Abstract
Background and Objectives
The natural history of pulmonary metastases from pancreatic ductal adenocarcinoma (PDAC) is not well studied. Limited evidence suggests patients with isolated pulmonary metastases from PDAC follow a more benign clinical course than those with other sites of metastases.
Methods
We performed a retrospective review of all patients with pulmonary metastases from PDAC from 2000-2010 and analyzed survival utilizing the Kaplan-Meier method based upon location of first metastasis (lung first, intra-abdominal first, or synchronous intra-abdominal and lung metastases).
Results
Median survival among subjects with lung as the only site of metastases was significantly longer than those with other metastatic patterns. In subjects that had undergone resection of their PDAC, survival in those with lung as a first site of recurrence remained significantly longer than those with abdominal first or synchronous intra-abdominal and lung recurrence. Among resected patients that developed lung only recurrence, survival was significantly prolonged (67.5 months) in those who underwent surgical resection/stereotactic radiosurgery compared to chemotherapy (33.8 months) or observation (29.9 months) for treatment of lung recurrence.
Conclusion
Patients with isolated pulmonary recurrence from PDAC may realize a survival benefit from surgical intervention or stereotactic radiosurgery compared to chemotherapy or observation for treatment of lung recurrence.
Keywords: pancreatic ductal adenocarcinoma, pulmonary metastases, lung metastases
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) remains a deadly disease with poor overall survival. In 2014, there will be an estimated 46, 420 new cases of pancreatic cancer and an estimated 39,590 deaths from the disease [1]. Even in patients with very early stage cancers that undergo resection, 5-year survival is only 31.4%, which represents an improvement from historical data [2]. Unfortunately, given the lack of effective screening methods for PDAC, patients often present at later stages with either locally advanced and unresectable or metastatic disease.
Recently, much attention has been given to the development of therapies that target molecular mechanisms in cancer progression. Initiatives such as International Cancer Genome Consortium and The Cancer Genome Atlas have focused on uncovering specific molecular aberrations in cancer [3, 4]. These have proven to be very varied and in pancreatic cancer specifically, have proven very heterogeneous, with most aberrations occurring with a frequency of 2% or less [5-7]. In conjunction with the lack of common molecular targets there is clinical data to suggest heterogeneity in the behavior of different patterns of pancreatic cancer recurrence [8, 9]. Pancreatic cancer recurs most commonly in the liver followed by the peritoneum and lung [8] [10]. Up to 80% of resected patients will develop disease recurrence within 2 years of surgery [9, 11] and will die of their recurrence at a median of 14-20 months [12]. However, among those who do achieve 5-year survival, recurrence is most commonly observed in the lungs [13]. Patients who recur late also most often recur in the lungs with metastatic lesions noted as late as 6.7 years after initial diagnosis [8]. Patients who develop lung metastases appear to have a prolonged time to development of metastases [14, 15] compared to much shorter times when evaluating time to development of all metastatic lesions. Given the known molecular heterogeneity of pancreatic cancer as well as this evidence indicating clinical heterogeneity, we hypothesized that patients with pulmonary metastases as a first site of pancreatic cancer metastases will have prolonged survival compared to those patients with other sites of first metastases. Identifying this population as a unique phenotype of metastatic PDAC may promote better targeted therapies for this deadly disease.
MATERIALS AND METHODS
This study is a retrospective review of all patients with pulmonary metastases from pancreatic cancer with a primary endpoint of survival based upon the site of first metastases. Following approval by the Institutional Review Board at the University of Pittsburgh, the electronic medical record was searched to uncover all patients diagnosed with pancreatic ductal adenocarcinoma and a diagnosis of pulmonary metastases between 2000 and 2010. In order to capture all patients with pulmonary metastases, a broad search was initiated for patients with a diagnosis of pancreatic cancer with the findings of lung or pulmonary and nodule, mass, lesion, metastases, metastasis, or metastatic in any radiology report or lung and adenocarcinoma, metastasis, or metastatic in any pathology report. Initially, 3200 patients were identified. Nearly all of these patients were excluded for either diagnosis outside the specified time period, incorrect primary diagnosis or absence of radiologic suspicion for lung metastases. The occurrence of metastases was confirmed by 1) radiologist interpretation based on increase in either size or number of lung lesions on serial CT or PET-CT imaging or 2) pathologic diagnosis after biopsy or resection. After analysis of the initial 3200 patients, 198 remained. 17 patients were excluded because they did not undergo serial CT scan confirming the presence of metastatic lung disease. One patient was excluded because despite pathologic evaluation, it was not known which of their three primary tumors (pancreatic, gastric, or renal) was the source of the lung metastasis. Five patients were excluded because they were ultimately found to have a primary lung cancer on pathology and one patient was excluded because it was unclear whether they had a metastatic or primary lung lesion based on their evaluation (radiologic only). Demographics, medical comorbidities, surgical treatment, imaging studies, clinicopathologic tumor characteristics and survival of the 174 patients found to have pulmonary metastases from pancreatic cancer were reviewed.
