Abstract
Immune function abnormalities have been reported in patients with Fanconi anemia (FA), dyskeratosis congenita (DC) and, rarely, in Shwachman-Diamond syndrome (SDS) and Diamond-Blackfan anemia (DBA), but large systematic studies are lacking. We assessed immunological parameters in 118 patients with these syndromes and 202 unaffected relatives. We compared results in patients with reference values, and with values in relatives after adjusting for age, sex, corticosteroid-treatment and severe bone marrow failure (BMF). Adult patients (≥18 years) with FA had significantly lower immunoglobulins (IgG, IgA and IgM), total lymphocytes and CD4 T-cells than reference values or adult relatives (p<0.001); children with FA had normal values. Both children and adults with FA had lower B- and NK-cells (p<0.01) than relatives or reference values. Patients with DC had essentially normal immunoglobulins but lower total lymphocytes than reference values or relatives, and lower T-, B- and NK-cells; these changes were more marked in children than adults (p<0.01). Most patients with DBA and SDS had normal immunoglobulins and lymphocytes. Lymphoproliferative responses, serum cytokine levels, including TNF-α and IFN-γ, and cytokine levels in supernatants from phytohemagglutinin-stimulated cultures were similar across patient groups and relatives. Only patients with severe BMF, particularly those with FA and DC, had higher serum G-CSF and Flt3-ligand and lower RANTES levels compared with all other groups or relatives (p<0.05). Overall, immune function abnormalities were seen mainly in adult patients with FA, which likely reflects their disease-related progression, and in children with DC, which may be a feature of early-onset severe disease phenotype.
Keywords: Inherited bone marrow failure syndromes, immune deficiency, cytokines
Introduction
Inherited bone marrow failure syndromes (IBMFS) comprise a heterogeneous group of rare cancer-prone genetic disorders with hematologic and physical abnormalities. The four major IBMFS are Fanconi anemia (FA), dyskeratosis congenita (DC), Diamond-Blackfan anemia (DBA), and Shwachman-Diamond syndrome (SDS). Patients with IBMFS often develop bone marrow failure (BMF) with resultant single- or multi-lineage cytopenias. They also have increased risks of myelodysplastic syndrome (MDS), acute leukemia, and specific solid tumors 1. Subtle immunologic abnormalities have been reported in each of these syndromes, without correlation with disease status 2–5.
FA is the most frequently studied syndrome. Earlier reports of immune function in FA included small numbers of cases, measured only a few parameters, and/or did not address differences in immune function in relation to patient ages or cancer history 6–12. The number of recent comprehensive immunological studies is small and findings inconsistent 13–16. The most frequent immune abnormalities reported are low B and NK cell numbers or decreased NK cell activity 4,15,16. Myers et al. found normal immunoglobulin IgG in children with FA 16, while Kortoff et al. studied patients with FA with severe BMF and reported low serum IgG and IgM, and high serum interleukin (IL)-6 and transforming growth factor (TGF)-β, and low soluble CD40 ligand 15. Justo et al. described elevated plasma levels of IL-10, tumor necrosis factor (TNF)-α and interferon (IFN)-γ, but normal TGF-β in a subset of patients with FA 13. Dufour et al. studied bone marrow mononuclear cells from patients with FA and reported increased expression of inflammatory cytokines TNF-α and IFN-γ 8. Matsui et al. observed increased sensitivity of bone marrow monocytes from FA and other IBMFS patients to lower dose (0.001 μg/mL) lipopolysaccharide stimulation than Dufour et al., resulting in a relative increase in TNF-α, IL-6 and IL-1β levels only at low dose 17.
Immune abnormalities reported in patients with DC include lymphopenia, variable levels of immunoglobulins, reduced T, B or NK cells, and diminished or absent responses to phytohemagglutinin 2,18–21. Patients with the Hoyeraal-Hreidarsson (HH) variant of DC may present with immunodeficiency, in addition to cerebellar hypoplasia, microcephaly, intrauterine growth retardation, and early onset aplastic anemia, as well as extremely short telomeres 22,23. Studies of immune function in DBA and SDS are limited to small numbers of patients and suggest hypogammaglobulinemia, lymphopenia and decreases in specific lymphocyte subsets in nearly half of the patients tested 3,5,24.
The pathophysiology of immune abnormalities in patients with IBMFS is unclear, since the germline mutations involve diverse pathways: DNA repair in FA, telomere biology in DC, and ribosome biogenesis in DBA, SDS and DC. Several mechanisms may be involved in immunodeficiency in IBMFS, such as defective hematopoietic cellular development as an integral component of the genetic syndrome, or increased apoptosis and/or immune dysregulation as a cause or consequence of an altered cytokine milieu related to inflammation and oxidative stress. Inflammation and oxidative stress have been associated with an increased risk of cancer in the general population 25,26 and have been proposed to be important mechanisms in the pathogenesis of BMF in FA 27.
The aim of this study was to comprehensively examine qualitative and quantitative immunologic findings in a cohort of well-characterized IBMFS patients of all ages, and compare those with each other and with age-matched unaffected relatives. We determined: 1) serum immunoglobulin levels and lymphocyte phenotypes, 2) serum cytokine profiles, and 3) lymphoproliferative responses and cytokine secretion by peripheral blood mononuclear cells (PBMCs) among case and control groups. We evaluated associations between abnormalities in immune parameters and age, and with adverse events such as severe BMF or prevalent cancers. We present raw data, and summary data for groups after adjustment for variables known to influence immune responses, including age, sex, corticosteroid-treatment, and severe BMF.
Methods
Subjects
The participants were untransplanted patients with FA, DC, DBA, and SDS and their unaffected first degree relatives, enrolled in the National Cancer Institute’s Institutional Review Board approved protocol [NCI Protocol 02-C-0052; NCT00027274] 28. All participants or their guardians provided written informed consent in accordance with Health and Human Services regulation 45 CFR 46.
