicine? Pain Manage. 2015;5:13–21. doi: 10.2217/pmt.14.49. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999–2008. MMWR. 2011;60:1487–1492. [PubMed] [Google Scholar]
- Cohen PJ. Medical marijuana 2010: it's time to fix the regulatory vacuum. J. Law Med. Ethics. 2010;38:654–666. doi: 10.1111/j.1748-720X.2010.00519.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Lintzeris N, Campbell G, Bruno R, Cohen M, Farrell M, Hall WD. Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend. 2014;147:144–150. doi: 10.1016/j.drugalcdep.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Elikkottil J, Gupta P, Gupta K. The analgesic potential of cannabinoids. J. Opioid Manage. 2009;5:341–357. [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F, Muller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int. 2012;109:495–501. doi: 10.3238/arztebl.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H. Cannabis in the treatment of rheumatic diseases: suggestions for a reasoned approach. J. Rheumatol. 2014;42:146–148. doi: 10.3899/jrheum.140683. [DOI] [PubMed] [Google Scholar]
- Kral AH, Malekinejad M, Vaudrey J, Martinez AN, Lorvick J, McFarland W, Raymond HF. Comparing respondent-driven sampling and targeted sampling methods of recruiting injection drug users in San Francisco. J. Urban Health. 2010;87:839–850. doi: 10.1007/s11524-010-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RN, Chambers WA, Pertwee RG. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia. 2001;56:1059–1068. doi: 10.1046/j.1365-2044.2001.02269.x. [DOI] [PubMed] [Google Scholar]
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–161. [PubMed] [Google Scholar]
- Li HL. An archaelogical and historical account of cannabis in China. Econ. Bot. 1974;28:437–448. [Google Scholar]
- Lucas P. Cannabis as an adjunct to or substitute for opiates in the treatment of chronic pain. J. Psychoactive Drugs. 2012;44:125–133. doi: 10.1080/02791072.2012.684624. [DOI] [PubMed] [Google Scholar]
- Merica D. Oregon, Alaska and Washington, D.C. legalize marijuana. CNN. 2014 [Google Scholar]
- O'Connell TJ, Bou-Matar CB. Long term marijuana users seeking medical cannabis in California (2001–2007): demographics, social characteristics, patterns of cannabis and other drug use of 4117 applicants. Harm Reduct. J. 2007;4:16. doi: 10.1186/1477-7517-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DC. Patients and caregivers report using medical marijuana to decrease prescription narcotics use. Humboldt J. Soc. Relat. 2013;35:24–40. [Google Scholar]
- Portal Labs. Medical Marijuana Laws for Patients. Public Health Law Research. 2014;2015 [Google Scholar]
- Raby WN, Carpenter KM, Rothenberg J, Brooks AC, Jiang H, Sullivan M, Bisaga A, Comer S, Nunes EV. Intermittent marijuana use is associated with improved retention in naltrexone treatment for opiate-dependence. Am. J. Addict. 2009;18:301–308. doi: 10.1080/10550490902927785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P. Therapeutic aspects of cannabis and cannabinoids. Br. J. Psychiatry. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat. Med. 2011;25:725–732. doi: 10.1177/0269216311398300. [DOI] [PubMed] [Google Scholar]
- Walsh Z, Callaway R, Belle-Isle L, Capler R, Kay R, Lucas P, Holtzman S. Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use. Int. J. Drug Policy. 2013;24:511–516. doi: 10.1016/j.drugpo.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Watters JK, Biernacki P. Targeted sampling: options for the study of hidden populations. Soc. Probl. 1989;36:416–430. [Google Scholar]
- Wenger LD, Lopez AM, Comfort M, Kral AH. The phenomenon of low-frequency heroin injection among street-based urban poor: drug user strategies and contexts of use. Int. J. Drug Policy. 2014;25:471–479. doi: 10.1016/j.drugpo.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J. Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]