All values are presented as mean and standard deviation or as frequency with percentages. Differences between groups were assessed with independent t-test for normally distributed continuous variables or analysis of variance (ANOVA) when testing the differences between more than two means. Differences in categorical variables were assessed by either Chi-square or Fisher-exact test as appropriate. Survival (presented as median value) was calculated from the time of diagnosis (unless noted) of primary pancreatic cancer to the time of death. Patients that did not die were censored at their last follow-up. Survival was estimated using Kaplan-Meier method and compared using the log-rank test. Comparing the survival among greater than 2 groups, we utilized dummy variables and Cox proportional-hazards regression. All calculations were based on using two-sided tests with significance set at p<0.05. Statistical analyses were performed using Stata software package, version 10 (StataCorp, Texas).
RESULTS
During the period of study, 174 patients with lung metastases from pancreatic cancer were identified. The diagnosis of lung metastases was made by imaging studies alone in 145 patients (83.3%) and by pathologic and radiologic findings together in 29 patients (16.7%). Patients were grouped according to their first site of metastases (lung first and only, n=58; lung first, n=25; abdominal first, n=48; or synchronous lung and abdominal metastases, n=43). There were no significant differences in baseline characteristics among these four groups including age at diagnosis, gender, race, BMI, ASA, age-adjusted CCI, or history of smoking or alcohol use. Among those patients who underwent resection of their primary pancreatic cancer, there was no difference in the grade of tumor, margin positivity, or use of neoadjuvant chemotherapy (Table 1).
Table 1.
Baseline characteristics of groups based on metastases type
| Lung first and only (n=58) | Lung First (n=25) | Abdominal First (n=43) | Synchronous (n=48) | p-value | |
|---|---|---|---|---|---|
| Age | 65.5±10.3 | 65.7±10.7 | 68.4±11.5 | 64.3±10.1 | 0.145 |
| Gender (Female) | 32 (55.2%) | 14 (56%) | 20 (46.5%) | 24 (50%) | 0.820 |
| Race (White) | 57 (98.3%) | 24 (96%) | 35 (87.5%) | 43 (89.6%) | 0.110 |
| BMI | 27.8±6.8 | 25.2±4.0 | 27.9±4.8 | 26.1±4.3 | 0.118 |
| ASA | 2.7±0.5 | 2.8±0.5 | 2.7±0.7 | 2.6±0.7 | 0.851 |
| Age-adjusted CCI | 6.2±2.6 | 6.6±2.9 | 7.6±3.4 | 5.9±2.9 | 0.070 |
| Never smoker | 28 (50%) | 8 (33.3%) | 17 (46%) | 26 (56.5%) | 0.261 |
| No or occasional alcohol use | 51 (91.1%) | 19 (79.2%) | 30 (81.1%) | 39 (84.8%) | 0.384 |