Each IBMFS was diagnosed according to standard criteria 1 and confirmed by syndrome-specific tests as described previously 28. All unaffected relatives were proven to be negative for an IBMFS by these tests. Normal blood count values were based on standard reference ranges for age and gender 29. BMF was defined according to clinical guidelines for the management of FA 30; severe: hemoglobin <8 g/dL, absolute neutrophil count <0.5 × 109/L, platelets <30 × 109/L, or on treatment; moderate: values below normal for age but not severe; or none: normal values for age.
Sample collection and processing
All blood samples were fasting and drawn in the morning. All patients and relatives were free of current or recent infection. Complete blood counts, serum immunoglobulins (IgG, IgA, IgM) and lymphocyte phenotyping (T, B and NK cells) were part of routine clinical testing. Abnormal immunoglobulin and lymphocyte subset values were below the 10th percentile or above the 90th percentile of age-established reference ranges (http://cclnprod.cc.nih.gov/dlm/testguide.nsf). Sera and PBMCs were obtained and processed according to standardized procedures of the IBMFS cohort study 28 and frozen (−80°C and liquid nitrogen, respectively) until testing.
Lymphoproliferation assays
Thawed PBMCs were cultured in triplicate (2×105 cells per well) in 96-well plates with or without stimuli as previously described 31,32. Phytohemagglutinin (PHA, T-cell mitogen) or pokeweed (PWM, B-cell mitogen), were used for polyclonal stimulation. Influenza A virus (Flu), and tetanus toxoid (Tet) were used as recall antigens, and HPV-16 L1 virus-like particles (VLP) were used for antigen-specific T cell responses. Cell-free supernatants were frozen (−80°C) to determine cytokines induced by stimulation. Cell cultures were pulsed with 1 μCi of 3H-thymidine (Perkin-Elmer, Wellesley, MA) for 18 hours, harvested and counted in an automated scintillation counter (Microbeta, Perkin-Elmer) to determine proliferation rates.
Cytokine determinations
Thawed serum samples and cell-free supernatants from PBMC cultures were tested in duplicate for 20 different cytokines and chemokines: IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-17, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), IFN-γ, G-CSF, interferon gamma-induced protein 10 (IP-10), Eotaxin, Flt3-ligand (Flt3L) and RANTES. The cytokine levels were determined by enzyme linked immunosorbent assays using Luminex-based Multiplex Human Cytokine/Chemokine Immunoassay kits by Millipore according to manufacturer’s guidelines. Data acquisition and analysis were performed using Bioplex software version 6 (Bio-Rad) as previously described 33. Net cytokine levels were obtained for supernatants by subtracting values in unstimulated cultures from values in PHA-stimulated cultures.
Statistical analyses
Data from children (age <18 years) and adults (age ≥18 years) were analyzed separately. To test for differences in demographic and clinical characteristics between patient groups and a pooled group of unaffected relatives, we used Kruskal-Wallis or Wilcoxon rank-sum tests for continuous variables, and Fisher’s exact test for binary variables, utilizing Stata 12 (StataCorp, College Station, TX). Adjustment for age, sex, corticosteroid treatment and severe BMF was done by multivariable regression analyses using ANOVA models [SAS Version 8.02 (SAS Institute Inc., Cary, NC)]. Data were log-transformed. Adjustment for cancer did not alter the findings; thus we do not present cancer-adjusted data. P value <0.05 was significant.
Results
Characteristics of participants
Features and disease status of the participants and treatments at the time of the study are summarized in Table I and in online supplementary Table Ia (children) and Ib (adults). The ages of patients with the four syndromes were similar. The ages among relatives of FA, DC and SDS patients were also similar, while relatives of DBA patients were younger (p=0.02). Overall, relatives were significantly older than patients (p=0.0001) because they included parents as well as siblings. There were relatively more females among patients with FA (71%; p=0.03) and relatively more males among patients with DC (79%; p=0.0003). The sex ratios among relatives were essentially equal.
Table I.
Characteristics of Participants.
| Participants | Patients (N=118) | Relatives (N=202) | p# | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA N (%) |
DC N (%) |
DBA N (%) |
SDS N (%) |
Total N (%) |
p* | FA | DC | DBA | SDS | Total | p* | ||
| Number | 31 | 42 | 33 | 12 | 118 | 74 | 79 | 37 | 12 | 202 | |||
| Age, median range, yrs. | 170.4-57 | 171.5-71 | 112-58 | 122-42 | 15.40.4-71 | 0.2* | 424-77 | 364-69 | 342-62 | 4510-68 | 39.52-77 | 0.02* | 0.0001 |
| Male:Female | 9:22 | 33:9 | 21:12 | 7:5 | 70:48 | 35:39 | 31:48 | 18:19 | 6:6 | 90:112 | |||
| P for M:F@ | 0.03 | <0.001 | 0.2 | 0.8 | <0.001 | 0.7 | 0.07 | 1 | 1 | 0.7 | |||
| Any BMF$ | 16 (52) | 32 (76) | 22 (67) | 4 (33) | 74 (63) | 0.04 | 0 | 0 | 0 | 0 | 0 | ||
| Severe BMF | 6 (19) | 20 (48) | 22 (67) | 0 | 48 (41) | <0.001 | 0 | 0 | 0 | 0 | 0 | ||
| Cancer | 6 (19) | 3 (7) | 1 (3) | 0 | 10 (8) | 0.06 | 1 | 1 | |||||
| Prednisone Rx | 0 | 0 | 12 (36) | 0 | 12 (10) | <0.001 | 0 | 0 | 0 | 0 | 0 | ||
| Androgen Rx | 2 (6) | 5 (12) | 0 | 0 | 7 (6) | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Transfusions | 3 (10) | 12 (29) | 10 (30) | 0 | 25 (21) | 0.2 | 0 | 0 | 0 | 0 | 0 | ||
| Any treatment | 4 (13) | 14 (33) | 20 (61) | 0 | 28 (24) | <0.001 | 0 | 0 | 0 | 0 | 0 | ||
FA, Fanconi anemia; DC, dyskeratosis congenita; DBA, Diamond-Blackfan anemia; SDS, Shwachman-Diamond syndrome; N, number; yrs., years; Rx, treatment.