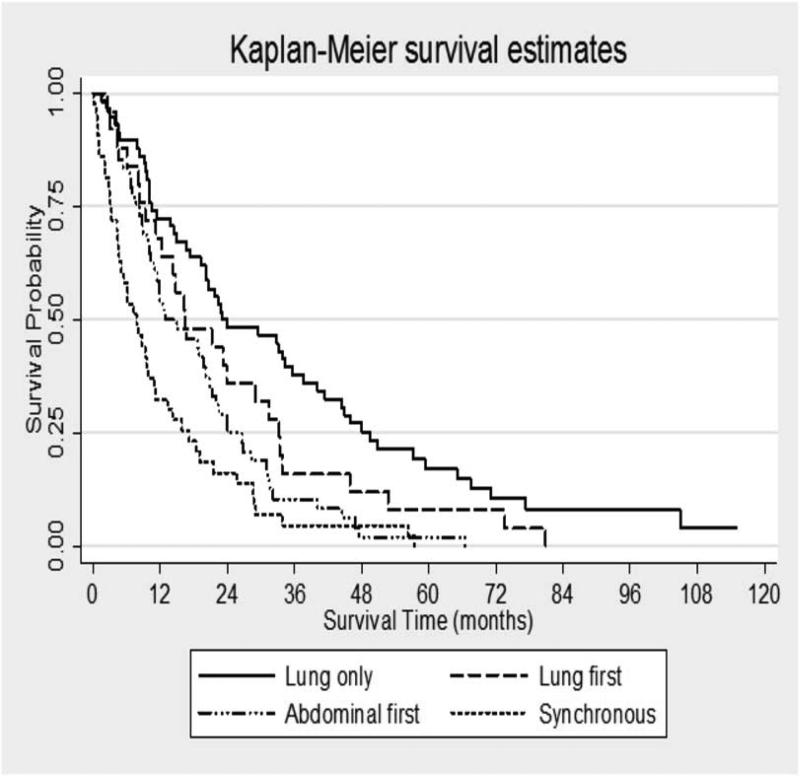
The overall median survival from time of diagnosis of pancreatic cancer was 15.9 months for all patients. In the entire patient cohort, median survival was 23.1 months (95% C.I. 19.2-37.5) for those with lung as the only site of metastases, compared to 16.5 months (95% C.I. 11.1-31.4) for those with lung first but not only metastases. For patients with abdominal metastases before lung metastases, median survival was 12.9 months (95% C.I. 9.97-20.7) and for those patients with synchronous intra-abdominal and lung metastases, median survival was 7.3 months (95% C.I. 4.47-10.97) (Figure 1). Utilizing patients with lung as the only site of metastases as a baseline for comparison, a hazard ratio of death for the remaining three groups was calculated utilizing Cox-proportional hazards technique. Compared to those with lung metastases only, patients with a diagnosis of pancreatic cancer and lung metastases followed by intra-abdominal metastases had a hazard ratio of death of 1.5 (p-value non-significant). However, using those with lung metastases only as a baseline, those with abdominal metastases before lung metastases had a hazard ratio of death of 2.15 (p<0.001, 95% C.I. 1.43-3.23) and those with synchronous intra-abdominal and lung metastases had a hazard ratio of death of 3.34 (p<0.001, 95% C.I. 2.19-5.09). The survival differences remained significant after controlling for age at diagnosis, gender, BMI, T stage of primary tumor, node positivity (if resected), and use of neoadjuvant therapy (if resected). After controlling for these patient factors, the hazard ratio of death for the abdominal metastases first group was 3.15 (p=0.003, 95% C.I. 1.46-6.8) and the synchronous metastases group hazard ratio was 6.01 (p=0.001, 95% C.I. 2.17-16.59).
Figure 1. Median survival in all patients with pulmonary metastases from PDAC grouped by site of first metastases.
Among those with lung as a first and only site of metastases, median survival was 23.1 months compared to 16.5 months for those with lung first but not only metastases, 12.9 months for those with abdominal metastases before lung metastases, and 7.3 months for those with synchronous intra-abdominal and lung metastases. Using patients with lung only metastases as a reference for comparison, the hazard ratios of death are 1.5 (95% C.I. 0.94-2.45, p=0.091) for patients with lung first but not only metastases, 2.2 (95% C.I. 1.43-3.23, p<0.0001) for patients with abdominal first metastases, and 3.3 (95% C.I. 2.2-5.1, p<0.0001) for patients with synchronous abdominal and lung metastases.
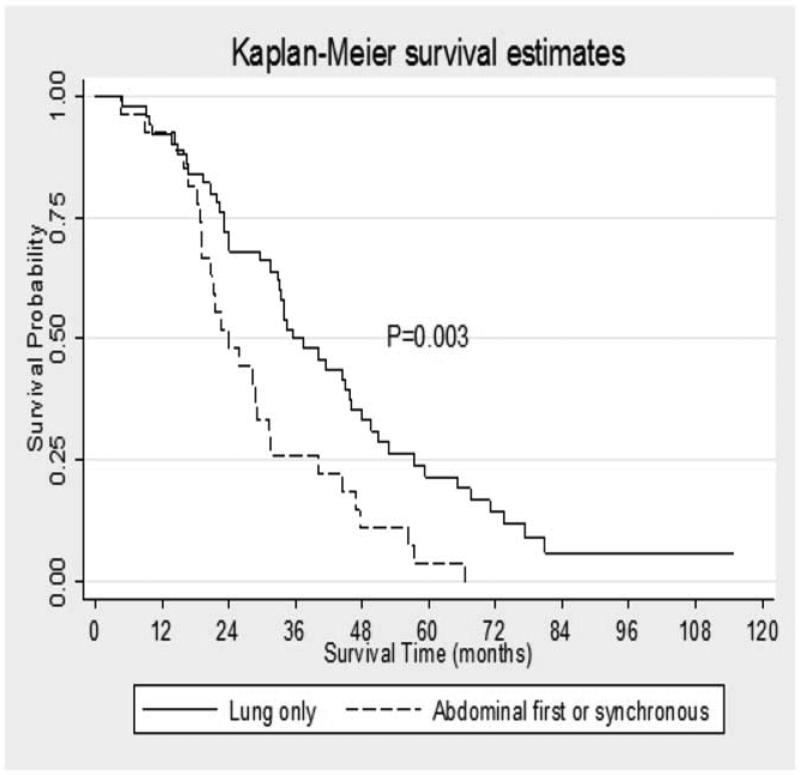
Of the 174 total patients, 96 (55.2%) patients did not undergo resection of their primary tumor. When evaluating only this subset of patients, those with lung as a first site of metastases compared to those with abdominal or synchronous metastatic disease had no difference in overall survival from the time of diagnosis (10.1 months versus 7.7 months, p=0.149). The remaining 78 (44.8 %) patients underwent resection of their primary tumor. Sixty-one (78%) patients underwent pancreaticoduodenectomy, 16 (20.5%) underwent distal pancreatectomy and 1 (1.2%) underwent total pancreatectomy. In these patients, those with lung first metastases experienced a significant survival benefit (Figure 2). The median survival from time of diagnosis for those patients with lung as a first recurrence after resection of their pancreatic primary was 35.6 months (95% C.I. 31.4-46.1) compared to 23.8 months (95% C.I. 18.97-31.2) for those with abdominal first or synchronous recurrence (p=0.003). This survival difference remained significant when controlling for age at diagnosis, gender, BMI, ASA, CCI, T stage of primary tumor, node positivity, tumor grade, treatment with neoadjuvant or adjuvant chemotherapy, and unilaterality of lung metastases. In this subset of patients who underwent resection of their primary pancreatic cancer, the time from surgical resection of their pancreatic primary to development of lung metastases was not different between groups (19.6 months for lung first and only, 22.6 months for lung first, 20.1 months for those with intra-abdominal metastases before lung metastases and 19.9 months for those who developed synchronous intra-abdominal and lung metastases).
Figure 2. Median survival in patients who underwent resection of primary PDAC.
Among those patients who underwent resection of their primary PDAC, the median survival of those with lung as a first recurrence was 35.6 months compared to 23.8 months among those with abdominal first or synchronous recurrence (p= 0.003).