Equality across the four syndromes (patient groups and relatives separately using Kruskal-Wallis analysis of variance);
Compares patient groups with combined relatives (Kruskal-Wallis).
Ratio of males to females (binomial distribution).
BMF: Any BMF: hemoglobin, neutrophil or platelet values below the normal for age; severe BMF: hemoglobin less than 8 g/dL, absolute neutrophil count less than 0.5×109/L, or platelets less than 30×109/L, or on treatment.
Children with DC and DBA were more likely to have any BMF or severe BMF than children with FA or SDS (p≤0.001; Supplementary Table Ia). The frequency of BMF among adults was similar across the syndromes (Supplementary Table Ib). Twelve children and one adult with DC were classified as HH variant. Two patients with FA and five with DC were on androgens, and 12 with DBA were on prednisone at the time of study. Six of 14 adults with FA (43%) had prevalent cancers: four had head and neck squamous cell carcinoma (HNSCC) and two had metastatic vulvar cancer. Two adults with DC had a history of resected HNSCC two and seven years prior to the study, and a third was in remission one year after treatment for acute myeloid leukemia (AML). One adult with DBA had completed treatment for lung SCC one year prior to study. Mutated genes were identified in 28/31 patients with FA, 39/42 patients with DC, 26/33 patients with DBA, and 11/12 patients with SDS (Supplementary Table I a-b).
Serum immunoglobulins
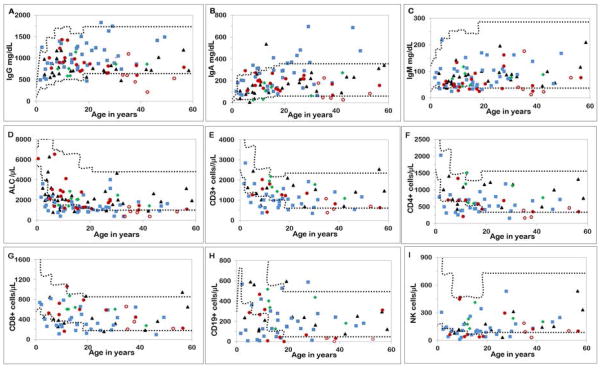
Values of immunoglobulins, total lymphocytes and lymphocyte subsets for patients with the four syndromes along with age-established normal ranges are shown in Figure 1; the adjusted mean values and confidence intervals for patient groups and relatives (after adjustment for age, sex, corticosteroid-treatment and severe BMF) are included in Figure 2.
Figure 1. Serum immunoglobulin and lymphocyte subsets in patients.
Fanconi anemia (FA), red circles (open circles are patients with cancer); dyskeratosis congenita (DC), blue squares (open squares are patients with Hoyerral-Hreidarsson syndrome); Diamond-Blackfan anemia (DBA), black triangles; Shwachman-Diamond syndrome (SDS), green diamonds.
Upper and lower dotted lines represent the 90th and the 10th percentile respectively, of the normal ranges for age as established by the National Institutes of Health Clinical Center Department of Laboratory Medicine (http://cclnprod.cc.nih.gov/dlm/testguide.nsf).
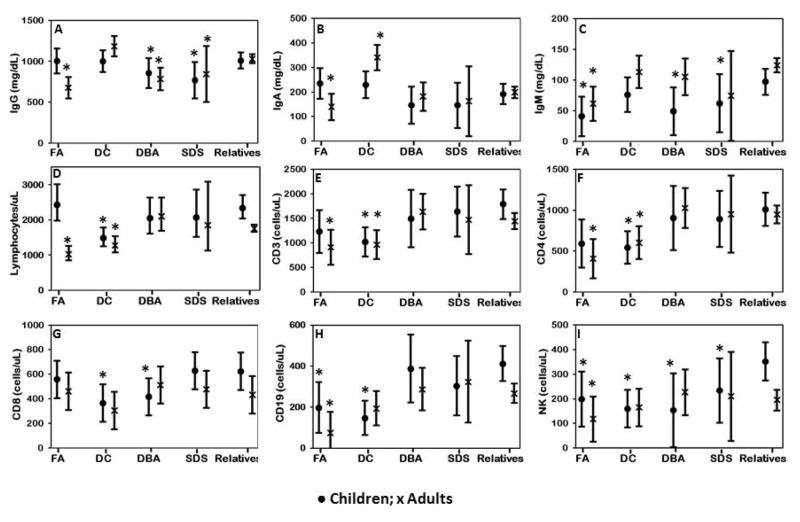
Figure 2. Comparison of serum immunoglobulins and lymphocyte subsets in patients with relatives.
● Children; x Adults. Adjusted mean (adjusted for age, sex, treatment with corticosteroids, and severe bone marrow failure) and 95% confidence intervals are shown. Group comparisons are between children (<18 year old) with each syndrome and combined pediatric relatives, and between adult patients (≥18 years) with syndromes and combined adult relatives. *Significant (p<0.05). A) IgG levels were low in adults with FA (p<0.0001) and children and adults with DBA and SDS (p<0.001) compared with patients with other syndromes and relatives within the same age range. B) IgA levels were lower in adults with FA and higher in adults with DC (p<0.0001). C) All children had lower IgM than all adults (p=0.03). Children with FA, DBA and SDS had low IgM, as did adults with FA compared with relatives within the same age groups (p=0.0001). D) Absolute lymphocyte counts (ALCs) were low in adults with FA and in children and adults with DC (p<0.001). E and F) Adults with FA and children and adults with DC had low CD3 and CD4+/CD3+ T cells (p<0.001). G) Children with DC and DBA had low CD8+/CD3+ T cells (p=0.005). H) Children and adults with FA and children with DC had low CD19+ B cells (p<0.001). I) Children with all four syndromes as well as adults with FA had low NK cells (p=0.004).
Among 26 patients with FA, six of 14 adults had low IgG (five with low IgG had cancer), three adults had low IgA, and six adults and three children had low IgM (Figure 1A–C). Eleven patients with FA had at least one low immunoglobulin value, while all immunoglobulins were below the normal range in three of six adults with cancer. Among 36 patients with DC, IgG was low in only one and high in two; IgA was high in five children and eight adults, and IgM was low in three children and one adult. Two of 27 patients with DBA had low IgG; one had low IgA; IgM was normal in all. All seven patients with SDS had normal IgG and IgM; two had low IgA (Figure 1A–C).
After adjustment for variables, adult patients with FA as a group had significantly lower IgG, IgA and IgM than all adult relatives (p≤0.0001; Figure 2A–C), while children with FA had only lower IgM (p=0.001) than all pediatric relatives (Figure 2A–C). Among DC, both children and adult patients had similar IgG and IgM levels as corresponding groups of relatives. Adult patients with DC had higher IgA than all adult relatives or adult patients with other syndromes (p<0.0001; Figure 2A–C). Immunoglobulin values were within age-established normal ranges in most patients with DBA and SDS (Figure 1A–C); however, both children and adult patients with DBA and SDS as groups had lower IgG than corresponding groups of relatives (Figure 2B; p<0.001). Children with DBA and SDS also had lower IgM than all pediatric relatives (p=0.001; Figure 2C).
Total lymphocyte counts
Absolute lymphocyte counts (ALC) were below normal in 10 of 29 patients with FA, 22 of 42 with DC, eight of 32 with DBA, and one of eight with SDS (Figure 1D). After adjustment, adult patients with FA as a group, and children and adults with DC had lower ALC than corresponding groups of relatives (p<0.001) while patients with DBA and SDS had similar ALC as relatives (Figure 2D).
Lymphocyte subsets
Eleven of 14 tested patients with FA had lower than normal T, B or NK cells: four had low CD3 T cells; three of these had low CD4 and one low CD8 T cell subsets; eight had low CD19 B cells, and six had low NK cells (CD16/CD56) (Figure 1 E–I). All subsets were reduced in two patients with FA with prevalent cancers. Twenty out of 30 patients with DC had below normal values of one or more lymphocyte subsets: 12 had low CD3 T cells, 12 low CD4 and 11 low CD8 subsets, 10 had low B cells, and 10 low NK cells (Figure 1 E–I). All subsets were reduced in four of these patients, (three were children with the HH variant and one was an adult with persistent pancytopenia one-year post-treatment for AML). Seven of 14 patients with DBA had below normal values for at least one lymphocyte subset: two had low CD3 T cells, two had low CD4 and four low CD8 subsets, four had low B cells, and three had low NK cells. Six of these patients with low subsets were children on treatment (three on prednisone and three on red blood cell transfusions) and one was an adult with no BMF. Lymphocyte subsets were normal in patients with SDS.
In the adjusted analysis, adult patients with FA as a group had lower CD3 T cells and CD4 subsets than relatives or adult patients with DBA and SDS (p<0.001); children with FA had values similar to pediatric relatives (Figure 2 E–G). Children as well as adults with FA had lower B and NK cells than corresponding groups of relatives (p<0.001; Figure 2 E–F). Patients with DC (children and adults) had lower CD3 T cells, CD4 subsets and B cells compared with relatives, while children with DC also had lower CD8 subsets and NK cells (p≤0.005 for all). Lymphocyte subsets in patients with DBA and SDS were in the same range as relatives except for CD8 T cell subsets which were lower in children with DBA and NK cells which were lower in children with DBA and SDS. Taken together, children with all four syndromes had lower NK cells than all pediatric relatives, but adults with FA had the lowest NK cells compared with all other groups (p=0.004; Figure 2I).
Overall, adult patients with FA had low immunoglobulins, total lymphocytes and lymphocyte subsets, while children had mostly normal values. Immunoglobulins were primarily within established normal ranges in patients with DC, while ALC and lymphocyte subsets were low, and these values were lower in children than in adults with DC, particularly in very young children with HH phenotype (Figure 1). Six of the seven patients with DBA with low lymphocyte subsets were children on prednisone or red blood cell transfusions. The patterns of immunoglobulins and lymphocyte abnormalities were generally similar in unadjusted and adjusted analyses. We did not see significant effects of BMF or cancer on immunoglobulins or lymphocytes.