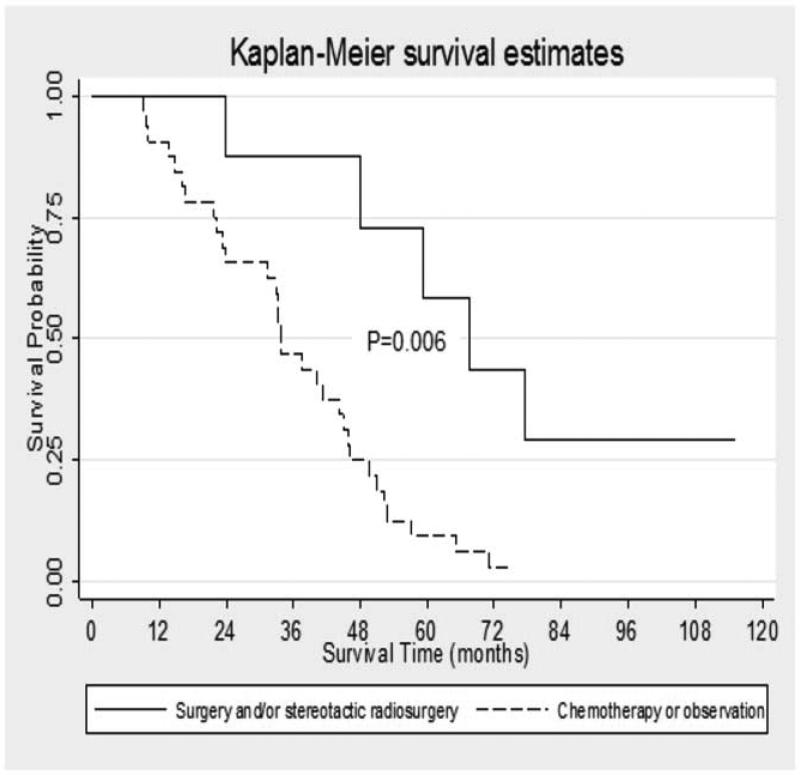
In the subset of patients who underwent resection of their primary pancreatic cancer and developed lung metastases as a first site of recurrence (n=49), median survival from the time of surgical resection of the primary PDAC was 33.3 months (35.6 months from time of diagnosis). Median metastases-free survival among this cohort was 14.9 months and nine of these patients (18.3%) were five-year survivors. Of the 40 patients in whom adjuvant treatment data was available, thirty-five (81.4%) underwent adjuvant therapy after resection of their primary pancreatic cancer. Twenty-seven patients (67.5%) underwent a median number of one regimen (range 0-6) of chemotherapy for treatment of their lung metastases. Of the 41 patients in whom full treatment data was available, five patients (12.2% of total) underwent surgical resection of lung metastases. (Table 2). Three of these patients had chemotherapy prior to resection. Four (9.6% of total) patients underwent stereotactic radiosurgery (one of whom also received surgical resection) of their lung metastases; one of these first received chemotherapy. Patients who had any treatment for metastatic disease (chemotherapy, surgery or stereotactic radiosurgery) had a trend toward improved survival compared to those who received no treatment (median survival 44.4 months versus 29.9 months from time of diagnosis, p-value 0.11; 43.2 versus 27.1 months from time of resection of primary, p-value 0.17, and 20.6 versus 11.5 months from time of diagnosis of lung metastases, p-value 0.17). When patients who underwent surgical resection of their lung metastases or stereotactic radiosurgery were combined (n=8, with one patient undergoing both treatments), their survival (67.5 months from time of diagnosis) was significantly better than those who underwent metastases-directed chemotherapy or observation (33.8 months, p-value 0.006) (Figure 3). When those treated with surgery or stereotactic radiosurgery (median survival 67.5 months) were compared to those undergoing metastases directed chemotherapy (median survival 33.8 months), there was a significant difference in survival (p-value 0.01). There was also a significant survival difference (p-value 0.008) amongst those treated surgically compared to those treated with observation alone (median survival 29.9 months). In the eight patients who underwent surgical resection or stereotactic radiosurgery for treatment of their lung metastases, median survival after this intervention was 27 months. Notably, those patients treated with metastases-directed chemotherapy did not demonstrate any survival benefit compared to those treated with observation alone when survival was evaluated from the time of diagnosis of PDAC or from resection of the primary lesion. There was a trend toward improved survival in those treated with chemotherapy versus observation when analyzed from the time of diagnosis of the first lung metastasis but this was not significant (18.9 months versus 11.5 months, p-value 0.69).
Table 2.