Serum cytokines
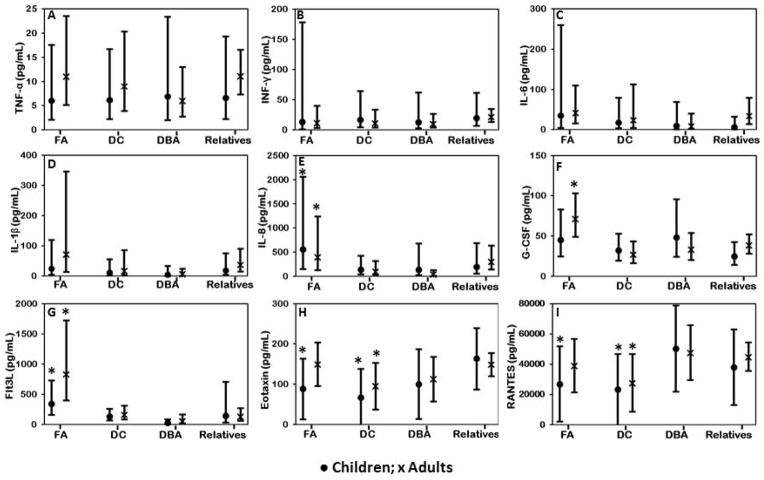
Sera from 25 patients with FA, 32 with DC, 22 with DBA and 78 relatives were tested to determine circulating proinflammatory cytokine levels. IL-4 was not detected in any sample; IL-1α, IL-7 and IL-12p70 were detected at very low levels and in less than 25% of samples and were not analyzed further. The raw values and adjusted means of IL-10, IL-17, GM-CSF, MCP-1 and MIP-1α were similar across all syndromes and relatives (data not shown). Unlike previous reports, we did not find significant abnormalities in serum levels of TNF-α, IFN-γ or IL-6 in any of the patient groups compared with relatives in both adjusted (Figure 3 A–C) and unadjusted analyses (Supplementary table S1a-b) 9,11,13. We did find higher levels of IL-8, G-CSF and Flt3L in adult patients with FA compared with adult relatives (p≤0.006) and higher levels of IL-8 and Flt3L in children with FA than in all pediatric relatives (p≤0.004) (Figure 3 D–I; Supplementary Table II a-b). Children with FA and adults and children with DC had lower eotaxin and RANTES levels than corresponding groups of relatives (p≤0.03 for all; Figure 3 H–I).
Figure 3. Comparison of serum cytokine levels in patients with relatives.
● Children; x Adults. Adjusted mean and 95% confidence intervals are shown. Group comparisons are between children (<18 year old) with each syndrome and pediatric relatives, and between adult patients (≥18 years) with syndromes and adult relatives. A, B, C, D) Serum TNF-α, IFN-γ, IL-6 and IL-1β were similar in patients and relatives. E) Children and adults with FA had high IL-8 than relatives or patients with other syndromes within the same age groups (p=0.004). F) Adults with FA had high G-CSF (p=0.006). G) Children and adults with FA had high Flt3L (p<0.0001). H) Children and adults with DC had low eotaxin (p=0.03). Children with FA, and children and adults with DC had low RANTES (p=0.02).
We further examined the effects of BMF and cancer on the levels of cytokines. Patients with FA and DC with severe BMF had higher G-CSF and Flt3L and lower RANTES levels compared with patients with moderate or no BMF or relatives (p≤0.05; Supplementary Figure 1). Because RANTES is mainly secreted by platelets 34, as expected, we found that lower RANTES levels correlated with lower platelet counts (p<0.001; R2 for combined FA, DC and DBA=0.47; Supplementary Figure 2a) (R2 for FA=0.36, DC=0.4, DBA=0.3; Supplementary Figure 2b). Patients with DC with severe BMF also had higher levels of IP-10 (p=0.01; Supplementary Figure 3). There were no significant effects of corticosteroid treatment (DBA) or prevalent cancers (FA) on the levels of cytokines (data not shown).
Lymphoproliferative responses
Lymphoproliferative responses to polyclonal stimuli (PHA and PWM) and standard recall antigens (Flu, Tet and VLP) were tested in 22 patients with FA, 21 with DC, 20 with DBA, 10 with SDS, and 58 relatives. In the analysis of raw data, children with all four syndromes showed lower PHA responses than pediatric relatives (p≤0.0002 for all; Supplementary Table IIIa), however, there were no differences in lymphoproliferative responses in the adjusted analyses (Supplementary Figure 4). All adult patient groups had similar lymphoproliferative responses as adult relatives in both unadjusted and adjusted analyses (Supplementary table IIIb and Supplementary Figure 4, respectively).
Cytokine levels in supernatants from PHA-stimulated cultures
Cytokine levels in cell-free supernatants from PHA-stimulated cultures appeared to be lower in pediatric patient groups than in relatives in the unadjusted analyses (Supplementary table IV a-b); however, there were no differences in responses among patient groups and relatives for both children and adults in the adjusted analyses (data not shown).
Discussion
The immune status of patients with an IBMFS is not entirely clear, due to patient heterogeneity, small sample size, and the wide variety of assays that have been used in different reports. We examined the immune function profile of children and adults with the four most common rare IBMFS, and compared findings in children and adults with each other, and with unaffected relatives of comparable ages. We describe results from parameters typically measured in the common clinical laboratory studies, as well as functional assays which are less frequently investigated due to sample and technical requirements. We present both raw data for individuals and summary data for groups after adjustment for variables known to influence immune responses. We observed abnormalities in multiple immune parameters most frequently in FA and DC, while patients with DBA or SDS had relatively normal immune markers.
Fanconi Anemia
Prior publications suggested that immunoglobulins were decreased 15 or normal 10,16 in patients with FA, but did not separate children and adults or adjust for other variables. In our study, most children with FA had normal levels of immunoglobulins, while half of the adults had decreased levels. Our data included patients with cancer, and although patients with cancer had low levels, many adults without cancer also had low values. Thus, decreased immunoglobulins appear to be a feature of adults with FA, both individually, and as a group after adjustment.
Studies by others have reported normal or decreased numbers of T cells in FA, while B cells were generally decreased 7,15,16. We found below normal numbers of absolute lymphocyte counts in one-third of children and adults with FA, but the adults as a group had lower ALC than normal relatives or children with FA. Similarly, adults with FA had lower numbers of CD3 T cells and CD4 subsets, and lower B and NK cells than relatives or children with FA, while children had lower B and NK cells than relatives. Our data suggest that T cell deficiencies develop later in life in FA while B and NK cell deficiencies may be present earlier. Since B-cells originate from early hematopoietic progenitors, stem cell deficits present early in life may contribute to low B-cell numbers in young patients 35. Furthermore, impaired terminal differentiation of B-cells into IgM-secreting cells may cause IgM deficiency 35 as was seen in 35% of our patients.
Published findings on cytokines in FA are inconsistent. Dufour et al. reported overexpression by marrow mononuclear cells of TNF-α and IFN-γ, negative modulators of hematopoiesis 8, while our previous studies of bone marrow cells did not corroborate these findings 17. There are also reports of increased plasma or serum levels of TNF-α and IFN-γ, although usually in less than half of the patients 9,13. We found no increase in TNF-α or IFN-γ in sera or supernatants from lymphocyte cultures in children or adults with FA. We did find abnormalities in some serum cytokine levels in patients with severe BMF. Patients with severe BMF had higher serum Flt3L and G-CSF and lower RANTES levels than those with no BMF. Flt3L is an early hematopoietic cytokine, the release of which may be triggered by stem cell deficiency, and high levels have previously been reported in FA 36. Likewise, G-CSF is involved in the regulation of hematopoiesis and elevated levels are seen in the setting of BMF in severe acquired aplastic anemia 37. RANTES is mainly platelet-derived, and as expected, decreased levels were seen in patients with low platelet counts 34. Overall, these results and those from our prior studies of bone marrow 17 do not demonstrate generalized cytokine dysregulation in patients with FA.
Our results indicate that FA is a syndrome without consistent and substantial immunological abnormalities. The types of immunodeficiency vary among patients, and are not all present in any single individual, and when present are more common in adults than in children. In contrast with Korthof et al 15, our findings were adjusted for the degree of BMF, and thus represent “all” FA, not just those with severe marrow failure. Further larger and longitudinal studies may determine whether more pronounced immunodeficiency seen in adults with FA is a harbinger of future malignancies.
Dyskeratosis congenita
Serum immunoglobulins were generally normal in patients with DC, although adults tended to have increased IgA levels. High IgA levels were reported previously in DC, but the reason for this is unclear 20. The majority of the children’s lymphocyte and subset numbers were below the normal range, while many of the adults’ values were normal, albeit at the low end (Figure 1). As a group, children and adults had lower lymphocytes and subsets than relatives (Figure 2). Unlike FA, immune alterations in DC were more marked in children than in adults, and most of those were very young children with HH subtype, as previously reported22.. DC has a highly variable and heterogeneous phenotype with most severe disease presentations seen in infancy and childhood whereas some adults may have milder or no abnormalities at all 38. We had previously shown the association of telomere length with disease severity in DC 23. In the present study, children with DC had significantly lower lymphocyte telomere length Z-scores than adults with DC (p<0.0001; data not shown) consistent with their early-onset severe disease phenotype. Children with DC also had more noticeable immunodeficiency than their adult counterparts. Thus, our results indicate that patients with DC with immunodeficiency are more likely to be the severely affected young children, as has also been reported previously19,20,22; many of whom are in the HH category; these patients have extremely short telomeres23. Similar to FA, we did not detect alteration in serum cytokines in DC except in association with severe BMF: low RANTES levels correlated with low platelet counts, while high serum G-CSF, Flt3L and IP-10 levels were seen in patients with severe BMF. Likewise, lymphoproliferative responses were similar in patients and unaffected relatives suggesting that most patients with DC would be able to mount adequate immune responses to vaccines.
Diamond-Blackfan anemia
There are no reported comprehensive studies of immune function in patients with DBA. In our study, the first of this nature, we found that patients with DBA had slightly lower levels of IgG than relatives, but within the established normal range. Adjusted lymphocyte counts, subset numbers, and serum cytokine levels were comparable to relatives. Although half of the children with DBA were on prednisone, their lymphocyte functions were normal after adjustment. Thus, our DBA cohort did not demonstrate significant immunodeficiency.
Shwachman-Diamond syndrome
More than half of the two dozen patients with SDS who had immune functions reported by others had normal results3,24. We found immunoglobulins and lymphocyte cell numbers to be within the normal range, although adjusted IgG was below the level in relatives. We did not have sufficient sera to examine cytokines or PBMC to evaluate lymphoproliferative responses. Our limited study suggests that immune function is not substantially altered in SDS.
All syndromes
Strengths of our study are that we investigated an extensive list of immune markers in more than 30 patients each with FA, DC, and DBA, and 12 with SDS. We examined immune parameters noted to be altered by others, as well as additional markers not previously reported. We scrutinized the results in children separately from adults, compared patients with unaffected relatives, and we report both crude individual and adjusted group data. We also compared data across the syndromes. These studies were conducted in parallel using the same procedures and assays. Limitations include relatively small numbers of individuals within each syndrome; not all studies could be performed in all patients due to insufficient material. We did not have sufficient numbers of patients to permit genotype/immune function associations. The adults with FA included six with prevalent cancer, but no active treatment except surgery. The children with DC included 12 with the severe HH phenotype, in which immunodeficiency has been previously described 22. Our study was biased by the fact that those with severe disease present in childhood. It is also possible that we lacked samples on severely affected patients due to non-enrolment of many such patients (needing active treatment) on our study. This may explain some of the inconsistencies between our results and published reports, particularly in patients with DBA or SDS 3,5,24.
Despite these limitations, we conclude that immune abnormalities are a feature of adults with FA more than children, that children with DC have more severe cellular immunodeficiency than adults, and that DBA and SDS are mostly unaltered in these parameters. Our studies were not designed to identify immune mechanisms responsible for the development of BMF in these syndromes. It can be noted, however, that most of the patients with FA were not on any treatment, while half of the children with DC and DBA were on androgens, prednisone or transfusions. These differences were taken into account during adjustment of data for severe BMF.
In summary, some patients with FA do have abnormal immune functions, and this occurs more frequently in adults than in children. The opposite occurs in DC, perhaps reflecting a more severe disease phenotype early in life. No marked alterations were seen in DBA and SDS. Longitudinal studies will help to elucidate the evolution of immune alterations over time during development, and the potential role of those changes in disease prognosis and survival.