Patients with resected primary PDAC with lung first metastases (n=41 patients in whom full treatment data was available)
| Surgery or Stereotactic Radiosurgery | Metastases-directed chemotherapy | No treatment | |
|---|---|---|---|
| Number (% of all) | 8 (16.3%)* | 23 (46%) | 10 (20.4%) |
| Median survival (months) | 67.5 | 33.8 | 22.3 |
| Neoadjuvant Chemotherapy (% of group) | 5 (62.5%) | 5 (21.7%) | 4 (40%) |
| Adjuvant Chemotherapy (% of group) | 7 (87.5%) | 20 (87%) | 4 (40%) |
| Metastases directed chemotherapy (% of group) | 4 (50%) | 23 (100%) | 0 (0%) |
| 5-year survivors (% of group) | 4 (50%) | 2 (8.7%) | 0 |
One patient received both therapies.
Figure 3. Median survival in patients who underwent resection of their primary PDAC and developed lung metastases as a first site of recurrence by treatment strategy.
Among those patients who underwent resection of their primary PDAC and developed lung recurrence first, those who underwent surgery or stereotactic radiosurgery had a median survival of 67.5 months (95% C.I. 23.9-) compared to 33.8 months (95% C.I. 23.2-44.9) among those who were treated metastases directed chemotherapy or observation alone (p=0.006).
DISCUSSION
In this retrospective review of patients treated at a large university health system, we have identified those patients with pancreatic cancer metastatic to the lung and described the natural history of their disease. Patients with pulmonary metastases as a site of first metastasis from PDAC have prolonged survival compared to those with abdominal metastases first or synchronous lung and abdominal metastases and this survival benefit appears to be based upon both the survival of patients who underwent resection of their primary PDAC and those who underwent surgical treatment for lung first recurrence after primary PDAC resection. When only patients who underwent resection of their primary PDAC were evaluated, those with pulmonary metastases first survived significantly longer (35.6 vs. 23.8 months) than those with intra-abdominal or synchronous lung and abdominal recurrence. Among patients who underwent resection of their primary PDAC and developed lung metastases as a first site of disease recurrence, a subset underwent either surgical resection of their lung metastases or stereotactic radiosurgery (with or without chemotherapy). These patients had significantly prolonged survival (67.5 months) compared to patients treated with either chemotherapy alone (33.8 months) or observation (29.9 months). These findings support our hypothesis that metastatic pancreatic cancer is a heterogeneous disease and that patients with pulmonary metastases as a first site of PDAC metastasis represent a unique clinical and likely biologic phenotype of the disease compared to those with other patterns of lung metastases from PDAC.
The natural history of pulmonary metastases from pancreatic cancer is not well described in the literature. Studies have demonstrated that a higher proportion of 5-year survivors from pancreatic cancer have lung metastases compared to other sites of metastasis, a propensity for late recurrence to occur in the lung, and a prolonged time to metastases in those who develop lung metastases [8, 13-15]. A review of 378 patients who underwent potentially curative resection for pancreatic cancer examining patterns of recurrence found that lung as a first site of recurrence occurred in 9.8% of patients and that patients with isolated lung recurrence had the longest median overall survival compared to patients with other patterns of recurrence [22]. Our results have shown a similar pattern among patients who develop lung metastases as their first site of metastasis. We have also shown however, that those patients who develop lung metastases first represent a different clinical phenotype than patients who develop intra-abdominal metastases first or synchronously. Among all patients who develop lung metastases from pancreatic cancer, those who develop them first have prolonged survival compared to those with other disease patterns and this is especially profound in those patients who underwent resection of their primary PDAC.