Supplementary Material
Acknowledgments
The study was supported by a research grant from Fanconi Anemia Research Fund (Fanconi.org) to N.G. This research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (B.P.A., N.G., S.A.S), and by contract HHSN261201100018C with Westat. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E (L.A.P). The authors would like to thank all the families who participate in the National Cancer Institute inherited bone marrow failure syndromes (IBMFS) cohort, the physicians who referred the patients, and the personnel in the Department of Laboratory Medicine at the NIH Clinical Center for performing clinical laboratory tests. We thank Lisa Leathwood, RN; Ann Carr, MS, CGC; Maureen Risch, RN and the other members of the IBMFS team at Westat, Inc. for their extensive efforts.
Footnotes
Authorship and Disclosures
NG, BPA and LAP designed the study and wrote the manuscript. KP, YP, MW, TJK and LAP performed the experiments on sera cytokine and lymphoproliferative studies. RTK, NG and YP performed data analyses. NG, BPA and SAS provided clinical characterization and diagnostic support to patients. All authors revised and approve the manuscript. The authors report no conflict of interest.
clinicaltrials.gov identifier: NCT00027274
Reference List
- 1.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24:101–122. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 3.Dror Y, Ginzberg H, Dalal I, Cherepanov V, Downey G, Durie P, Roifman CM, Freedman MH. Immune function in patients with Shwachman-Diamond syndrome. Br J Haematol. 2001;114:712–717. doi: 10.1046/j.1365-2141.2001.02996.x. [DOI] [PubMed] [Google Scholar]
- 4.Fagerlie SR, Bagby GC. Immune defects in Fanconi anemia. Crit Rev Immunol. 2006;26:81–96. doi: 10.1615/critrevimmunol.v26.i1.40. [DOI] [PubMed] [Google Scholar]
- 5.Khan S, Pereira J, Darbyshire PJ, Holding S, Dore PC, Sewell WA, Huissoon A. Do ribosomopathies explain some cases of common variable immunodeficiency? Clin Exp Immunol. 2011;163:96–103. doi: 10.1111/j.1365-2249.2010.04280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnara GP, Bonsi L, Strippoli P, Ramenghi U, Timeus F, Bonifazi F, Bonafe M, Tonelli R, Bubola G, Brizzi MF. Production of interleukin 6, leukemia inhibitory factor and granulocyte-macrophage colony stimulating factor by peripheral blood mononuclear cells in Fanconi’s anemia. Stem Cells. 1993;11(Suppl 2):137–143. doi: 10.1002/stem.5530110822. [DOI] [PubMed] [Google Scholar]
- 7.Castello G, Gallo C, Napolitano M, Ascierto PA. Immunological phenotype analysis of patients with Fanconi’s anaemia and their family members. Acta Haematol. 1998;100:39–43. doi: 10.1159/000040861. [DOI] [PubMed] [Google Scholar]
- 8.Dufour C, Corcione A, Svahn J, Haupt R, Poggi V, Beka’ssy AN, Scime R, Pistorio A, Pistoia V. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 2003;102:2053–2059. doi: 10.1182/blood-2003-01-0114. [DOI] [PubMed] [Google Scholar]
- 9.Rosselli F, Sanceau J, Gluckman E, Wietzerbin J, Moustacchi E. Abnormal lymphokine production: a novel feature of the genetic disease Fanconi anemia. II. In vitro and in vivo spontaneous overproduction of tumor necrosis factor alpha. Blood. 1994;83:1216–1225. [PubMed] [Google Scholar]
- 10.Roxo P, Jr, Arruda LK, Nagao AT, Carneiro-Sampaio MM, Ferriani VP. Allergic and immunologic parameters in patients with Fanconi’s anemia. Int Arch Allergy Immunol. 2001;125:349–355. doi: 10.1159/000053837. [DOI] [PubMed] [Google Scholar]
- 11.Schultz JC, Shahidi NT. Tumor necrosis factor-alpha overproduction in Fanconi’s anemia. Am J Hematol. 1993;42:196–201. doi: 10.1002/ajh.2830420211. [DOI] [PubMed] [Google Scholar]
- 12.Stark R, Thierry D, Richard P, Gluckman E. Long-term bone marrow culture in Fanconi’s anaemia. Br J Haematol. 1993;83:554–559. doi: 10.1111/j.1365-2141.1993.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 13.Justo GA, Bitencourt MA, Pasquini R, Castelo-Branco MT, Rumjanek VM. Increased IL10 plasmatic levels in Fanconi anemia patients. Cytokine. 2013 doi: 10.1016/j.cyto.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Justo GA, Bitencourt MA, Pasquini R, Castelo-Branco MT, Almeida-Oliveira A, Diamond HR, Rumjanek VM. Immune status of Fanconi anemia patients: decrease in T CD8 and CD56 CD16 NK lymphocytes. Ann Hematol. 2013 doi: 10.1007/s00277-013-1953-4. [DOI] [PubMed] [Google Scholar]
- 15.Korthof ET, Svahn J, Peffault de LR, Terranova P, Moins-Teisserenc H, Socie G, Soulier J, Kok M, Bredius RG, van TM, Jol-van der Zijde EC, Pistorio A, Corsolini F, Parodi A, Battaglia F, Pistoia V, Dufour C, Cappelli E. Immunological profile of Fanconi anemia: a multicentric retrospective analysis of 61 patients. Am J Hematol. 2013;88:472–476. doi: 10.1002/ajh.23435. [DOI] [PubMed] [Google Scholar]
- 16.Myers KC, Bleesing JJ, Davies SM, Zhang X, Martin LJ, Mueller R, Harris RE, Filipovich AH, Kovacic MB, Wells SI, Mehta PA. Impaired immune function in children with Fanconi anaemia. Br J Haematol. 2011;154:234–240. doi: 10.1111/j.1365-2141.2011.08721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui K, Giri N, Alter BP, Pinto LA. Cytokine production by bone marrow mononuclear cells in inherited bone marrow failure syndromes. Br J Haematol. 2013;163:81–92. doi: 10.1111/bjh.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solder B, Weiss M, Jager A, Belohradsky BH. Dyskeratosis congenita: multisystemic disorder with special consideration of immunologic aspects. A review of the literature. Clin Pediatr (Phila) 1998;37:521–530. doi: 10.1177/000992289803700901. [DOI] [PubMed] [Google Scholar]