There is a paucity of data regarding the treatment of isolated lung metastases from PDAC. In a retrospective case review of all patients who underwent lung resection for their metastatic pancreatic cancer over a 10 year period, 9 patients were identified and their median survival was 51 months compared to 23 months in matched controls [14]. The authors stress that these patients were highly selected and were required to have isolated and stable disease, a long interval between pancreatic resection and development of lung metastases, and a response to systemic chemotherapy suggestive of “good biology,” and stress the safety and feasibility of pulmonary metastasectomy for this group of patients. In a second evaluation of patients who underwent surgical resection for PDAC, 430 (61.4%) developed recurrent disease and 21 of these patients were selected for reoperation with curative intent [15]. Seven of these patients underwent lung resection and the initial disease free interval in these patients was significantly longer than those who were operated upon for liver or regional recurrence. Overall survival was also better (92.3 months compared to 32.5 months) compared to those who developed a liver recurrence. Notably, time to second recurrence was significantly longer in patients operated upon for lung metastases than those who underwent reoperation for liver or locoregional recurrence. Based on these data, the authors suggest that operations for recurrent pancreatic cancer appear to benefit only those with isolated pulmonary metastases. Our study evaluates a slightly different group of patients and includes all patients with any pulmonary metastases from pancreatic cancer. Forty-four percent of these patients underwent resection of their primary PDAC and of these, 62% developed recurrence in the lung first. Of these patients, only 10% (n=5) underwent surgical resection of their lung recurrence. We confirm the findings from the aforementioned studies: resection for patients with isolated pulmonary recurrence from PDAC is associated with improved survival. We also demonstrate its very limited clinical use even in a high-volume tertiary care center. Additionally, we demonstrate the benefit of stereotactic radiosurgery in these patients (n=4), perhaps suggestive of the highly selected nature of those patients chosen for intervention for recurrent PDAC in lungs. While we were able to demonstrate survival superiority compared to chemotherapy alone or watchful waiting, (the median survival after intervention for patients who underwent surgery or stereotactic radiosurgery for lung metastases was 27 months), the selection bias associated with those undergoing surgical intervention (non-disseminated lung metastases, increased likelihood of solitary or oligometastases) contributes to this group's improved survival. It is with caution, therefore, that we conclude that surgical resection is of benefit to all patients with first lung recurrence from pulmonary metastases and duly note the necessity to carefully choose these patients before proceeding with any metastasectomy.
A multitude of studies have attempted to identify molecular factors that contribute to the metastatic potential of pancreatic cancer. These have identified many factors such as variant expression of MAPK/ERK pathway, Kap-1 and MUC1, mutant p53 induced changes in cell signaling as well as MYC protein stabilization that may contribute to the metastatic potential of pancreatic cancer [16] [17, 18] [19]. Some attempts have been made to uncover how the heterogeneity of these drivers of pancreatic cancer may result in unique patterns of disease recurrence. Genomic studies have demonstrated significant heterogeneity in PDAC, but with individual mutations occurring with very low frequency [5-7]. More specifically, in a study using genomic sequencing techniques in human patients, those with Her-2 amplified cancer were noted to have a lack of liver metastases and increased frequency of lung and brain metastases [20]. In a murine orthotopic model of pancreatic cancer, it was demonstrated that overexpression of MUC1 with either the cytoplasmic tail or tandem repeat deleted resulted in more metastases to lung and lymph nodes compared to overexpression of full-length MUC1 [17]. Finally, a study examining patients who underwent pulmonary metastasectomy suggested that DPC4 loss was more common among patients with widespread metastatic disease than among those with isolated lung metastases [14, 21]. The clinical data we present here support a biologic difference in those patients with lung first or lung only metastases. Even among patients who all develop lung metastases, there appears to be at least two unique phenotypes: those who develop lung metastases as a first site of metastases and those who develop lung metastases at the same time or after intra-abdominal metastases. This clinical knowledge, combined with ongoing discoveries of the biologic heterogeneity of pancreatic cancer may provide a platform for more patient-specific therapy.
Despite the broad inclusion criteria in our study, there are several limitations. The percentage of patients who develop pulmonary metastases from PDAC remains unknown, as we were only able to capture those with lung metastases and not all patients with pancreatic cancer. Many of the patients in our cohort do not have a pathologic diagnosis of their pulmonary metastases, and while our radiologic criteria for identifying metastases were strict, there may still be misdiagnoses. Additionally, likely a reflection of the relative lack of data regarding management of isolated lung metastases from pancreatic cancer, few patients in our cohort underwent surgical treatment for their lung metastases. Only 8 patients were identified even when those patients who underwent surgical resection or stereotactic radiosurgery were combined. So while the differences in survival between these patients and those who underwent chemotherapy or observation after development of a lung recurrence are impressive, the number of patients is quite small and there is certainly a selection bias.
CONCLUSIONS
In conclusion we report here that patients who develop pulmonary metastases as a site of first recurrence from pancreatic cancer have improved survival compared to those who develop lung metastases as a second or synchronous site of recurrence. This difference is highlighted even further in the group of patients who undergo surgical resection of their primary PDAC. This clinical heterogeneity is suggestive of biologic variation in pancreatic cancer between individual patients and further studies are likely to elucidate molecular markers or perhaps even targets for treatment in different groups of patients. Among those patients with presumed favorable biology and isolated pulmonary metastases, surgical intervention or stereotactic radiosurgery may confer a survival benefit compared to adjuvant chemotherapy or observation of lung recurrence from PDAC.
SYNOPSIS.
Pulmonary metastases from pancreatic adenocarcinoma (PDAC) may represent a clinically and biologically unique phenotype of pancreatic cancer as patients with pulmonary metastases as a first site of metastasis have prolonged survival compared to those with intra-abdominal metastasis first. Among those patients with presumed favorable biology and isolated pulmonary metastases after resection of their primary PDAC, surgical intervention or stereotactic radiosurgery may confer a survival benefit compared to chemotherapy or observation of lung recurrence from PDAC.
ACKNOWLEDGEMENTS
Financial Support: SDC and BAB are supported by Grant Number T32CA113263 from the National Cancer Institute. PRV is supported by Grant Number 2T32GM008516-21 from the National Institute of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
ABBREVIATIONS LIST
- PDAC
Pancreatic ductal adenocarcinoma
Footnotes
Disclosures: None
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 3.Hede K. Superhighway or blind alley? The cancer genome atlas releases first results. Journal of the National Cancer Institute. 2008;100:1566–1569. doi: 10.1093/jnci/djn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Cancer Genome, C. Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, et al. International network of cancer genome projects. Nature. 2010;464:993–998. doi: 10.1038/nature08987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel N, Hudson TJ. The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nature reviews Gastroenterology & hepatology. 2012;9:77–87. doi: 10.1038/nrgastro.2011.215. [DOI] [PubMed] [Google Scholar]
- 6.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asiyanbola B, Gleisner A, Herman JM, Choti MA, Wolfgang CL, Swartz M, et al. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2009;13:752–759. doi: 10.1007/s11605-008-0762-x. [DOI] [PubMed] [Google Scholar]
- 9.Hattangadi JA, Hong TS, Yeap BY, Mamon HJ. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115:3640–3650. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2006;10:511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez JM, Morton CA, Al-Saadi S, Villadolid D, Cooper J, Bowers C, et al. The natural history of resected pancreatic cancer without adjuvant chemotherapy. The American surgeon. 2010;76:480–485. [PubMed] [Google Scholar]
- 12.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Annals of surgery. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Annals of surgical oncology. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnaoutakis GJ, Rangachari D, Laheru DA, Iacobuzio-Donahue CA, Hruban RH, Herman JM, et al. Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2011;15:1611–1617. doi: 10.1007/s11605-011-1605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas RM, Truty MJ, Nogueras-Gonzalez GM, Fleming JB, Vauthey JN, Pisters PW, et al. Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012;16:1696–1704. doi: 10.1007/s11605-012-1912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C, Zhan L, Jiang J, Pan Y, Zhang H, Li X, et al. KAP-1 is overexpressed and correlates with increased metastatic ability and tumorigenicity in pancreatic cancer. Medical oncology. 2014;31:25. doi: 10.1007/s12032-014-0025-5. [DOI] [PubMed] [Google Scholar]
- 17.Kohlgraf KG, Gawron AJ, Higashi M, Meza JL, Burdick MD, Kitajima S, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer research. 2003;63:5011–5020. [PubMed] [Google Scholar]
- 18.Weissmueller S, Manchado E, Saborowski M, Morris JPt, Wagenblast E, Davis CA, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell. 2014;157:382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ischenko I, Petrenko O, Hayman MJ. Analysis of the tumor-initiating and metastatic capacity of PDX1-positive cells from the adult pancreas. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3466–3471. doi: 10.1073/pnas.1319911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou A, Waddell N, Cowley MJ, Gill AJ, Chang DK, Patch AM, et al. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome medicine. 2013;5:78. doi: 10.1186/gm482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen KT,SS, Papavasiliou P, Arrangoiz R, Gaughan JP, Hoffman JP. Patterns of recurrence and outcomes in pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;(Suppl 4) [Google Scholar]