- 19.Jyonouchi S, Forbes L, Ruchelli E, Sullivan KE. Dyskeratosis congenita: a combined immunodeficiency with broad clinical spectrum--a single-center pediatric experience. Pediatr Allergy Immunol. 2011;22:313–319. doi: 10.1111/j.1399-3038.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- 20.Knudson M, Kulkarni S, Ballas ZK, Bessler M, Goldman F. Association of immune abnormalities with telomere shortening in autosomal-dominant dyskeratosis congenita. Blood. 2005;105:682–688. doi: 10.1182/blood-2004-04-1673. [DOI] [PubMed] [Google Scholar]
- 21.Lee BW, Yap HK, Quah TC, Chong A, Seah CC. T cell immunodeficiency in dyskeratosis congenita. Arch Dis Child. 1992;67:524–526. doi: 10.1136/adc.67.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sznajer Y, Baumann C, David A, Journel H, Lacombe D, Perel Y, Blouin P, Segura JF, Cezard JP, Peuchmaur M, Vulliamy T, Dokal I, Verloes A. Further delineation of the congenital form of X-linked dyskeratosis congenita (Hoyeraal-Hreidarsson syndrome) Eur J Pediatr. 2003;162:863–867. doi: 10.1007/s00431-003-1317-5. [DOI] [PubMed] [Google Scholar]
- 23.Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97:353–359. doi: 10.3324/haematol.2011.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaballie H, Renard M, Vermylen C, Scheers I, Revencu N, Regal L, Cassiman D, Sevenants L, Hoffman I, Corveleyn A, Bordon V, Haerynck F, Allegaert K, De BK, Roskams T, Boeckx N, Bossuyt X, Meyts I. Misdiagnosis as asphyxiating thoracic dystrophy and CMV-associated haemophagocytic lymphohistiocytosis in Shwachman-Diamond syndrome. Eur J Pediatr. 2013;172:613–622. doi: 10.1007/s00431-012-1908-0. [DOI] [PubMed] [Google Scholar]
- 25.Burstein E, Fearon ER. Colitis and cancer: a tale of inflammatory cells and their cytokines. J Clin Invest. 2008;118:464–467. doi: 10.1172/JCI34831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dougan M, Dranoff G. Inciting inflammation: the RAGE about tumor promotion. J Exp Med. 2008;205:267–270. doi: 10.1084/jem.20080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Sejas DP, Qiu Y, Williams DA, Pang Q. Inflammatory ROS promote and cooperate with the Fanconi anemia mutation for hematopoietic senescence. J Cell Sci. 2007;120:1572–1583. doi: 10.1242/jcs.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, Carr AG, Greene MH, Rosenberg PS. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150:179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brugnara C. Reference values in infancy and childhood. In: Orkin SH, Nathan DG, Ginsberg D, Look AT, Fisher DE, Lux SE, editors. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia: Saunders; 2009. pp. 1774–1784. [Google Scholar]
- 30.Shimamura A. Treatment of Hematologic Abnormalities in FA. In: Eiler ME, Frohnmayer D, Frohnmayer L, Larsen K, Owen J, editors. Fanconi Anemia: Guidelines for Diagnosis and Management. Eugene, OR: Fanconi Anemia Research Fund, Inc; 8 A.D. pp. 49–75. [Google Scholar]
- 31.Pinto LA, Edwards J, Castle PE, Harro CD, Lowy DR, Schiller JT, Wallace D, Kopp W, Adelsberger JW, Baseler MW, Berzofsky JA, Hildesheim A. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188:327–338. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- 32.Pinto LA, Viscidi R, Harro CD, Kemp TJ, Garcia-Pineres AJ, Trivett M, Demuth F, Lowy DR, Schiller JT, Berzofsky JA, Hildesheim A. Cellular immune responses to HPV-18, -31, and -53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006;353:451–462. doi: 10.1016/j.virol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Pinto LA, Castle PE, Roden RB, Harro CD, Lowy DR, Schiller JT, Wallace D, Williams M, Kopp W, Frazer IH, Berzofsky JA, Hildesheim A. HPV-16 L1 VLP vaccine elicits a broad-spectrum of cytokine responses in whole blood. Vaccine. 2005;23:3555–3564. doi: 10.1016/j.vaccine.2005.01.146. [DOI] [PubMed] [Google Scholar]
- 34.Feng X, Scheinberg P, Samsel L, Rios O, Chen J, McCoy JP, Jr, Ghanima W, Bussel JB, Young NS. Decreased plasma cytokines are associated with low platelet counts in aplastic anemia and immune thrombocytopenic purpura. J Thromb Haemost. 2012;10:1616–1623. doi: 10.1111/j.1538-7836.2012.04757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, Wang G, Zou W. Bone marrow and the control of immunity. Cell Mol Immunol. 2012;9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyman SD, Seaberg M, Hanna R, Zappone J, Brasel K, Abkowitz JL, Prchal JT, Schultz JC, Shahidi NT. Plasma/serum levels of flt3 ligand are low in normal individuals and highly elevated in patients with Fanconi anemia and acquired aplastic anemia. Blood. 1995;86:4091–4096. [PubMed] [Google Scholar]
- 37.Omori F, Okamura S, Shimoda K, Otsuka T, Harada M, Niho Y. Levels of human serum granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor under pathological conditions. Biotherapy. 1992;4:147–153. doi: 10.1007/BF02171759. [DOI] [PubMed] [Google Scholar]
- 38.Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol. 2013;6:327–337. doi: 10.1586/ehm.13.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.